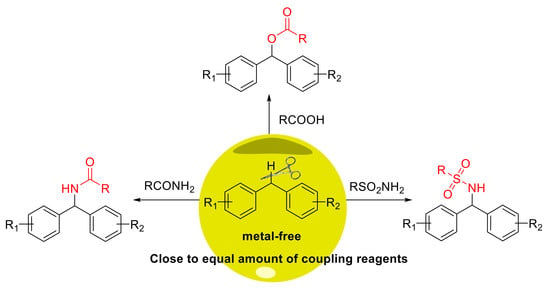
An Efficient Metal-Free Oxidative Esterification and Amination of Benzyl C–H Bond
Abstract
1. Introduction
2. Results
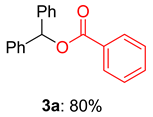
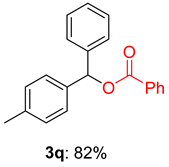
2.1. Optimization of Reaction Conditions for Synthesis of Benzhydryl Benzoate 3a
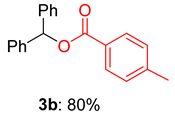
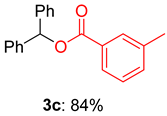
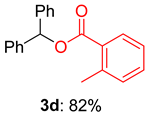
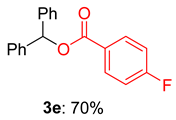
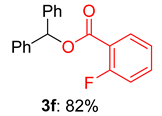
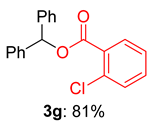
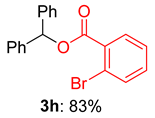
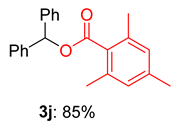
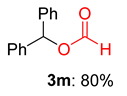
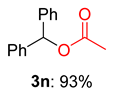
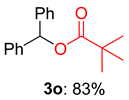
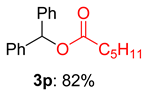
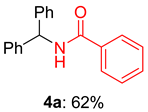
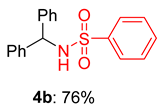
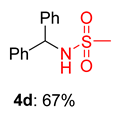
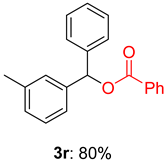
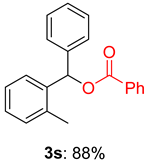
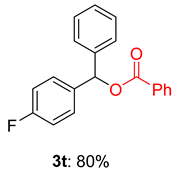
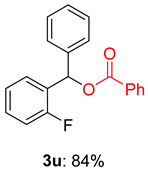
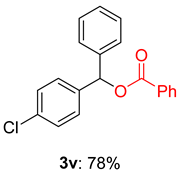
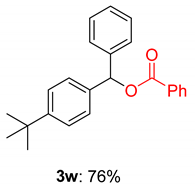
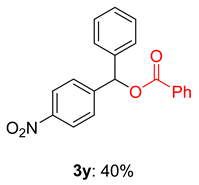
2.2. Substrate Scope for the Carboxylic Acids and Amines
2.3. Substrate Scope for the Diarylmethanes
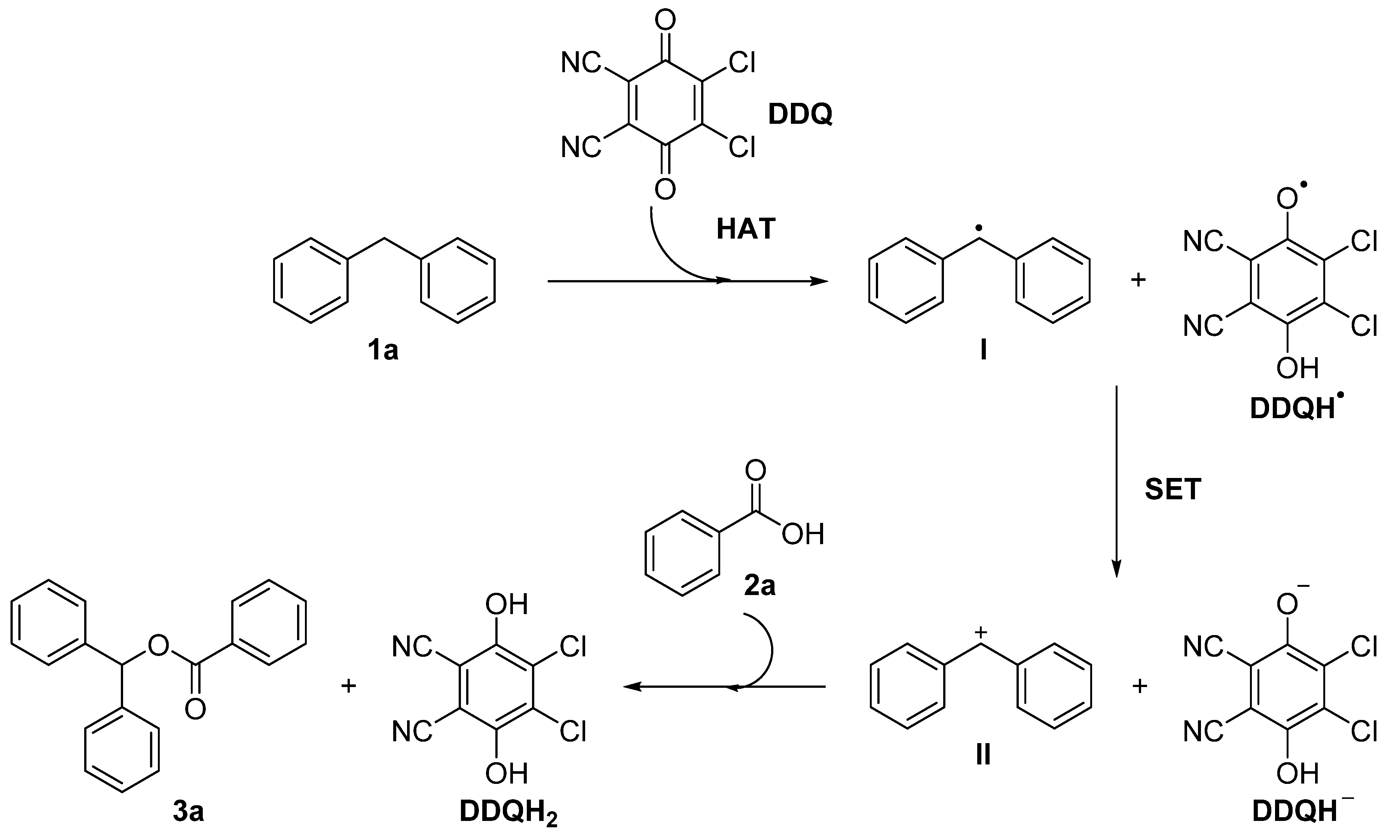
2.4. Mechanism
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Benzhydryl Benzoate
3.3. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Otera, J.; Nishikido, J. Esterification. Methods, Reactions, and Applications, 2nd ed.; WILEY-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Aliboni, A.; D’Andrea, A.; Massanisso, P. Propolis Specimens from Different Locations of Central Italy: Chemical Profiling and Gas Chromatography-mass Spectrometry (GC-MS) Quantitative Analysis of the Allergenic Esters Benzyl Cinnamate and Benzyl Salicylate. J. Agric. Food Chem. 2011, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Amino Acid-Protecting Groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [PubMed]
- Tzouras, N.V.; Stamatopoulos, I.K.; Papastavrou, A.T.; Liori, A.A.; Vougioukalakis, G.C. Sustainable Metal Catalysis in C–H Activation. Coord. Chem. Rev. 2017, 343, 25–138. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Gianpiero, C.; Warratz, S.; Ackermann, L. 3d Transition Metals for C-H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef]
- Kantam, M.L.; Gadipelly, C.; Deshmukh, G.; Reddy, K.R.; Bhargava, S. Copper Catalyzed C−H Activation. Chem. Rec. 2019, 19, 1302–1318. [Google Scholar] [CrossRef]
- Kumar, S.; Pradhan, S.; Roy, S.; De, P.B. Iron-Catalyzed Regioselective Remote C(sp2)-H Carboxylation of Naphthyl and Quinoline Amides. J. Org. Chem. 2019, 84, 10481–10489. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jing, K.; Li, Q.L.; Wang, G.W. Palladium-Catalyzed Decarboxylative Coupling of Potassium Oxalate Monoester with 2-Aryloxypyridines. Acta Chim. Sinica 2019, 77, 729–734. [Google Scholar] [CrossRef]
- Long, J.G.; Le, L.Y.; Iwasaki, T.; Qiu, R.H.; Kambe, N. Copper-Catalyzed Amination of C(sp3)-H bonds: From Anilides to Indolines. J. Org. Chem. 2020, 85, 482–492. [Google Scholar] [CrossRef]
- Tong, H.R.; Zheng, W.R.; Lv, X.Y.; He, G.; Liu, P.; Chen, G. Asymmetric Synthesis of β-Lactam via Palladium-Catalyzed Enantioselective Intramolecular C(sp3)-H Amidation. ACS Catal. 2020, 10, 114–120. [Google Scholar] [CrossRef]
- Ye, L.; Tian, Y.; Meng, X.; Gu, Q.S.; Liu, X.Y. Enantioselective Copper(I)/Chiral Phosphoric Acid Catalyzed Intramolecular Amination of Allylic and Benzylic C-H Bonds. Angew. Chem. Int. Ed. 2020, 59, 1129–1133. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Sharma, M.; Sharma, U.K.; Sinha, A.K. DDQ Catalyzed Benzylic Acetoxylation of Arylalkanes: A Case of Exquisitely Controlled Oxidation under Sonochemical Activation. Tetrahedron 2007, 63, 9718–9723. [Google Scholar] [CrossRef]
- Chen, L.; Shi, E.B.; Wan, X.B. Bu4NI-Catalyzed C-O Bond Formation by Using a Cross-Dehydrogenative Coupling (CDC) Reaction. Chem. Eur. J. 2011, 17, 4085–4089. [Google Scholar] [CrossRef]
- Uyanik, M.; Suzuki, D.; Yasui, T.; Ishihara, K. In Situ Generated (Hypo)Iodite Catalysts for the Direct α-Oxyacylation of Carbonyl Compounds with Carboxylic Acids. Angew. Chem. Int. Ed. 2011, 50, 5331–5334. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liang, S.; Chen, S.Y.; Zhang, J.; Fu, S.S.; Yu, X.Q. A Metal-Free Oxidative Esterification of the Benzyl CH Bond. Adv. Synth. Catal. 2012, 354, 1287–1292. [Google Scholar] [CrossRef]
- Baba, H.; Moriyama, K.; Togo, H. Benzylic-Acetoxylation of Alkylbenzenes with PhI(OAc)2 in the Presence of Catalytic Amounts of TsNH2 and I2. Tetrahedron Lett. 2011, 52, 4303–4307. [Google Scholar] [CrossRef]
- Pan, D.C.; Pan, Z.L.; Hu, Z.M.; Li, M.C.; Hu, X.Q.; Jin, L.Q.; Sun, N.; Hu, B.X.; Shen, Z.L. Metal-Free Aerobic Oxidative C-O Coupling of C(sp3)-H with Carboxylic Acids Catalyzed by DDQ and tert-Butyl Nitrite. Eur. J. Org. Chem. 2019, 5650–5655. [Google Scholar] [CrossRef]
- Yi, H.; Liu, Q.; Liu, J.; Zeng, Z.; Yang, Y.H.; Lei, A.W. DDQ-Catalyzed Oxidative C-O Coupling Of sp3 C-H Bonds with Carboxylic Acids. ChemSusChem 2012, 5, 2143–2146. [Google Scholar] [CrossRef]
- Nobre, S.M.; Monteiro, A.L. Synthesis of Diarylmethane Derivatives from Pd-Catalyzed Cross-Coupling Reactions of Benzylic Halides with Arylboronic Acids. Tetrahedron Lett. 2004, 45, 8225–8228. [Google Scholar] [CrossRef]
- Perusquía-Hernández, C.; Lara-Issasi, G.R.; Frontana-Uribe, B.A.; Cuevas-Yañez, E. Synthesis and Esterification Reactions of Aryl Diazomethanes Derived from Hydrazone Oxidations Catalyzed by TEMPO. Tetrahedron Lett. 2013, 54, 3302–3305. [Google Scholar] [CrossRef]
- Tran, V.H.; La, M.T.; Kim, H.K. Iron(III)-Catalyzed Direct Synthesis of Diphenylmethyl Esters from 2-Diphenylmethoxypyridine. Synthetic Commun. 2019, 49, 2379–2387. [Google Scholar] [CrossRef]
- La, M.T.; Kim, H.K. Facile Synthesis of Diphenylmethyl Esters from 2-Diphenylmethoxypyridine Using Catalytic Boron Trifluoridediethyl Etherate. Tetrahedron Lett. 2018, 59, 1855–1859. [Google Scholar] [CrossRef]
- Noto, R.; Buscemi, S.; Consiglio, G.; Spinelli, D. Linear Free Energy ortho-Correlations in the Thiophene Series. Part IX. Kinetics of Esterification with Diazodiphenylmethane of Some 3-, 4-, and 5-Substituted Thiophene-2-Carboxylic Acids in Methanol. J. Heterocyclic Chem. 1981, 18, 735–738. [Google Scholar] [CrossRef]
- Adhikari, A.A.; Shah, J.P.; Howard, K.T.; Russo, C.M.; Wallach, D.R.; Linaburg, M.R.; Chisholm, J.D. Convenient Formation of Diphenylmethyl Esters Using Diphenylmethyl Trichloroacetimidate. Synlett 2014, 25, 283–287. [Google Scholar]
- Mahajani, N.S.; Meador, R.I.L.; Smith, T.J.; Canarelli, S.E.; Adhikari, A.A.; Shah, J.P.; Russo, C.M.; Wallach, D.R.; Howard, K.T.; Millimaci, A.M.; et al. Ester Formation via Symbiotic Activation Utilizing Trichloroacetimidate Electrophiles. J. Org. Chem. 2019, 84, 7871–7882. [Google Scholar] [CrossRef]
- Richter, S.C.; Oestreich, M. Bioinspired Metal-Free Formal Decarbonylation of α-Branched Aliphatic Aldehydes at Ambient Temperature. Chem. Eur. J. 2019, 25, 8508–8512. [Google Scholar] [CrossRef]
- Peterson, P.E.; Stepanian, M. Hydroboration of Vinyl Ethers with Diisopinocampheylborane. J. Org. Chem. 1988, 53, 1903–1907. [Google Scholar] [CrossRef]
- Ju, T.; Fu, Q.; Ye, J.H.; Zhang, Z.; Liao, L.L.; Yan, S.S.; Tian, X.Y.; Luo, S.P.; Li, J.; Yu, D.G. Selective and Catalytic Hydrocarboxylation of Enamides and Imines with CO2 to Generate α, α-Disubstituted α-Amino Acids. Angew. Chem. Int. Ed. 2018, 57, 13897–13901. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, H.M.; Zhang, E.L.; Ma, X.T.; Chen, J.H.; Yu, X.C.; Li, H. Selective Catalytic Hofmann N-alkylation of Poor Nucleophilic Amines and Amides with Catalytic Amounts of Alkyl Halides. Green Chem. 2016, 18, 3940–3944. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Ni, Y.X.; Liu, R.; Song, K.X.; Lin, S.H.; Pan, Q.M. In Situ Generated Cationic Pd(II)/Bipyridine-Catalyzed Addition of Arylboronic Acids to N-Sulfonyl-Arylaldimines. Tetrahedron Lett. 2017, 58, 2034–2037. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

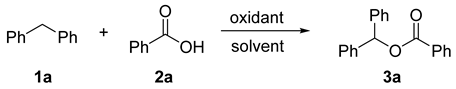
| Entry | Oxidant (equiv.) | Solvent | Yield b (%) |
|---|---|---|---|
| 1 | DDQ (1.2) | DCE | 95 |
| 2 | BQ (1.2) | DCE | 0 |
| 3 | TBHP (1.2) | DCE | 0 |
| 4 | tBuOOtBu (1.2) | DCE | 0 |
| 5 | H2O2 (1.2) | DCE | 0 |
| 6 | dicumyl peroxide (1.2) | DCE | 0 |
| 7 | DDQ (1.2) | CHCl3 | 94 |
| 8 | DDQ (1.2) | CH2Cl2 | 93 |
| 9 | DDQ (1.2) | Cl2CHCHCl2 | 80 |
| 10 | DDQ (1.2) | EtOAc | 10 |
| 11 | DDQ (1.2) | CH3CN | 12 |
| 12 | DDQ (1.2) | H2O | 0 |
| 13 | DDQ (1.2) | pyridine | 0 |
| 14c | DDQ (1.2) | DCE | 72 |
| 15d | DDQ (0.5) | DCE | 51 |
| 16e | DDQ (1.2) | DCE | 70 |
| 17f | DDQ (1.2) | DCE | 84 |

 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |

 |  |  |
 |  |  |
 |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, R.; He, G.; Zhang, J. An Efficient Metal-Free Oxidative Esterification and Amination of Benzyl C–H Bond. Molecules 2020, 25, 1527. https://doi.org/10.3390/molecules25071527
Liu S, Chen R, He G, Zhang J. An Efficient Metal-Free Oxidative Esterification and Amination of Benzyl C–H Bond. Molecules. 2020; 25(7):1527. https://doi.org/10.3390/molecules25071527
Chicago/Turabian StyleLiu, Saiwen, Ru Chen, Guowen He, and Jin Zhang. 2020. "An Efficient Metal-Free Oxidative Esterification and Amination of Benzyl C–H Bond" Molecules 25, no. 7: 1527. https://doi.org/10.3390/molecules25071527
APA StyleLiu, S., Chen, R., He, G., & Zhang, J. (2020). An Efficient Metal-Free Oxidative Esterification and Amination of Benzyl C–H Bond. Molecules, 25(7), 1527. https://doi.org/10.3390/molecules25071527