Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.)
Abstract
1. Introduction
2. Material and Methods
2.1. Commercial Reagents
2.2. General Experimental Procedures
2.3. Plant Material
2.4. Extraction and Fractionation
2.5. Chromatographic Analysis
2.6. In Vitro Pharmacological Evaluation
2.6.1. Cell Lines and Culture Conditions
2.6.2. Sample Dilution
2.6.3. Anti-Proliferative Assay
2.6.4. E-Screen Assay
2.6.5. Cell Cycle Analysis
2.7. In Vivo Assays
2.7.1. Animals
Samples and Drugs Preparation
2.8. Acute oral Toxicity
2.9. In Vivo Anti-Cancer Evaluation
2.9.1. Ehrlich Solid Tumor
2.9.2. Hollow Fiber Assay
2.10. In Vivo Anti-Estrogenic Evaluation
Uterothropic Assay
2.11. Statistical Analysis
3. Results
3.1. Chromatographic Analysis of FFINAL
3.2. In Vitro Assays
3.2.1. In Vitro Anti-Proliferative Screening
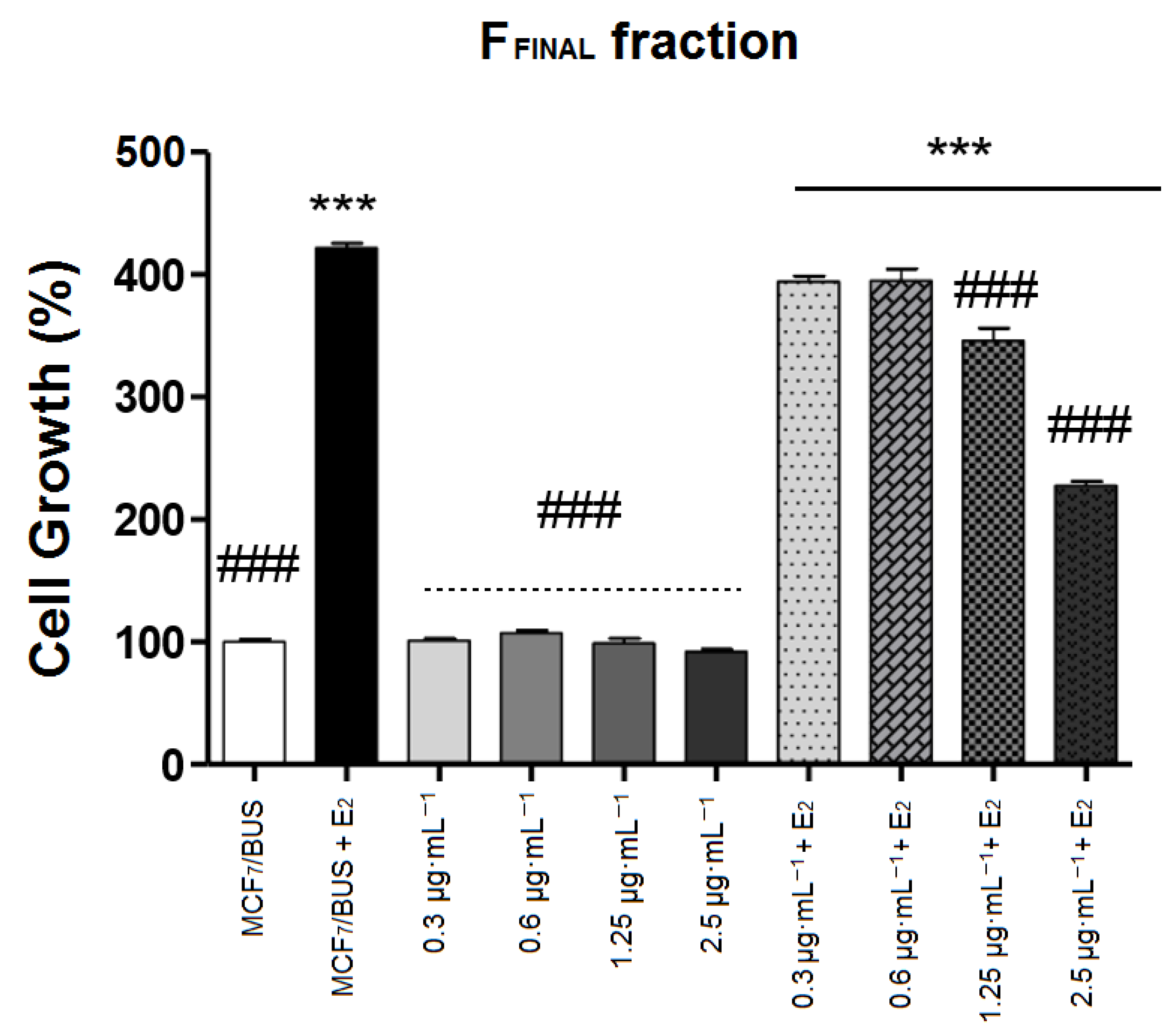
3.2.2. E-Screen Assay in MCF-7 BUS Cells
3.2.3. MCF-7 BUS Cell Cycle by Flow Cytometry
3.3. In Vivo Assays
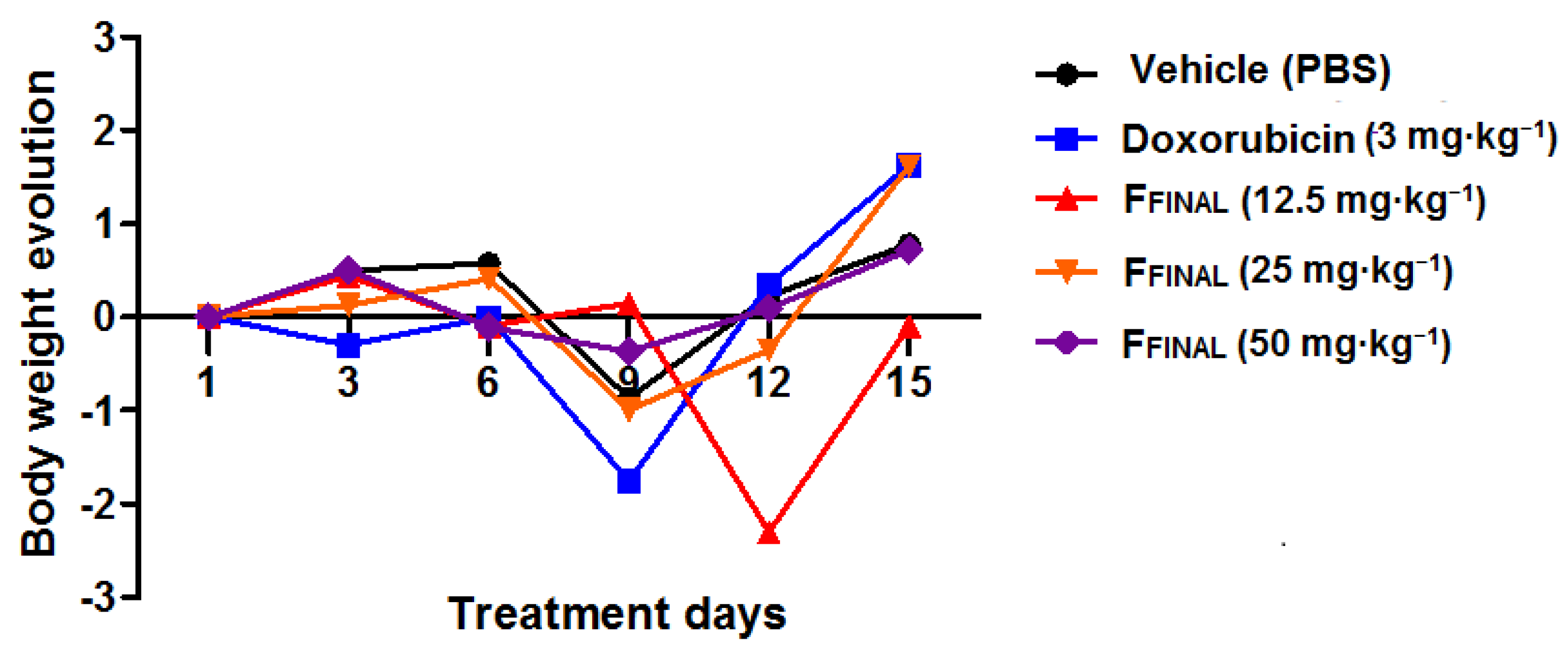
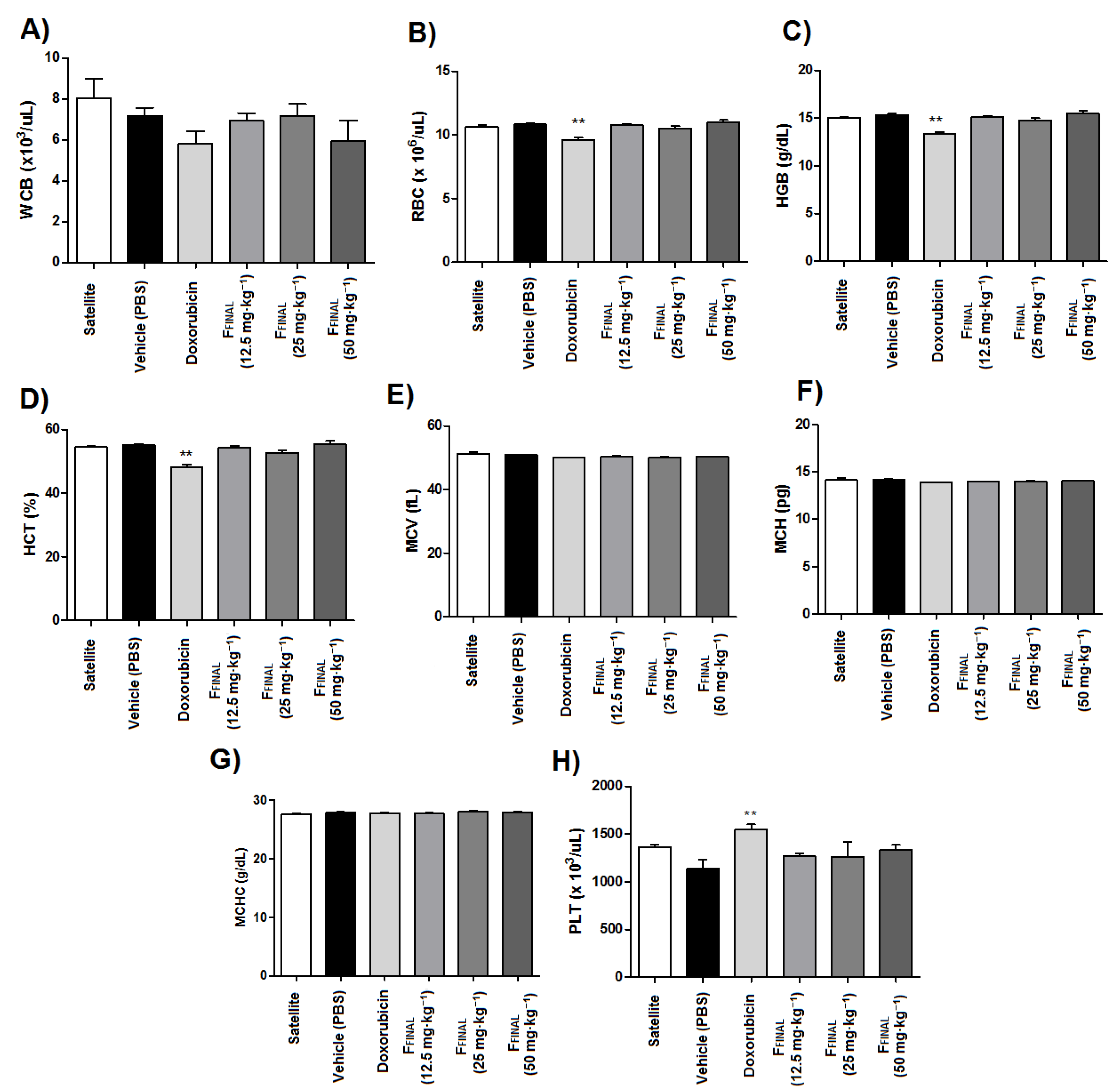
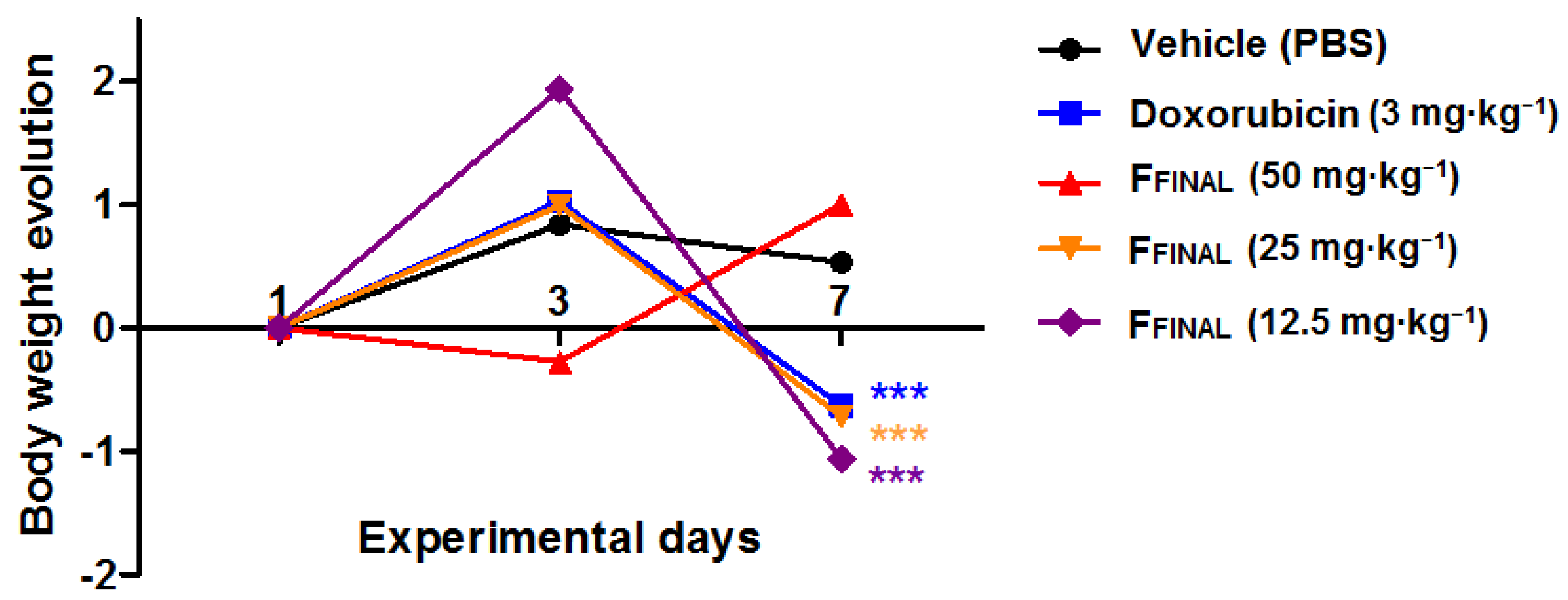
3.3.1. Oral Acute Toxicity
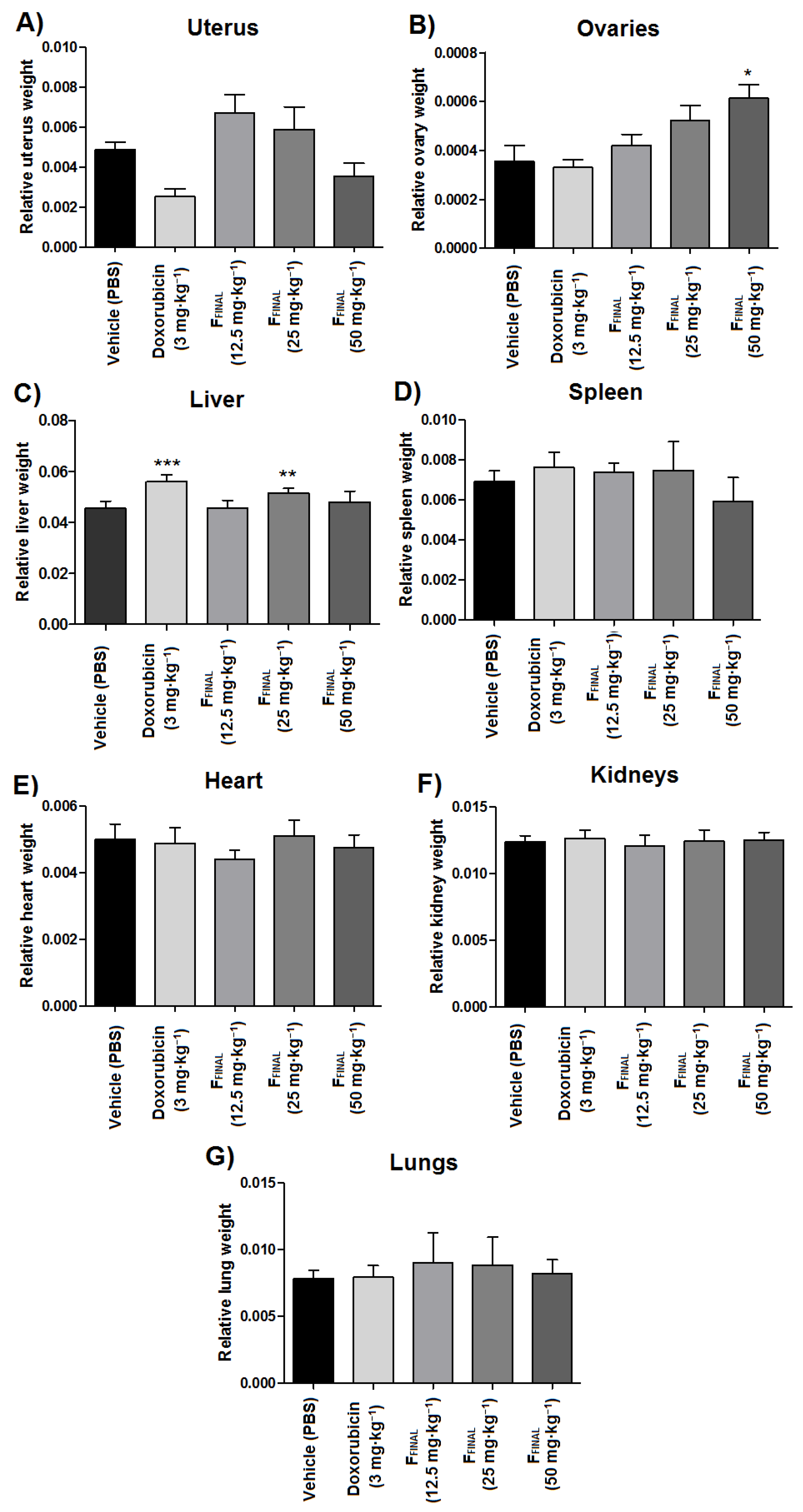
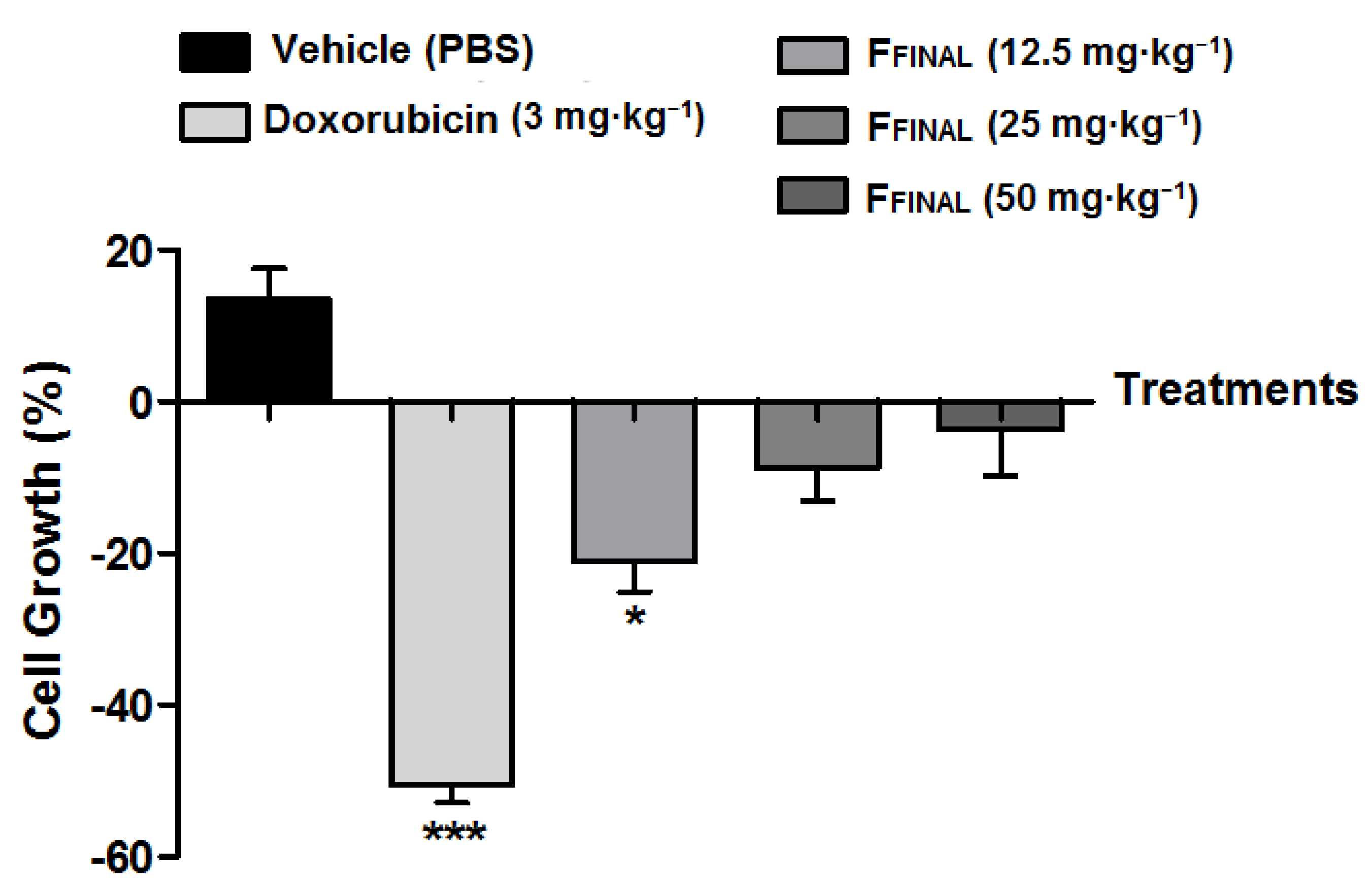
3.3.2. Ehrlich Solid Tumor (Back)
3.3.3. Hollow Fiber Assays with MCF-7
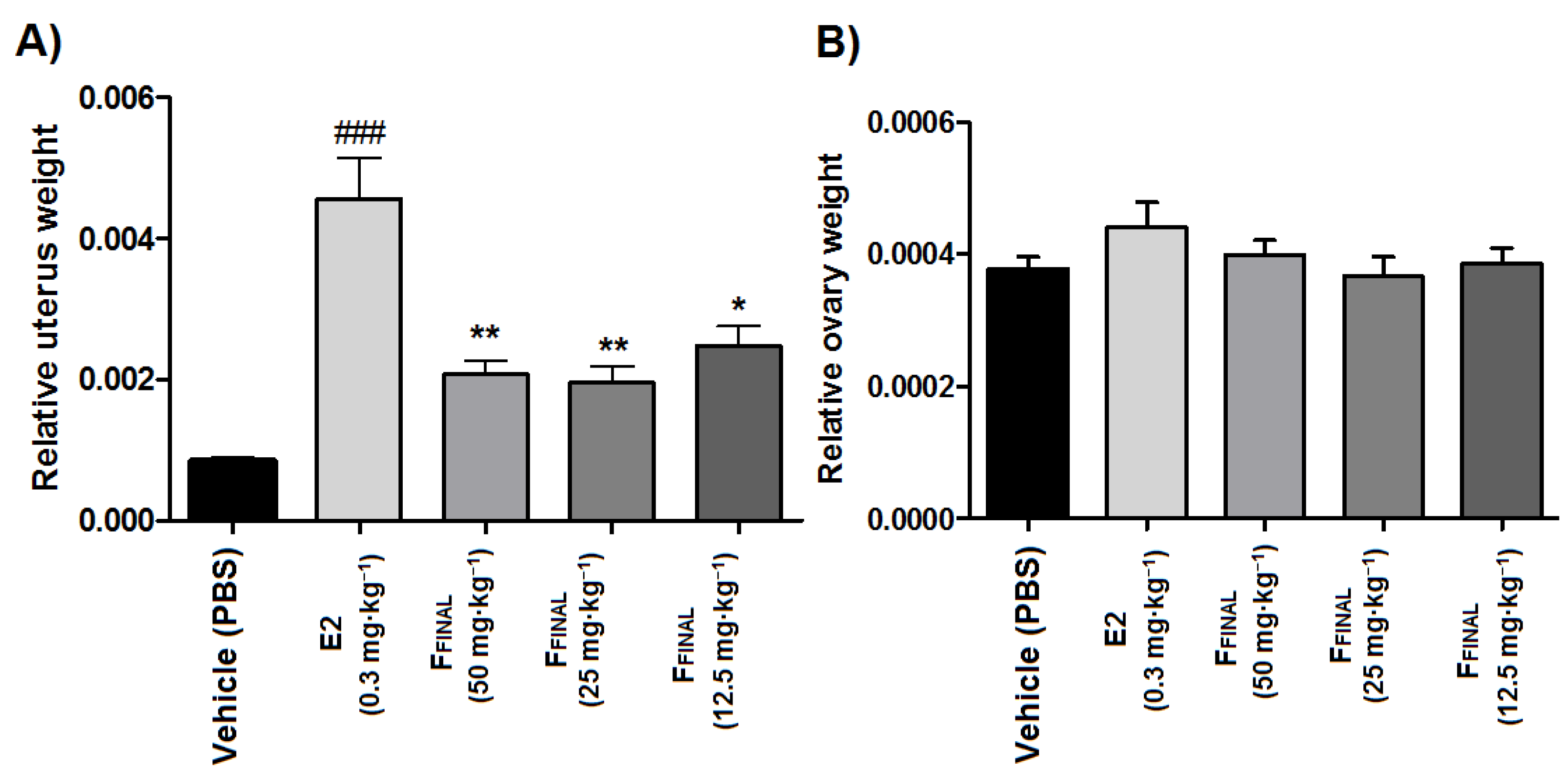
3.3.4. Uterotrophic Activity in Prepubertal Rats
In vivo Anti-Estrogenic Activity of the FFINAL Fraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Salomone, S. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Mondal, S.; Bandyopadhyay, S.K.; Ghosh, M.; Mukhopadhyay, S.; Roy, S.; Mandal, C. Natural Products: Promising Resources for Cancer Drug Discovery. Anticancer Agents Med. Chem. 2012, 12, 49–75. [Google Scholar] [CrossRef]
- John, M. Natural Products in Drug Discovery: The Past, Present and Future. Nat. Rev. Cancer 2002, 2, 3–8. [Google Scholar]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Ngbolua, J.-P.K.-N. A review on the Phytochemistry and Pharmacology of Psidium guajava L. (Myrtaceae) and Future direction. Discov. Phytomedicine 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Joseph, B.; Priya, R.M. Phytochemical and biopharmaceutical aspects of Psidium guajava (L.) essential oil: A review. Res. J. Med. Plant 2011, 5, 432–442. [Google Scholar] [CrossRef]
- El-Ahmady, S.H.; Ashour, M.L.; Wink, M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013, 25, 475–481. [Google Scholar] [CrossRef]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Hirudkar, J.R.; Parmar, K.M.; Prasad, R.S.; Sinha, S.K.; Lomte, A.D.; Itankar, P.R.; Prasad, S.K. The antidiarrhoeal evaluation of Psidium guajava L. against enteropathogenic Escherichia coli induced infectious diarrhoea. J. Ethnopharmacol. 2020, 251, 112561. [Google Scholar] [CrossRef]
- Chen, K.C.; Peng, C.C.; Chiu, W.T.; Cheng, Y.T.; Huang, G.T.; Hsieh, C.L.; Peng, R.Y. Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr. Cancer 2010, 62, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.H.; Park, K.R.; Kim, S.M.; Yun, H.M.; Nam, D.; Lee, S.G.; Jang, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, P.; Doto, A.; Miceli, M.; Mita, L.; Benedetti, R.; Nebbioso, A.; Veglione, M.; Rigano, D.; Cioffi, M.; Sica, V.; et al. Psidium guajava L. anti-neoplastic effects: Induction of apoptosis and cell differentiation. Cell Prolif. 2012, 45, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Park, H.R. Anticancer activity of guava (Psidium guajava L.) branch extracts against HT-29 human colon cancer cells. J. Med. Plants Res. 2010, 4, 891–896. [Google Scholar]
- Correa, M.G.; Couto, J.S.; Teodoro, A.J. Anticancer properties of Psidium guajava—A mini-review. Asian Pacific J. Cancer Prev. 2016, 17, 4199–4204. [Google Scholar]
- Rizzo, L.Y.; Longato, G.B.; Ruiz, A.L.G.; Tinti, S.V.; Possenti, A.; Vendramini-Costa, D.B.; Sartoratto, A.; Figueira, G.M.; Silva, F.L.N.; Eberlin, M.N.; et al. In Vitro, In Vivo and In Silico Analysis of the Anticancer and Estrogen-like Activity of Guava Leaf Extracts. Curr. Med. Chem. 2014, 21, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- Lapa, A.J.; Souccar, C.; Lima-Landman, M.T.R.; Castro, M.A.S.; Godinho, R.O.; Lima, T.C.M. Farmacologia e toxicologia de produtos naturais. In Farmacognosia: Da Planta ao Medicamento, 5th ed.; Universidade UFRGS: Porto Alegre, Brazil, 2004; pp. 247–262. [Google Scholar]
- Iwamoto, L.H.; Vendramini-Costa, D.B.; Monteiro, P.A.; Ruiz, A.L.T.G.; de Oliveira Sousa, I.M.; Foglio, M.A.; De Carvalho, J.E.; Rodrigues, R.A.F. Anticancer and anti-inflammatory activities of a standardized dichloromethane extract from Piper umbellatum L. leaves. Evidence-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Hollingshead, M.G.; Alley, M.C.; Camalier, R.F.; Abbott, B.J.; Mayo, J.G.; Malspeis, L.; Grever, M.R. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995, 57, 131–141. [Google Scholar] [CrossRef]
- Decker, S.; Hollingshead, M.; Bonomi, C.A.; Carter, J.P.; Sausville, E.A. The hollow fibre model in cancer drug screening: The NCI experience. Eur. J. Cancer 2004, 40, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.; Pezzuto, J.M.; Farnsworth, N.R.; Wani, M.C.; Kinghorn, A.D.; Swanson, S.M. Use of the in vivo hollow fiber assay in natural products anticancer drug discovery. J. Nat. Prod. 2009, 72, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Geiser, A.G.; Hummel, C.W.; Draper, M.W.; Henck, J.W.; Cohen, I.R.; Rudmann, D.G.; Donnelly, K.B.; Adrian, M.D.; Shepherd, T.A.; Wallace, O.B.; et al. A new selective estrogen receptor modulator with potent uterine antagonist activity, agonist activity in bone, and minimal ovarian stimulation. Endocrinology 2005, 146, 4524–4535. [Google Scholar] [CrossRef] [PubMed]
- Hummel, C.W.; Geiser, A.G.; Bryant, H.U.; Cohen, I.R.; Dally, R.D.; Fong, K.C.; Frank, S.A.; Hinklin, R.; Jones, S.A.; Lewis, G.; et al. A selective estrogen receptor modulator designed for the treatment of uterine leiomyoma with unique tissue specificity for uterus and ovaries in rats. J. Med. Chem. 2005, 48, 6772–6775. [Google Scholar] [CrossRef]
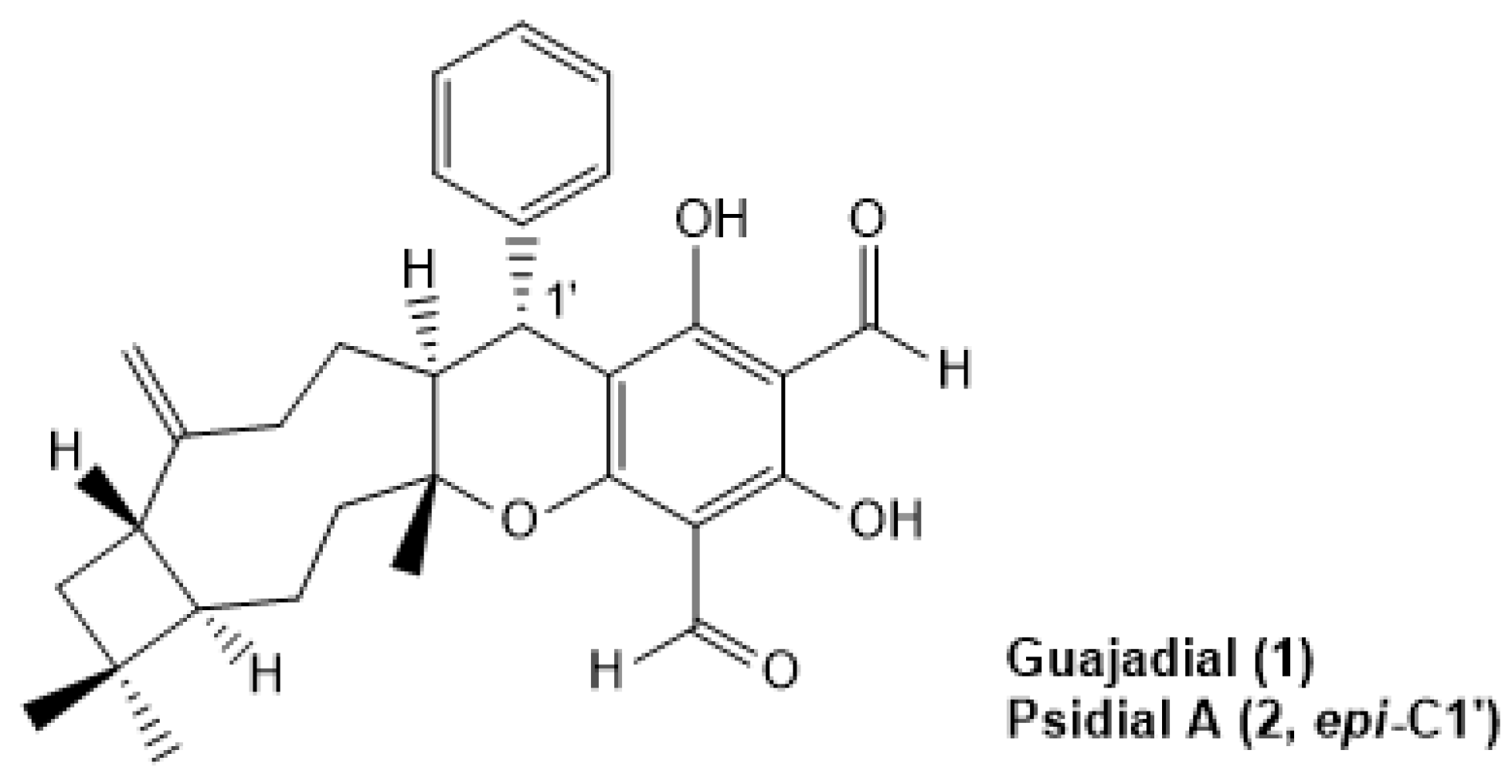
- Yang, X.L.; Hsieh, K.L.; Liu, J.K. Guajadial: An unusual meroterpenoid from guava leaves Psidium guajava. Org. Lett. 2007, 9, 5135–5138. [Google Scholar] [CrossRef]
- Chapman, L.M.; Beck, J.C.; Lacker, C.R.; Wu, L.; Reisman, S.E. Evolution of a Strategy for the Enantioselective Total Synthesis of (+)-Psiguadial B. J. Org. Chem. 2018, 83, 6066–6085. [Google Scholar] [CrossRef]
- Rieseberg, M.; Kasper, C.; Reardon, K.F.; Scheper, T. Flow cytometry in biotechnology. Appl. Microbiol. Biotechnol. 2001, 56, 350–360. [Google Scholar] [CrossRef]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2018, 60, 524–536. [Google Scholar] [CrossRef]
- Osborne, C.K.; Trent, J.M.; Boldt, D.H.; Clark, G.M. Effects of tamoxifen on human breast cancer cell cycle kinetics: Accumulation of cells in early g1 phase. Cancer Res. 1983, 43, 3583–3585. [Google Scholar]
- Ehrlich, P. Experimentelle carcinomstudien an Mäusen. Arb. Inst. Exp. Ther. Frankfurt 1906, 1, 78–80. [Google Scholar]
- Graeser, R.; Esser, N.; Unger, H.; Fichtner, I.; Zhu, A.; Unger, C.; Kratz, F. INNO-206, the (6-maleimidocaproyl hydrazone derivative of doxorubicin), shows superior antitumor efficacy compared to doxorubicin in different tumor xenograft models and in an orthotopic pancreas carcinoma model. Invest. New Drugs 2010, 28, 14–19. [Google Scholar] [CrossRef]
- Uboh, F.E.; Edet, E.E.; Eteng, M.U.; Eyong, E.U. Comparative effect of aqueous extract of P. Guajava leaves and ascorbic acid on serum sex hormones levels in male and female rats. J. Appl. Sci. Res. 2010, 6, 275–279. [Google Scholar]
- Rang, H.; Dale, M.; Ritter, M.; Flower, R.; Henderson, G. Anticancer drugs. In Rang and Dale’s Pharmacology, 7th ed.; Hyde, M., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; ISBN 978-0-7020-3471-8. [Google Scholar]
- Zhang, Y.B.; Ye, Y.P.; Wu, X.D.; Sun, H.X. Astilbotriterpenic acid induces growth arrest and apoptosis in HeLa cells through mitochondria-related pathways and reactive oxygen species (ROS) production. Chem. Biodivers. 2009, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Luo, N.; Wu, X.; Xu, Z.; Wang, X.; Pan, X. The modulatory role of low concentrations of bisphenol A on tamoxifen-induced proliferation and apoptosis in breast cancer cells. Environ. Sci. Pollut. Res. 2019, 26, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Hall, J. Pituitary Hormones and Their Control by Hypothalamus. In Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier Science: Amsterdam, The Netherlands, 2015; ISBN 9781455770168. [Google Scholar]
- Costanzo, L.S. Fisiología endócrina. In Fisiología, 6th ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; ISBN 9788491132738. [Google Scholar]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Cell Lines | Histology a | D.I. h (104 cell mL−1) | |
|---|---|---|---|
| Tumor cell lines | U251 b | CNS, Glioblastoma | 4.0 |
| MCF 7 b | Adenocarcinoma-mammary gland | 6.0 | |
| MCF7 BUS c | Adenocarcinoma-mammary gland | 6.0 | |
| MDA-MB-231d | Adenocarcinoma-mammary gland | 4.0 | |
| NCI-ADR/RES b, e | Adenocarcinoma-ovary | 5.0 | |
| OVCAR-3 b | Adenocarcinoma-ovary | 7.0 | |
| 786-0 b | Adenocarcinoma-kidney | 5.0 | |
| NCI-H460 b | Large Cell Carcinoma-lung | 4.0 | |
| PC-3 b | Adenocarcinoma-prostate | 4.5 | |
| HT29 b | Adenocarcinoma-colon | 5.0 | |
| Immortalized cell lines | HaCaT f | Spontaneously transformed keratinocytes from histologically normal skin | 4.0 |
| MCF 10A g | non-tumorigenic epithelial cells from mammary gland | 4.0 |
| Retention Time (RT) (min) | Relative Percentage (%) |
|---|---|
| 28.08 | 50.7 |
| 45.01 | 6.4 |
| 46.71 | 42.9 |
| *TGI (µg·mL−1) | |||
|---|---|---|---|
| FFINAL | DCE | Doxorubicin | |
| U251 | 3.90 | 61.5 | 1.22 |
| MCF-7 | 5.59 | 64.61 | 11.39 |
| NCI/ADR-RES | 6.19 | 50.51 | −0.12 |
| 786-0 | 7.69 | 62.88 | 4.20 |
| NCI-H460 | 2.79 | 57.82 | 0.44 |
| PC-3 | 3.70 | 39.94 | 1.52 |
| OVCAR-3 | 2.66 | 27.23 | 2.12 |
| HT-29 | 6.65 | 101.78 | 10.34 |
| HaCat | 2.91 | 58.39 | 0.35 |
| TGI (µg·mL−1) | |
|---|---|
| FFINAL | |
| MCF-7 | 8.86 |
| MCF-7 BUS | 2.27 |
| MDA MB 231 | 5.13 |
| MCF 10A | 8.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazioli, J.M.; Costa, J.H.; Shiozawa, L.; Ruiz, A.L.T.G.; Foglio, M.A.; Carvalho, J.E.d. Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.). Molecules 2020, 25, 1525. https://doi.org/10.3390/molecules25071525
Bazioli JM, Costa JH, Shiozawa L, Ruiz ALTG, Foglio MA, Carvalho JEd. Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.). Molecules. 2020; 25(7):1525. https://doi.org/10.3390/molecules25071525
Chicago/Turabian StyleBazioli, Jaqueline Moraes, Jonas Henrique Costa, Larissa Shiozawa, Ana Lúcia Tasca Gois Ruiz, Mary Ann Foglio, and João Ernesto de Carvalho. 2020. "Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.)" Molecules 25, no. 7: 1525. https://doi.org/10.3390/molecules25071525
APA StyleBazioli, J. M., Costa, J. H., Shiozawa, L., Ruiz, A. L. T. G., Foglio, M. A., & Carvalho, J. E. d. (2020). Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.). Molecules, 25(7), 1525. https://doi.org/10.3390/molecules25071525





