(Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties
Abstract
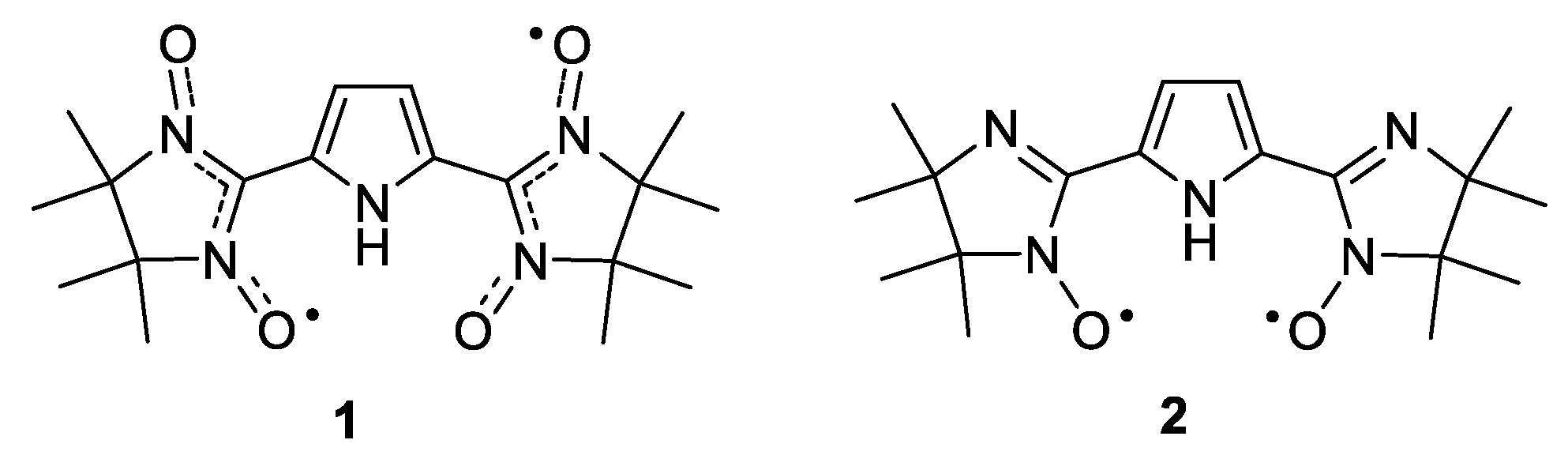
1. Introduction
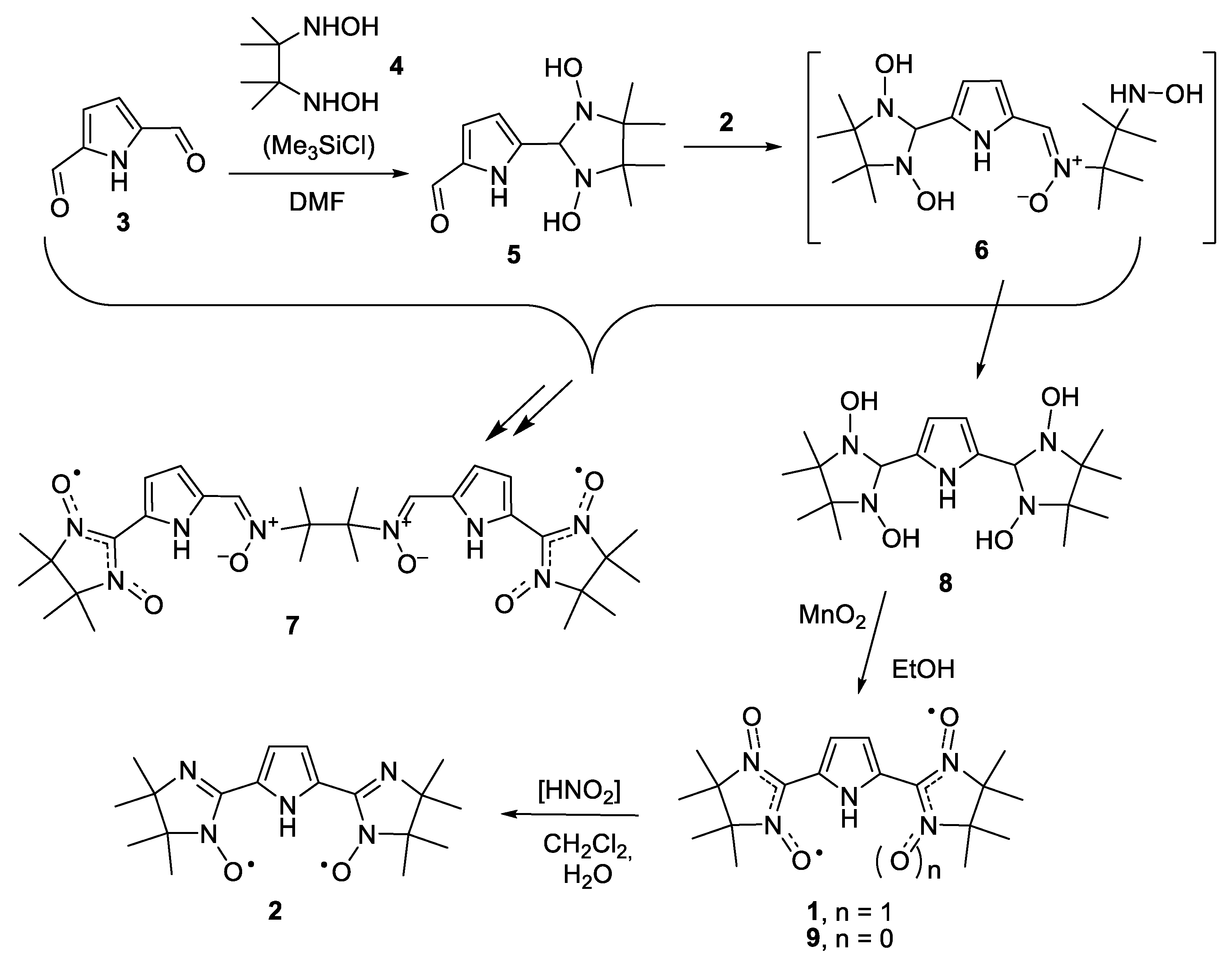
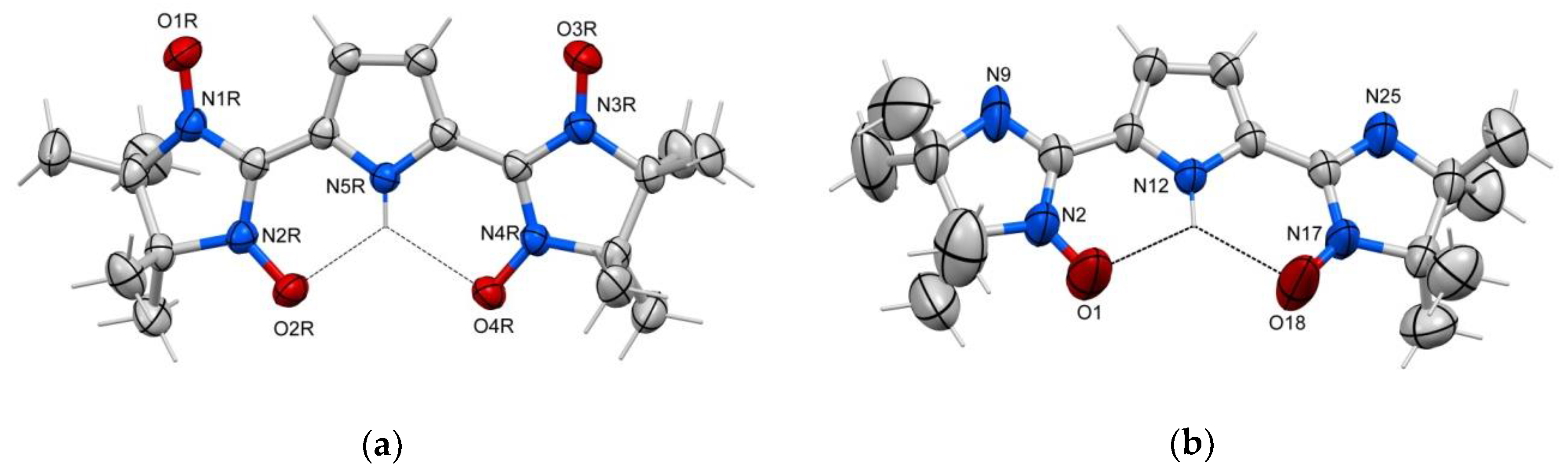
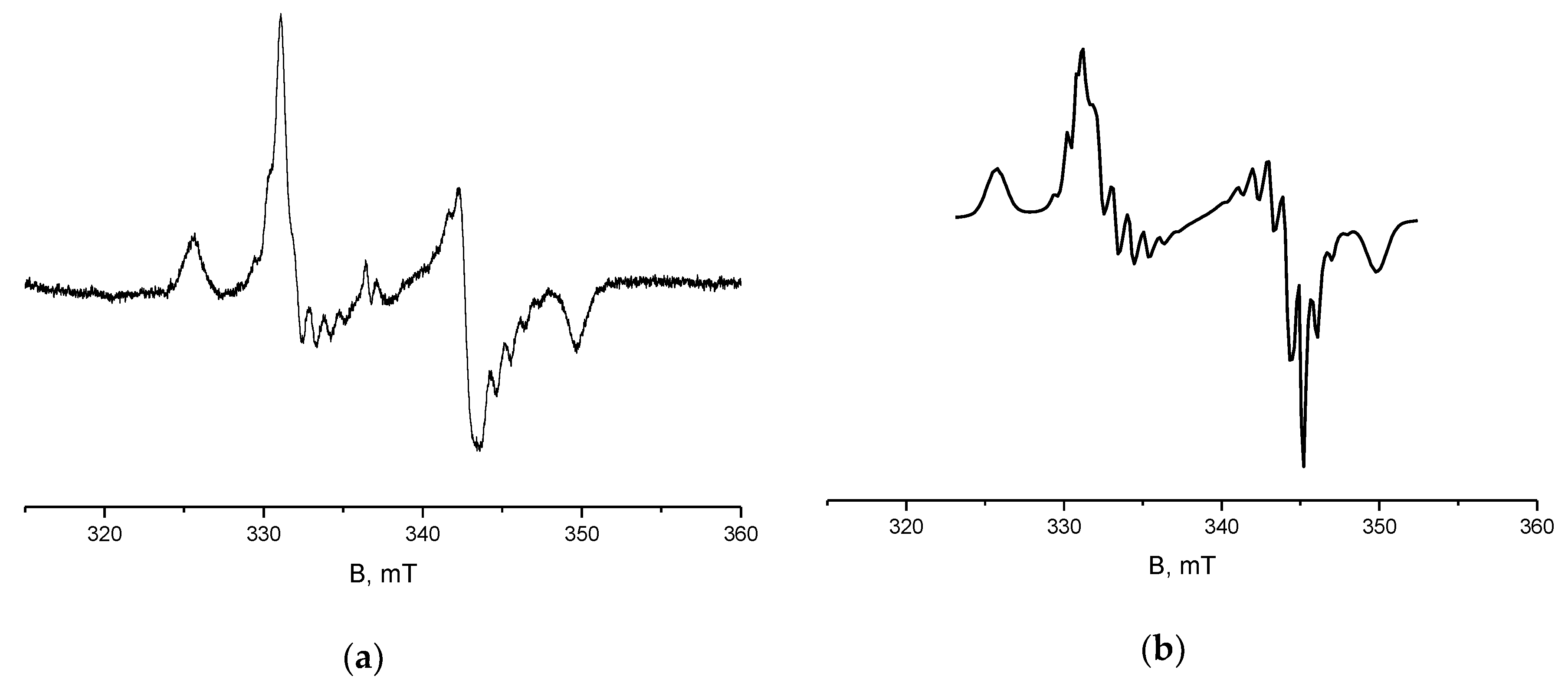
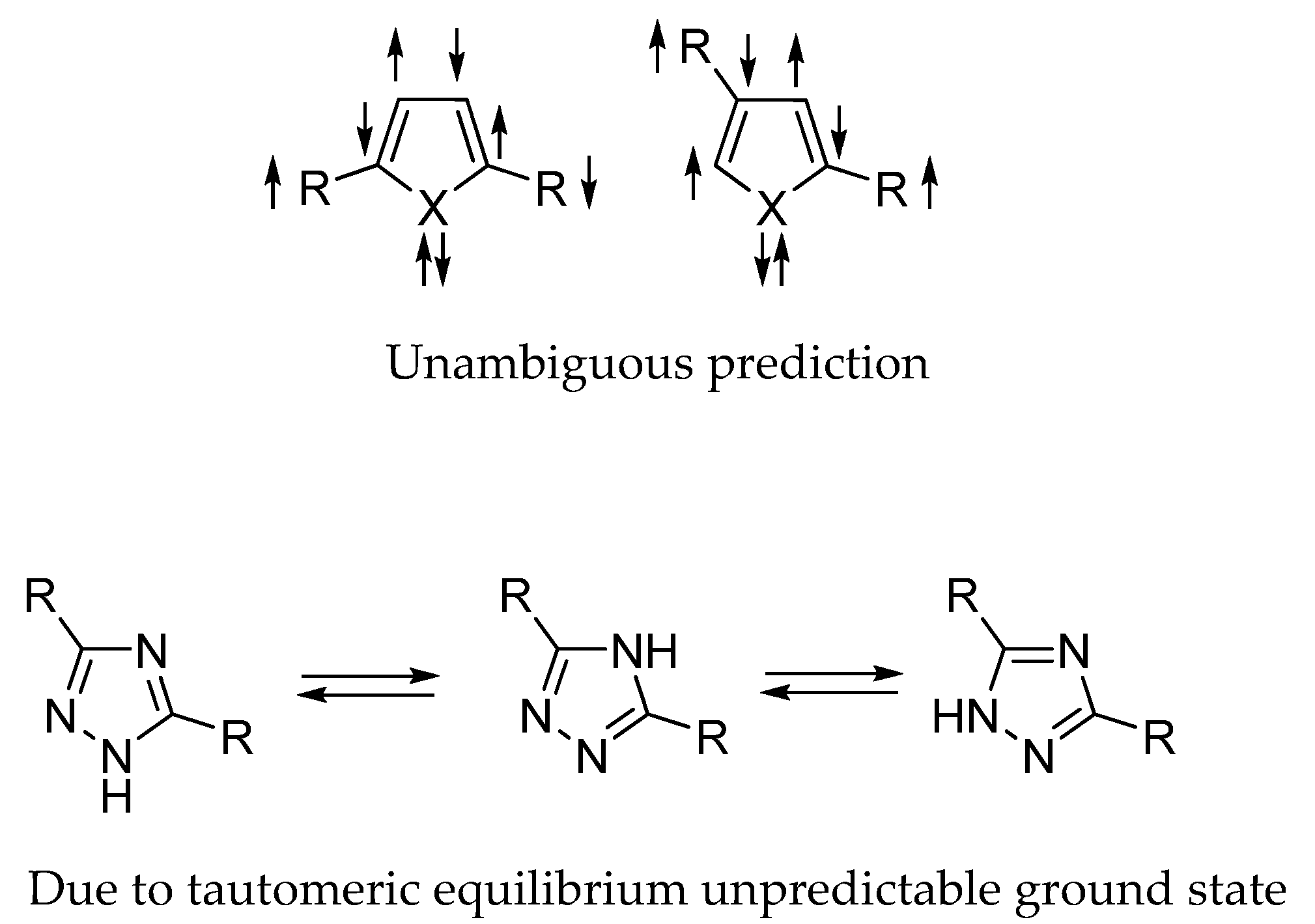
2. Results and Discussion
3. Conclusions
4. Experimental Part
4.1. Reagents and General Methods
4.2. Procedures for Preparation of 2,2′-(1H-pyrrole-2,5-diyl)-bis(4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl) (1)
4.3. Synthesis of 2,2′-(1H-pyrrole-2,5-diyl)bis(4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl) (2)
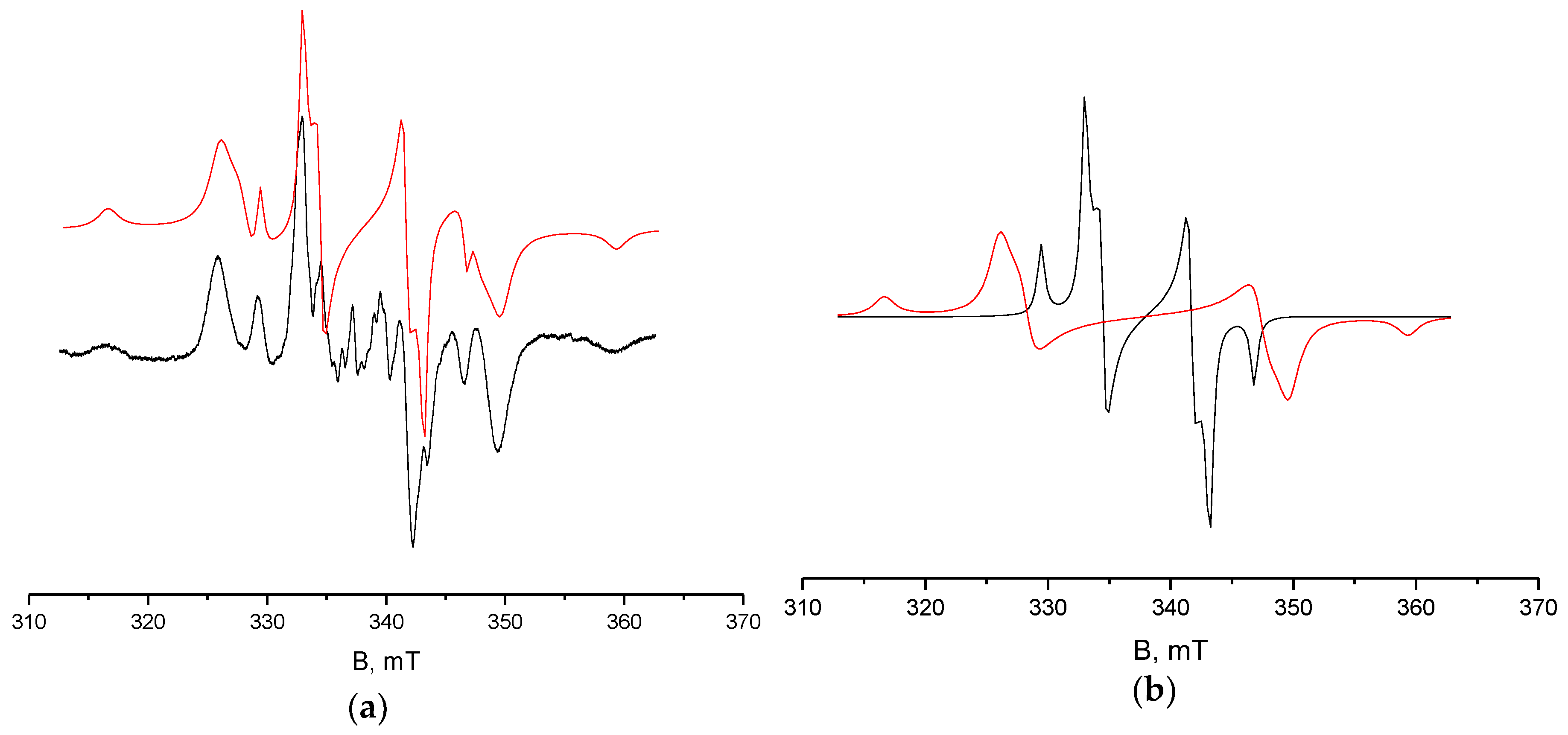
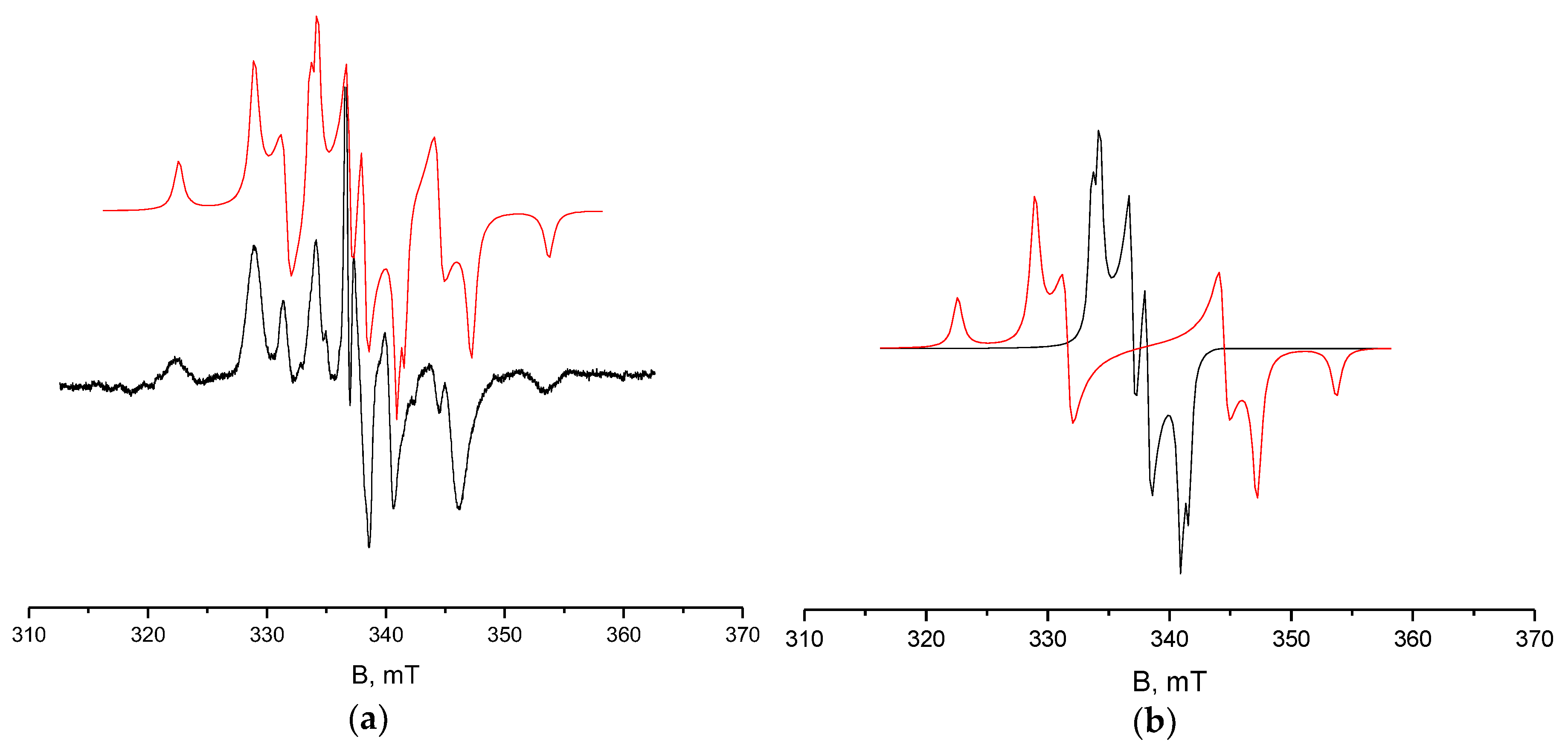
4.4. X-Band ESR Measurements
4.5. Crystallographic Analysis
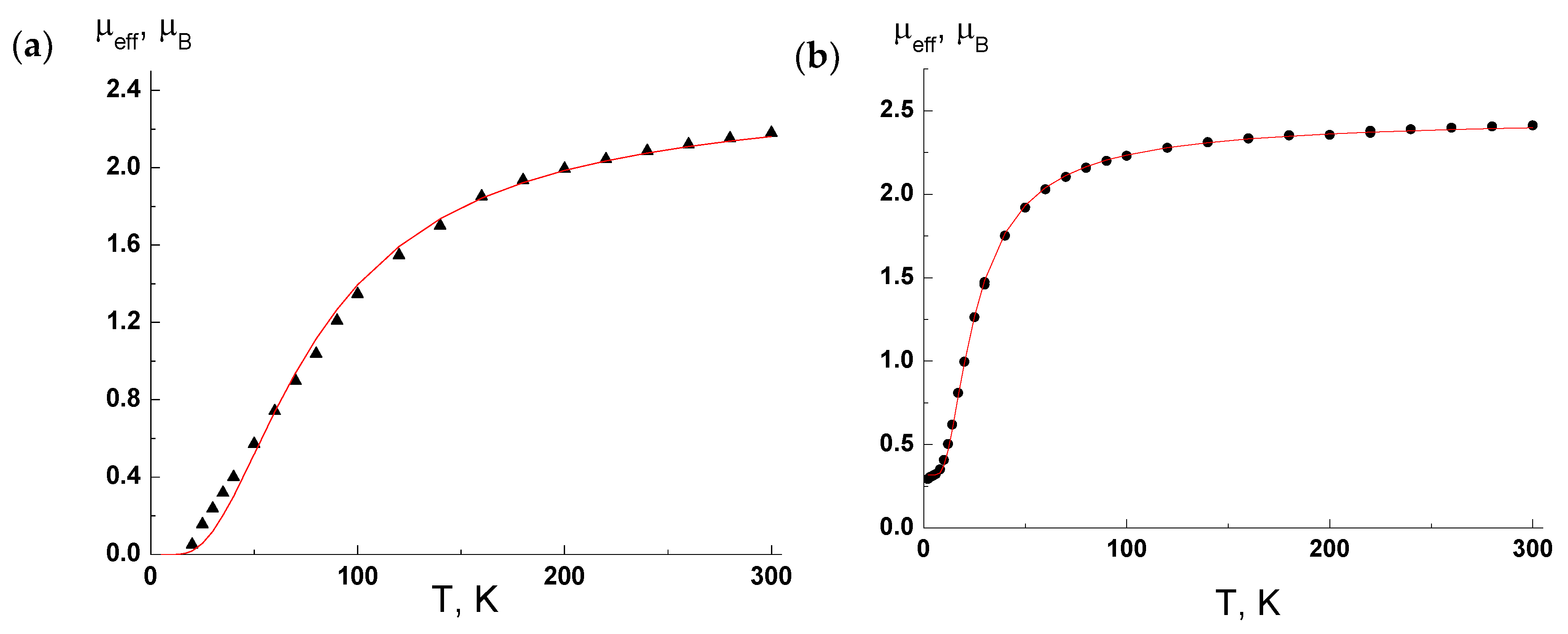
4.6. Magnetic Measurements
4.7. Quantum-Chemical Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pal, S.K.; Itkis, M.E.; Tham, F.S.; Reed, R.W.; Oakley, R.T.; Haddon, R.C. Resonating valence-bond ground state in a phenalenyl-based neutral radical conductor. Science 2005, 309, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Hu, L.; Suizu, R.; Nomura, K.; Yoshikawa, H.; Awaga, K.; Noda, Y.; Kanai, K.; Ouchi, Y.; Seki, K.; et al. Interactive radical dimers in photoconductive organic thin films. Angew. Chem. Int. Ed. 2009, 48, 4022–4024. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Komatsu, H.; Suzuki, K. Interplay between magnetism and conductivity derived from spin-polarized donor radicals. Chem. Soc. Rev. 2011, 40, 3105–3118. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Kinoshita, M. Molecular Magnetism; Itoh, K., Kinoshita, M., Eds.; Kodansha & Gordon and Breach: Tokyo, Japan, 2000. [Google Scholar]
- Miller, J.S.; Drillon, M. Magnetism: Molecules to Materials II–V; Miller, J.S., Drillon, M., Eds.; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Veciana, J. π-Electron. Magnetism from Molecules to Magnetic Materials; Veciana, J., Ed.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Hicks, R.G. Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron. Compounds; Hicks, R.G., Ed.; John Wiley and Sons: Chichester, UK, 2010. [Google Scholar]
- Kuratsu, M.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Kanzawa, K.; Hosokoshi, Y.; Lan, X.-Z.; Miyazaki, Y.; et al. (Nitronyl nitroxide)-substituted trioxytriphenylamine radical cation tetrachlorogallate salt: A 2p-electron-based weak ferromagnet composed of a triplet diradical cation. Chem. Asian. J. 2012, 7, 1604–1609. [Google Scholar] [CrossRef]
- Barskaya, I.; Tretyakov, E.; Sagdeev, R.; Ovcharenko, V.; Bagryanskaya, E.; Maryunina, K.; Takui, T.; Sato, K.; Fedin, M. Photoswitching of a thermally unswitchable molecular magnet Cu(hfac)2L(i-Pr) evidenced by steady-state and time-resolved electron paramagnetic resonance. J. Am. Chem. Soc. 2014, 136, 10132–10138. [Google Scholar] [CrossRef]
- Sato, K.; Nakazawa, S.; Rahimi, R.; Ise, T.; Nishida, S.; Yoshino, T.; Mori, N.; Toyota, K.; Shiomi, D.; Yakiyama, Y.; et al. Molecular electron-spin quantum computers and quantum information processing: Pulse-based electron magnetic resonance spin technology applied to matter spin-qubits. J. Mater. Chem. 2009, 19, 3739–3754. [Google Scholar] [CrossRef]
- Nakazawa, S.; Nishida, S.; Ise, T.; Yoshino, T.; Mori, N.; Rahimi, R.D.; Sato, K.; Morita, Y.; Toyota, K.; Shiomi, D.; et al. A synthetic two-spin quantum bit: G-engineered exchange-coupled biradical designed for controlled-NOT gate operationsngew. Angew. Chem. Int. Ed. 2012, 51, 9860–9864. [Google Scholar] [CrossRef]
- Ayabe, K.; Sato, K.; Nakazawa, S.; Nishida, S.; Sugisaki, K.; Ise, T.; Morita, Y.; Toyota, K.; Shiomi, D.; Kitagawa, M.; et al. Pulsed electron spin nutation spectroscopy for weakly exchange-coupled multi-spin molecular systems with nuclear hyperfine couplings: A general approach to bi- and triradicals and determination of their spin dipolar and exchange interactions. Mol. Phys. 2013, 111, 2767–2787. [Google Scholar] [CrossRef]
- Coronado, E.; Epstein, A.J. Molecular spintronics and quantum computing. J. Mater. Chem. 2009, 19, 1670–1671. [Google Scholar]
- Coronado, E.; Day, P. Magnetic molecular conducto. Chem. Rev. 2004, 104, 5419–5548. [Google Scholar] [CrossRef]
- Enoki, T.; Miyazaki, A. Magnetic TTF-based charge-transfer complexes. Chem. Rev. 2004, 104, 5449–5477. [Google Scholar] [CrossRef] [PubMed]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, E.; Okada, K.; Suzuki, S.; Baumgarten, M.; Romanenko, G.; Bogomyakov, A.; Ovcharenko, V. Synthesis, structure and properties of nitronyl nitroxide diradicals with fused-thiophene couplers. J. Phys. Org. Chem. 2016, 29, 725–734. [Google Scholar] [CrossRef]
- Borden, W.T.; Davidson, E.R. Effects of electron repulsion in conjugated hydrocarbon diradicals. J. Am. Chem. Soc. 1977, 99, 4587–4594. [Google Scholar] [CrossRef]
- Borden, W.T. Diradicals; Wiley-Interscience: New York, NY, USA, 1982; pp. 1–72. [Google Scholar]
- Dougherty, D.A. Spin control in organic molecules. Acc. Chem. Res. 1991, 24, 88–94. [Google Scholar] [CrossRef]
- Lahti, P.M. Molecule-Based Magnetic Materials; Turnbull, M.M., Sugimoto, T., Thompson, L.K., Eds.; ACS Symposium Series 644; American Chemical Society: Washington, DC, USA, 1996; pp. 218–235. [Google Scholar]
- Bushby, R.J.; McGill, D.R.; Ng, K.M.; Taylor, N. Disjoint and coextensive diradical diions. J. Chem. Soc. Perkin Trans. 2 1997, 1405–1414. [Google Scholar] [CrossRef]
- Suzuki, S.; Okada, K. Organic Redox Systems Synthesis, Properties, and Applications; Nishinaga, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 269–285. [Google Scholar]
- Ueda, A.; Nishida, S.; Fukui, K.; Ise, T.; Shiomi, D.; Sato, K.; Takui, T.; Nakasuji, K.; Morita, M. Three-dimensional intramolecular exchange interaction in a curved and nonalternant pi-conjugated system: Corannulene with two phenoxyl radicals. Angew. Chem. Int. Ed. 2010, 49, 1678–1682. [Google Scholar] [CrossRef]
- Dias, J.R. Disjoint molecular orbitals in nonalternant conjugated diradical hydrocarbons. J. Chem. Inf. Comput. Sci. 2003, 43, 1494–1501. [Google Scholar] [CrossRef]
- Okada, K.; Imamura, K.; Oda, M.; Kozaki, M.; Morimoto, Y.; Ishino, K.; Tashiro, K. Novel dimers of 2,2’-(m-phenylene)bis(4,5-diphenyl-1-imidazolyl) Diradical. Chem. Lett. 1998, 891–892. [Google Scholar] [CrossRef]
- Cho, D.; Ko, K.C.; Lee, J.Y. Organic magnetic diradicals (radical-coupler-radical): Standardization of couplers for strong ferromagnetism. J. Phys. Chem. A 2014, 118, 5112–5121. [Google Scholar] [CrossRef]
- Haraguchi, M.; Tretyakov, E.; Gritsan, N.; Romanenko, G.; Gorbunov, D.; Bogomyakov, A.; Maryunina, K.; Suzuki, S.; Kozaki, M.; Shiomi, D.; et al. (Azulene-1,3-diyl)-bis(nitronyl nitroxide) and (Azulene-1,3-diyl)-bis(iminonitroxide) and Their Copper Complexes. Chem. Asian J. 2017, 12, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.A.; Bolton, J.R.; Wertz, J.E. Electron. Paramagnetic Resonance; Wiley: New York, NY, USA, 1994; Eq. 6.34 from Chapter 6, 164. [Google Scholar]
- Datta, S.N.; Trindle, C.O.; Illas, F. Theoretical and Computational Aspects of Magnetic Organic Molecules; Imperial College Press: London, UK, 2013; pp. 151–152. [Google Scholar]
- Ali, M.E.; Datta, S.N. Broken-symmetry density functional theory investigation on bis-nitronyl nitroxide diradicals: Influence of length and aromaticity of couplers. J. Phys. Chem. A 2006, 110, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, T.; Inoue, K.; Koga, N.; Iwamura, H. Exchange Interactions between Two Nitronyl Nitroxide or Iminyl Nitroxide Radicals Attached to Thiophene and 2,2’-Bithienyl Rings. J. Am. Chem. Soc. 1995, 117, 2467–2478. [Google Scholar] [CrossRef]
- Poderoso, J.L.; González-Cabello, A.; Jürgens, O.; Vidal-Gancedo, J.; Veciana, J.; Torres, T.; Vázquez, P. Synthesis and magnetic coupling of a bis(α-nitronyl nitroxide) radical derived from 1,2,4-triazole. Synth. Met. 2001, 121, 1830–1831. [Google Scholar] [CrossRef]
- Knizhnikov, V.A.; Borisova, N.E.; Yurashevich, N.Y.; Popova, L.A.; Chernyad’ev, A.Y.; Zubreichuk, Z.P.; Reshetova, M.D. Pincer ligands based on α-amino acids: I. Synthesis of polydentate ligands from pyrrole-2,5-dicarbaldehyde. Russ. J. Org. Chem. 2007, 43, 855–860. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Romanenko, G.V.; Korobkov, I.V.; Rey, P. Synthesis of vicinal bishydroxylamine. Russ. Chem. Bull. 1999, 48, 1519–1525. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Tolstikov, S.E.; Romanenko, G.V.; Bogomyakov, A.S.; Stass, D.V.; Maryasov, A.G.; Gritsan, N.P.; Ovcharenko, V.I. Method for the synthesis of a stable heteroatom analog of trimethylenemethane. Russ. Chem. Bull. 2011, 60, 2608–2612. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327–e1332. [Google Scholar]
- Shoji, M.; Koizumi, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. A general algorithm for calculation of Heisenberg exchange integrals J in multispin systems. Chem. Phys. Lett. 2006, 432, 343–347. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |

| SS-MOs | SA-MOs | |||
|---|---|---|---|---|
| (n,m) a | CASSCF | NEVPT2 | CASSCF | NEVPT2 |
| (a) 1 | ||||
| (2,2) | 0.2 | −140 | 0.2 | −140 |
| (6,6) | −61 | −173 | −61 | −55 |
| (10,10) | −135 | −188 | −135 | −106 |
| (16,13) | −124 | −138 | −124 | −93 |
| (20,15) | −116 | −114 | −116 | −96 |
| (b) 2 | ||||
| (2,2) | 1.2 | 2.8 | 1.2 | −3.5 |
| (6,6) | −21 | −35 | −21 | −22 |
| (10,10) | −48 | −58 | −48 | −41 |
| (16,13) | −47 | −39 | −47 | −31 |
| Paramagnetic Parts | Linkers | Angles, ° | J Value, K a | Reference |
|---|---|---|---|---|
| NN b |  | 7.8, 10.5 | −115 | [32] |
| NN |  | 0 | −111 | This work |
| NN | 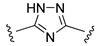 | – | −1.6 | [33] |
| NN | 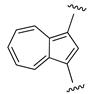 | ~43 | 10 | [28] |
| NN |  | 40 | [32] | |
| IN |  | – | −30 | [32] |
| IN | 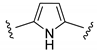 | 0 | −32 | This work |
| IN | 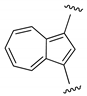 | ~5, ~11 | 0–1 | [28] |
| IN | 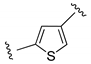 | – | 16 | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretyakov, E.; Tkacheva, A.; Romanenko, G.; Bogomyakov, A.; Stass, D.; Maryasov, A.; Zueva, E.; Trofimov, B.; Ovcharenko, V. (Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties. Molecules 2020, 25, 1503. https://doi.org/10.3390/molecules25071503
Tretyakov E, Tkacheva A, Romanenko G, Bogomyakov A, Stass D, Maryasov A, Zueva E, Trofimov B, Ovcharenko V. (Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties. Molecules. 2020; 25(7):1503. https://doi.org/10.3390/molecules25071503
Chicago/Turabian StyleTretyakov, Evgeny, Anastasia Tkacheva, Galina Romanenko, Artem Bogomyakov, Dmitri Stass, Alexander Maryasov, Ekaterina Zueva, Boris Trofimov, and Victor Ovcharenko. 2020. "(Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties" Molecules 25, no. 7: 1503. https://doi.org/10.3390/molecules25071503
APA StyleTretyakov, E., Tkacheva, A., Romanenko, G., Bogomyakov, A., Stass, D., Maryasov, A., Zueva, E., Trofimov, B., & Ovcharenko, V. (2020). (Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties. Molecules, 25(7), 1503. https://doi.org/10.3390/molecules25071503







