Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance
Abstract
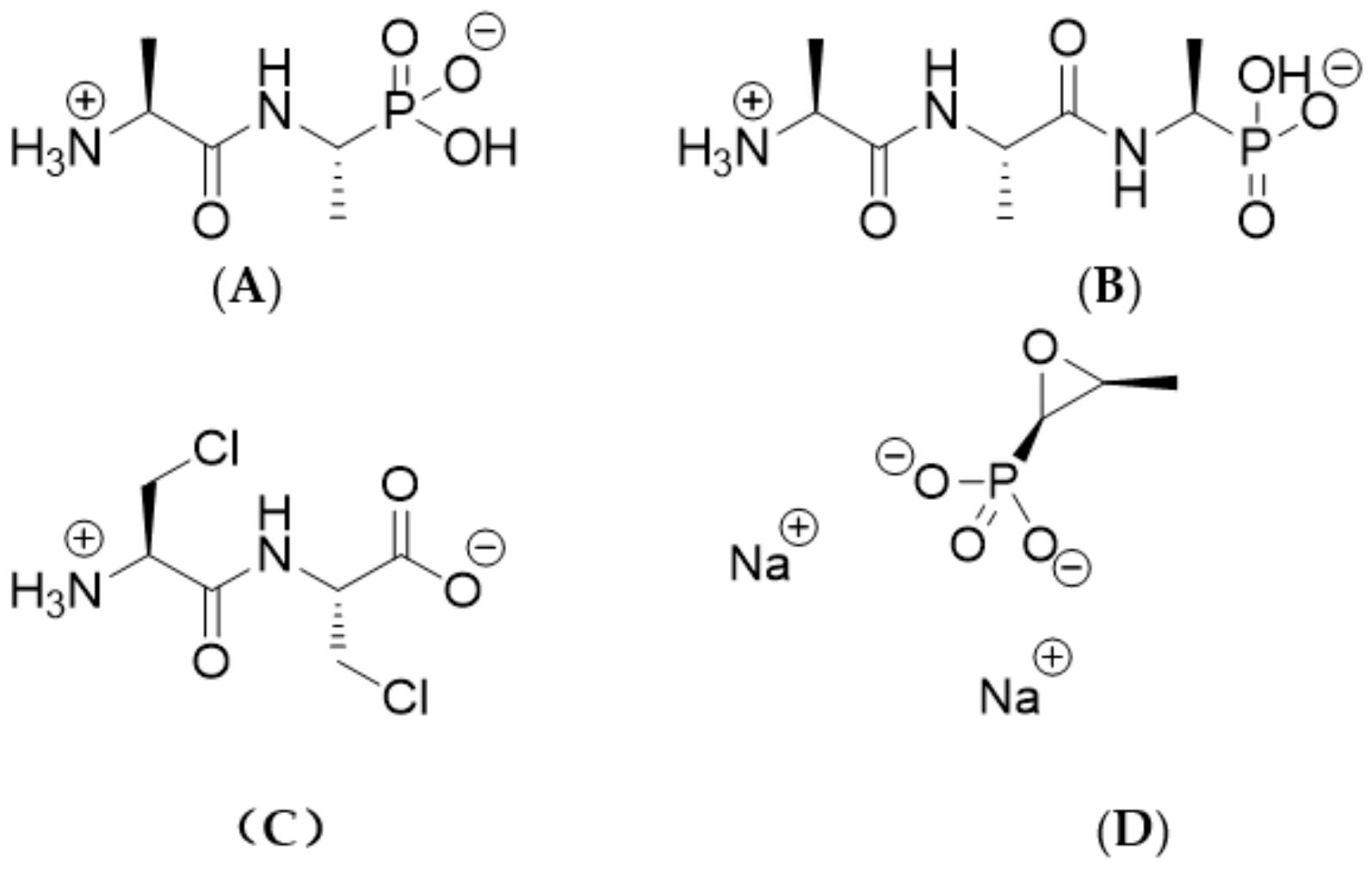
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Antibacterial Agents and Media
4.2. Bacterial Isolates
4.3. Determination of MICs
4.4. Study of the Interaction between Alafosfalin and Meropenem Against Carbapenemase-Producing Enterobacterales
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Appelbaum, P.C. 2012 and beyond: Potential for the start of a second pre-antibiotic era? J. Antimicrob. Chemother. 2012, 67, 2062–2068. [Google Scholar] [CrossRef]
- Piddock, L.J. The crisis of no new antibiotics - what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Livermore, D.M. Fourteen years in resistance. Int. J. Antimicrob. Agents 2012, 39, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Bush, K.; Craig, W.A.; Frimodt-Møller, N.; Grayson, M.L.; Mouton, J.W.; Turnidge, J.; Harbarth, S.; Gyssens, I.C. ESCMID Study Group for Antibiotic Policies.. Forgotten antibiotics: An inventory in Europe, the United States, Canada, and Australia. Clin. Infect. Dis. 2012, 15, 268–274. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, J.; Bhally, H.S.; Salmon, A.H.; de Zoysa, J.R. Successful treatment of carbapenemase producing Enterobacteriaceae peritonitis: ‘Old therapy for a new bug’. Perit. Dial. Int. 2020, 40, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, e732–739. [Google Scholar] [CrossRef] [PubMed]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Rationale, chemistry, and structure-activity relationships. Antimicrob. Agents Chemother. 1979, 15, 677–683. [Google Scholar] [CrossRef]
- Neuman, M. Recent developments in the field of phosphonic acid antibiotics. J. Antimicrob. Chemother. 1984, 14, 309–311. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert., R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Alaphosphin and related phosphonopeptides. Antimicrob. Agents Chemother. 1979, 15, 684–695. [Google Scholar] [CrossRef]
- Cheung, K.S.; Boisvert, W.; Lerner, S.A.; Johnston, M. Chloroalanyl antibiotic peptides: Antagonism of their antimicrobial effects by L-alanine and L-alanyl peptides in Gram-negative bacteria. J. Med. Chem. 1986, 29, 2060–2068. [Google Scholar] [CrossRef]
- Allen, J.G.; Havas, L.; Leicht, E.; Lenox-Smith, I.; Nisbet, L.J. Phosphonopeptides as antibacterial agents: Metabolism and pharmacokinetics of alafosfalin in animals and humans. Antimicrob. Agents Chemother. 1979, 16, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Lees, L.J. Pharmacokinetics of alafosfalin, alone and in combination with cephalexin, in humans. Antimicrob. Agents Chemother. 1980, 7, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Lloyd, W.J.; Nisbet, L.J.; Ringrose, P.S.; Westmacott, D. Antibacterial properties of alafosfalin combined with cephalexin. Antimicrob. Agents Chemother. 1981, 20, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Welling, P.G.; Kendall, M.J.; Dean, S.; Wise, R.; Andrews, J.M. Effect of food on the bioavailability of alafosfalin, a new antibacterial agent. J. Antimicrob. Chemother. 1980, 6, 373–379. [Google Scholar] [CrossRef]
- Neumann, J.; Bruch, M.; Gebauer, S.; Brandsch, M. Transport of the phosphonodipeptide alafosfalin by the H+/peptide cotransporters PEPT1 and PEPT2 in intestinal and renal epithelial cells. Eur. J. Biochem. 2004, 271, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Ohshima, J.; Ohsawa, E.; Maruyama, H.B. In vitro potentiation of cephalosporins by alafosfalin against urinary tract bacteria. Antimicrob. Agents Chemother. 1982, 21, 706–710. [Google Scholar] [CrossRef]
- Maruyama, H.B.; Arisawa, M.; Sawada, T. Alafosfalin, a new inhibitor of cell wall biosynthesis: in vitro activity against urinary isolates in Japan and potentiation with beta-lactams. Antimicrob. Agents Chemother. 1979, 16, 444–451. [Google Scholar] [CrossRef]
- Gibson, M.M.; Price, M.; Higgins, C.F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J. Bacteriol. 1984, 160, 122–130. [Google Scholar] [CrossRef]
- Ringrose, P.S. Warhead delivery and suicide substrates as concepts in antimicrobial drug design. In The Scientific Basis of Antimicrobial Therapy, Society of General Microbiology Symposium 38; Greenwood, D., O’Grady, F., Eds.; Cambridge: Cambridge, UK, 1985. [Google Scholar]
- Atherton, F.R.; Hassall, C.H.; Lambert, R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)phosphonic acid. J. Med. Chem. 1986, 29, 29–40. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100-S22 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Giakkoupi, P.; Vourli, S.; Polemis, M.; Kalapothaki, V.; Tzouvelekis, L.S.; Vatopoulos, A.C. Supplementation of growth media with Zn2+ facilitates detection of VIM-2-producing Pseudomonas aeruginosa. J. Clin. Microbiol. 2008, 46, 1568–1569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trotter, A.J.; Aydin, A.; Strinden, M.J.; O’Grady, J. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance. Curr. Opin. Microbiol. 2019, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Timbrook, T.T.; Spivak, E.S.; Hanson, K.E. Current and future opportunities for rapid diagnostics in antimicrobial stewardship. Med. Clin. North Am. 2018, 102, 899–911. [Google Scholar] [CrossRef]
- Perry, J.D.; Riley, G.; Gould, F.K.; Perez, J.M.; Boissier, E.; Ouedraogo, R.T.; Freydière, A.M. Alafosfalin as a selective agent for isolation of Salmonella from clinical samples. J. Clin. Microbiol. 2002, 40, 3913–3916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kondacs, L.A.; Orenga, S.; Anderson, R.J.; Marrs, E.C.; Perry, J.D.; Gray, M. C-Terminal 1-aminoethyltetrazole-containing oligopeptides as novel alanine racemase inhibitors. Molecules 2020, 25, 1315. [Google Scholar] [CrossRef]
- Ng, K.T.; Perry, J.D.; Marrs, E.C.L.; Orenga, S.; Anderson, R.J.; Grey, M. Synthesis and antimicrobial activity of phosphonopeptide derivatives incorporating single and dual inhibitors. Molecules 2020. Unpublished Proof. [Google Scholar]
Sample Availability: If not available from a commercial source, samples of the compounds are available from the authors. |
| Organism (No. Tested) and Antimicrobial Agent | Concentration (mg/L) | |||
|---|---|---|---|---|
| Mode | MIC50 | MIC90 | Range | |
| Enterobacterales (n = 197) | ||||
| Alafosfalin | 2 | 2 | > 8 | ≤0.031–>8 |
| Di-alanyl fosfalin | > 8 | 8 | > 8 | ≤ 0.031–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 2–> 8 |
| Fosfomycin | 4 | 4 | > 32 | 0.125–> 32 |
| E. coli (n = 53) | ||||
| Alafosfalin | 0.063 | 0.125 | 0.25 | ≤ 0.031–2 |
| Di-alanyl fosfalin | 0.25 | 0.5 | 2 | ≤ 0.031–> 8 |
| β-Cl-Ala-β-Cl-Ala | 8 | 8 | > 8 | 2–> 8 |
| Fosfomycin | 0.5 | 0.5 | 1 | 0.125–8 |
| K. pneumoniae (n = 87) | ||||
| Alafosfalin | 2 | 2 | > 8 | 0.25–> 8 |
| Di-alanyl fosfalin | > 8 | > 8 | > 8 | 0.5–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 8–> 8 |
| Fosfomycin | 4 | 8 | > 32 | 2–> 32 |
| E. cloacae (n = 27) | ||||
| Alafosfalin | 1 | 1 | 1 | 0.125–4 |
| Di-alanyl fosfalin | 4 | > 8 | > 8 | 0.25–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 8–> 8 |
| Fosfomycin | 16 | 16 | 32 | 4–> 32 |
| CPE (n = 128) | ||||
| Alafosfalin | 2 | 1 | 4 | ≤ 0.031–> 8 |
| Di-alanyl fosfalin | > 8 | 8 | > 8 | 0.063–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 2–> 8 |
| Fosfomycin | 16 | 4 | 32 | 0.125–> 32 |
| ESBL (n = 47) | ||||
| Alafosfalin | 2 | 2 | > 8 | ≤ 0.031–> 8 |
| Di-alanyl fosfalin | > 8 | 8 | > 8 | ≤ 0.031–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 2–> 8 |
| Fosfomycin | > 32 | 8 | > 32 | 0.125–> 32 |
| AmpC (n = 22) | ||||
| Alafosfalin | > 8 | 4 | > 8 | 0.063–> 8 |
| Di-alanyl fosfalin | > 8 | > 8 | > 8 | 0.063–> 8 |
| β-Cl-Ala-β-Cl-Ala | > 8 | > 8 | > 8 | 8–> 8 |
| Fosfomycin | 32 | 8 | > 32 | 0.125–> 32 |
| Organism (No. Tested) and Antimicrobial Agent | Concentration (mg/L) | |||
|---|---|---|---|---|
| Mode | MIC50 | MIC90 | Range | |
| All S. aureus (n = 50) | ||||
| Alafosfalin | 4 | 4 | 8 | 0.125–16 |
| Di-alanyl fosfalin | 4 | 8 | 16 | 0.5–32 |
| β-Cl-Ala-β-Cl-Ala | 2 | 2 | 4 | 0.125–16 |
| Fosfomycin | 8 | 4 | 16 | 0.5–> 32 |
| MRSA (n = 37) | ||||
| Alafosfalin | 4 | 4 | 8 | 0.125–16 |
| Di-alanyl fosfalin | 4 | 8 | 16 | 0.5–32 |
| β-Cl-Ala-β-Cl-Ala | 2 | 2 | 4 | 0.125–16 |
| Fosfomycin | 8 | 4 | 16 | 0.5–> 32 |
| MSSA (n = 13) | ||||
| Alafosfalin | 4 | 4 | 8 | 0.25–16 |
| Di-alanyl fosfalin | 16 | 8 | 16 | 0.5–32 |
| β-Cl-Ala-β-Cl-Ala | 1 | 1 | 2 | 0.5–2 |
| Fosfomycin | 4 | 4 | 16 | 2–16 |
| All Enterococci (n = 50) | ||||
| Alafosfalin | 8 | 16 | > 32 | 4–> 32 |
| Di-alanyl fosfalin | 0.5 | 0.5 | 2 | ≤ 0.016–> 32 |
| β-Cl-Ala-β-Cl-Ala | 16 | 16 | 32 | 2–16 |
| Fosfomycin | > 32 | > 32 | > 32 | 16–> 32 |
| E. faecalis (n = 11) | ||||
| Alafosfalin | 8 | 8 | 32 | 4–> 32 |
| Di-alanyl fosfalin | 0.031 | 0.063 | 0.5 | ≤ 0.016–> 32 |
| β-Cl-Ala-β-Cl-Ala | 8 | 8 | 16 | 4–16 |
| Fosfomycin | 32 | 32 | > 32 | 32–> 32 |
| E. faecium (n = 34) | ||||
| Alafosfalin | 16 | 16 | 16 | 4–32 |
| Di-alanyl fosfalin | 0.5 | 0.5 | 2 | ≤ 0.016–4 |
| β-Cl-Ala-β-Cl-Ala | 16 | 16 | 32 | 2–> 32 |
| Fosfomycin | > 32 | > 32 | > 32 | 16–> 32 |
| GRE (n = 43) | ||||
| Alafosfalin | 16 | 16 | > 32 | 4–> 32 |
| Di-alanyl fosfalin | 0.5 | 0.5 | > 32 | ≤ 0.016–> 32 |
| β-Cl-Ala-β-Cl-Ala | 16 | 16 | > 32 | 4–> 32 |
| Fosfomycin | > 32 | > 32 | > 32 | 32–> 32 |
| Species | Carbapenemase | Alafosfalin MIC (mg/L) | Meropenem MIC (mg/L) | FICI | Interpretation |
|---|---|---|---|---|---|
| K. oxytoca | KPC-2 | 4 | 0.25 | 0.16 | Synergy |
| K. pneumoniae | KPC-3 | 1 | 1 | 0.25 | Synergy |
| K. pneumoniae | KPC-3 | 1 | 16 | 0.19 | Synergy |
| K. pneumoniae | KPC-4 | 0.5 | 4 | 0.38 | Synergy |
| K. pneumoniae | KPC | 1 | 0.25 | 0.38 | Synergy |
| E. coli | NDM-1 | 0.125 | 8 | 0.75 | No interaction |
| E. coli | NDM-1 | 0.031 | 0.125 | 0.76 | No interaction |
| E. cloacae | NDM-1 | 1 | 4 | 1 | No interaction |
| K. pneumoniae | NDM-1 | 2 | 2 | 0.27 | Synergy |
| E. coli | NDM-1 | 0.125 | 16 | 0.25 | Synergy |
| E. cloacae | VIM-1 | 2 | 4 | 0.19 | Synergy |
| K. pneumoniae | VIM-1 | 2 | 16 | 0.28 | Synergy |
| K. pneumoniae | IMP | 0.25 | 1 | 0.37 | Synergy |
| K. pneumoniae | IMP | 0.25 | 1 | 0.50 | Synergy |
| K. oxytoca | IMP | 1 | 0.5 | 0.13 | Synergy |
| K. pneumoniae | OXA-48 | 1 | 8 | 0.38 | Synergy |
| E. coli | OXA-48 | 0.125 | 0.25 | 0.37 | Synergy |
| K. pneumoniae | OXA-48 | 2 | 1 | 0.5 | Synergy |
| K. pneumoniae | OXA-48 | 2 | 4 | 0.26 | Synergy |
| K. pneumoniae | OXA-48 | 1 | 2 | 0.63 | No interaction |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrs, E.C.L.; Varadi, L.; Bedernjak, A.F.; Day, K.M.; Gray, M.; Jones, A.L.; Cummings, S.P.; Anderson, R.J.; Perry, J.D. Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance. Molecules 2020, 25, 1445. https://doi.org/10.3390/molecules25061445
Marrs ECL, Varadi L, Bedernjak AF, Day KM, Gray M, Jones AL, Cummings SP, Anderson RJ, Perry JD. Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance. Molecules. 2020; 25(6):1445. https://doi.org/10.3390/molecules25061445
Chicago/Turabian StyleMarrs, Emma C.L., Linda Varadi, Alexandre F. Bedernjak, Kathryn M. Day, Mark Gray, Amanda L. Jones, Stephen P. Cummings, Rosaleen J. Anderson, and John D. Perry. 2020. "Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance" Molecules 25, no. 6: 1445. https://doi.org/10.3390/molecules25061445
APA StyleMarrs, E. C. L., Varadi, L., Bedernjak, A. F., Day, K. M., Gray, M., Jones, A. L., Cummings, S. P., Anderson, R. J., & Perry, J. D. (2020). Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance. Molecules, 25(6), 1445. https://doi.org/10.3390/molecules25061445







