Glycosylation of Stilbene Compounds by Cultured Plant Cells
Abstract
1. Introduction
2. Results
2.1. Glycosylation of Oxyresveratrol (1) and Gnetol (2) by Cultured Plant Cells of Phytolacca americana
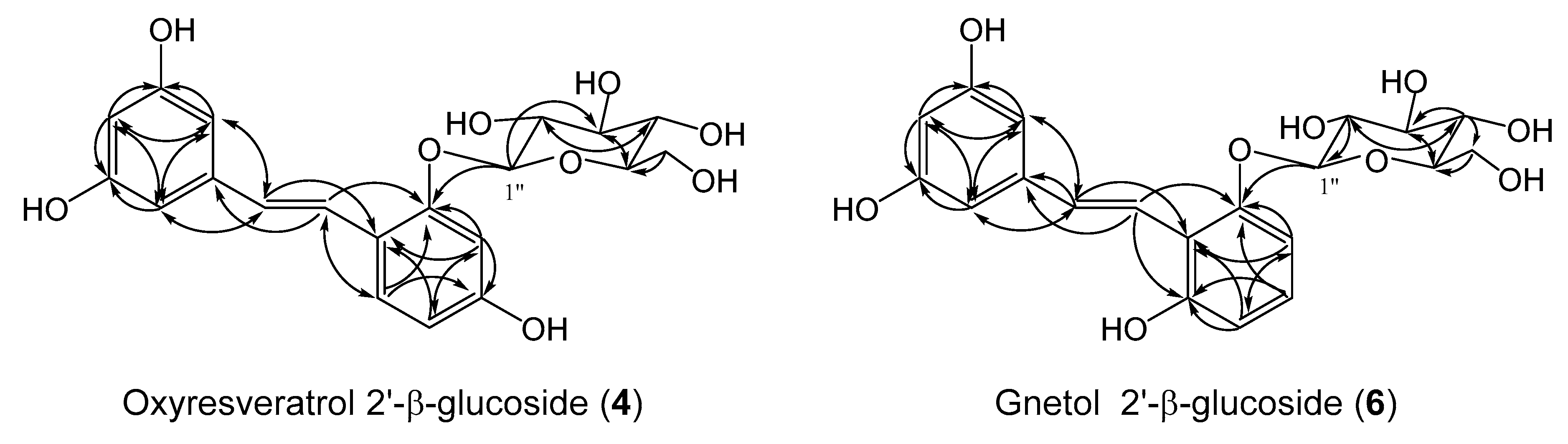
2.2. Determination of the Chemical Structures of New Compounds, Oxyresveratrol 2′-β-Glucoside (4) and Gnetol 2′-β-Glucoside (6)
3. Discussion
4. Materials and Methods
4.1. General
4.2. Analyses
4.3. Cultivation of Plant Callus
4.4. Glycosylation by Cultured Plant Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Junsaeng, D.; Anukunwithaya, T.; Songvut, P.; Sritularak, B.; Likhitwitayawuid, K.; Khemawoot, P. Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats. BMC Compl. Alt. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C. Determination of phytochemical compounds, and tyrosinase inhibitory and antimicrobial activities of bioactive compounds from Streblus ilicifolius (S Vidal) corner. Trop. J. Pharm. Res. 2016, 15, 497–506. [Google Scholar] [CrossRef][Green Version]
- Likhitwitayawuid, K.; Sornsute, A.; Sritularak, B.; Ploypradith, P. Chemical transformations of oxyresveratrol (trans–2,4,3′,5′–tetrahydroxystilbene) into a potent tyrosinase inhibitor and a strong cytotoxic agent. Bioorg. Med. Chem. Lett. 2006, 16, 5650–5653. [Google Scholar] [CrossRef] [PubMed]
- Tengamnuay, P.; Pengrungruangwong, K.; Pheansri, I.; Likhitwitayawuid, K. Artocarpus lakoocha heartwood extract as a novel cosmetic ingredient: Evaluation of the in vitro anti–tyrosinase and in vivo skin whitening activities. Int. J. Cosmet. Sci. 2006, 28, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.T.; Lamont, M.; Chibrikova, L.; Fekkes, D.; Vlug, A.S.; Lorenz, P.; Kreutzmann, P.; Slemmer, J.E. Potential neuroprotective effects of oxyresveratrol against traumatic injury. Eur. J. Pharmacol. 2012, 680, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Aftab, N.; Likhitwitayawuid, K.; Vieira, A. Comparative antioxidant activities and synergism of resveratrol and oxyresveratrol. Nat. Prod. Res. 2010, 24, 1726–1733. [Google Scholar] [CrossRef]
- Ashraf, M.I.; Shahzad, M.; Shabbir, A. Oxyresveratrol ameliorates allergic airway inflammation via attenuation of IL–4, IL–5, and IL–13 expression levels. Cytokine 2010, 76, 375–381. [Google Scholar] [CrossRef]
- Chung, K.O.; Kim, B.Y.; Lee, M.H.; Kim, Y.R.; Chung, H.Y.; Park, J.H.; Moon, J.O. In–vitro and in–vivo anti–inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003, 55, 1695–1700. [Google Scholar] [CrossRef]
- Galindo, I.; Hernáez, B.; Berná, J.; Fenoll, J.; Cenis, J.L.; Escribano, J.M.; Alonso, C. Comparative inhibitory activity of the stilbenes resveratrol and oxyresveratrol on African swine fever virus replication. Antivir. Res. 2011, 91, 57–63. [Google Scholar] [CrossRef]
- Lipipun, V.; Sasivimolphan, P.; Yoshida, Y.; Daikoku, T.; Sritularak, B.; Ritthidej, G.; Likhitwitayawuid, K.; Pramyothin, P.; Hattori, M.; Shiraki, K. Topical cream–based oxyresveratrol in the treatment of cutaneous HSV–1 infection in mice. Antivir. Res. 2011, 91, 154–160. [Google Scholar] [CrossRef]
- Suwannalert, P.; Povichit, N.; Puchadapirom, P.; Junking, M. Anti–aging activity and non–toxic dose of phytooxyresveratrol from Artocarpus lakoocha Roxb. Trop. J. Pharm. Res. 2012, 11, 69–74. [Google Scholar] [CrossRef]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Lin, H.S. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods 2016, 22, 122–131. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Martinez, S.E.; Akinwumi, B.C.; Anderson, H.D.; Takemoto, J.K.; Sayre, C.L.; Davies, N.M. Preclinical pharmacokinetics and pharmacodynamics and content analysis of gnetol in foodstuffs. Phytotherapy Res. 2015, 29, 1168–1179. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Bolanle, C. Akinwumi 1,2,* ID, Kimberly-Ann M. Bordun 2 and Hope D. Anderson. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cull suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Devries, J.H.M.; Vanleeeuwen, S.D.; Mengelers, M.J.B.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-Obeta- glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radical Res. 2000, 33, 667–676. [Google Scholar] [CrossRef]
- Ishihara, K.; Hamada, H.; Hirata, T.; Nakajima, N. Biotransformation using plant cultured cells. J. Mol. Catal. B: Enz. 2003, 23, 145–170. [Google Scholar] [CrossRef]
- Tabata, M.; Ikeda, F.; Hiraoka, N.; Konoshima, M. Glucosylation of phenolic compounds by Datura innoxia suspension cultures. Phytochemistry 1976, 15, 1225–1229. [Google Scholar] [CrossRef]
- Mizukami, H.; Terao, T.; Miura, H.; Ohashi, H. Glucosylation of salicyl alcohol in cultured plant cells. Phytochemistry 1983, 22, 679–680. [Google Scholar] [CrossRef]
- Tabata, M.; Umetani, Y.; Ooya, M.; Tanaka, S. Glucosylation of phenolic compounds by plant cell cultures. Phytochemistry 1988, 27, 809–813. [Google Scholar] [CrossRef]
- Ushiyama, M.; Furuya, T. Glycosylation of phenolic compounds by root culture of Panax ginseng. Phytochemistry 1989, 28, 3009–3013. [Google Scholar] [CrossRef]
- Lewinson, E.; Berman, E.; Mazur, Y.; Gressel, J. Glucosylation of exogenous flavanones by grapefruit (Citrus paradisi) cell cultures. Phytochemistry 1986, 25, 2531–2535. [Google Scholar] [CrossRef]
- Shimoda, K.; Sato, N.; Kobayashi, T.; Hamada, H.; Hamada, H. Glycosylation of daidzein by the Eucalyptus cell cultures. Phytochemistry 2008, 69, 2303–2306. [Google Scholar] [CrossRef]
- Imai, H.; Kitagawa, M.; Ishihara, K.; Masuoka, N.; Shimoda, K.; Nakajima, N.; Hamada, H. Glycosylation of trans-resveratrol by plant-cultured cells. Biosci. Biotechnol. Biochem. 2012, 8, 1552–1554. [Google Scholar] [CrossRef]
- Sato, D.; Shimizu, N.; Shimizu, Y.; Akagi, M.; Eshita, Y.; Ozaki, S.; Nakajima, N.; Ishihara, K.; Masuoka, N.; Hamada, H.; et al. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci., Biotechnol, Biochemi. 2014, 78, 1123–1128. [Google Scholar] [CrossRef]
- Iwakiri, T.; Imai, H.; Hamada, H.; Nakayama, T.; Ozaki, S. Synthesis of 3,5,3′,4′-tetrahydroxy- trans-stilbene-4′-O-beta-d-glucopyranoside by glucosyltransferases from Phytolacca americana. Nat. Prod. Commun. 2013, 8, 119–120. [Google Scholar]
- Uesugi, D.; Hamada, H.; Shimoda, K.; Kubota, N.; Ozaki, S.; Nagatani, N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci., Biotechnol, Biochemi. 2017, 81, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, F.; Tahara, S.; Mizutani, J. Antifungal stress compounds from Veratrum grandiflorum leaves treated with cupric chloride. Phytochemistry 1992, 31, 3005–3007. [Google Scholar] [CrossRef]
- Venkataraman, K. Wood phenolics in the chemotaxonomy of the moraceae. Phytochemistry 1972, 11, 1571–1586. [Google Scholar] [CrossRef]
- Nyemba, A.M.; Ngando Mpondo, T.; Kimbu, S.F.; Connolly, J.D. Stilbene glycosides from Guibourtia tessmannii. Phytochemistry 1995, 39, 895–898. [Google Scholar] [CrossRef]
- Mannila, E.; Talvitie, A. Stilbenes from Picea abies bark. Phytochemistry 1992, 31, 3288–3289. [Google Scholar] [CrossRef]
- Ponrasu, T.; Charles, R.E.; Sivakumar, R.; Divakar, S. Syntheses of α-tocopheryl glycosides by glucosidases. Biotechnol. Lett. 2008, 30, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-P.; Cheng, K.-W.; Zhu, Q.; Wang, X.-C.; Lin, Z.-X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Hsu, C.-L.; Yen, G.-C. Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus. J. Agric. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef]
- Hirakura, K.; Fujimoto, Y.; Fukai, T.; Nomura, T. Two phenolic glycosides from the root bark of the cultivated mulberry tree (Morus lhou). J. Nat. Prod. 1986, 49, 218–224. [Google Scholar] [CrossRef]
- Jia, Y.-N.; Peng, Y.-L.; hao, Y.-P.; Cheng, X.-F.; Zhou, Y.; Chai, C.-L.; Zeng, L.-S.; Pan, M.-H.; Xu, L. Comparison of the hepatoprotective effects of the three main stilbenes from mulberry twigs. J. Agric. Food Chem. 2019, 67, 5521–5529. [Google Scholar] [CrossRef]
- Ali, Z.; Tanaka, T.; lliya, I.; Iinuma, M.; Furusawa, M.; Ito, T.; Nakaya, K.; Murata, J.; Darnaedi, D. Phenolic constituents of Gnetum klossii. J. Nat. Prod. 2003, 66, 558–560. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Position | 4 | 6 | |||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| Aglycon | 1 | 139.7 | 140.8 | ||
| 2 | 104.3 | 6.34 d (1.6) | 104.3 | 6.38 d (2.0) | |
| 3 | 158.3 | 158.4 | |||
| 4 | 101.4 | 6.03 s | 101.6 | 6.10 t (1.6) | |
| 5 | 158.3 | 158.4 | |||
| 6 | 104.3 | 6.34 d (1.6) | 104.3 | 6.38 d (2.0) | |
| 7 | 125.7 | 6.74 d (16) | 131.7 | 7.46 d (17.2) | |
| 8 | 122.5 | 7.26 d (16.4) | 119.9 | 7.35 d (16.4) | |
| 1′ | 117.6 | 113.5 | |||
| 2′ | 155.6 | 156.4 | |||
| 3′ | 102.9 | 6.51 d (1.6) | 106.0 | 6.57 d (8.4) | |
| 4′ | 158.1 | 127.8 | 6.97 t (8.4) | ||
| 5′ | 109.4 | 6.40 dd (8.8, 1.6) | 109.6 | 6.64 d (8.4) | |
| 6′ | 126.5 | 7.40 d (8.4) | 156.5 | ||
| Glc | 1″ | 100.7 | 4.74 d (6.8) | 100.6 | 4.89 d (7.6) |
| 2″ | 73.3 | 3.44 m | 73.6 | 3.30 m | |
| 3″ | 76.5 | 3.26 m | 77.0 | 3.19 t (8.8) | |
| 4″ | 69.5 | 3.26 m | 69.7 | 3.31 m | |
| 5″ | 76.9 | 3.18 m | 77.1 | 3.38 m | |
| 6″ | 60.5 | 3.45 m, | 60.7 | 3.69 d (10.8), | |
| 3.73 m | 3.47 dd (12.0, 5.6) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules 2020, 25, 1437. https://doi.org/10.3390/molecules25061437
Shimoda K, Kubota N, Uesugi D, Kobayashi Y, Hamada H, Hamada H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules. 2020; 25(6):1437. https://doi.org/10.3390/molecules25061437
Chicago/Turabian StyleShimoda, Kei, Naoji Kubota, Daisuke Uesugi, Yusuke Kobayashi, Hatsuyuki Hamada, and Hiroki Hamada. 2020. "Glycosylation of Stilbene Compounds by Cultured Plant Cells" Molecules 25, no. 6: 1437. https://doi.org/10.3390/molecules25061437
APA StyleShimoda, K., Kubota, N., Uesugi, D., Kobayashi, Y., Hamada, H., & Hamada, H. (2020). Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules, 25(6), 1437. https://doi.org/10.3390/molecules25061437




