Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review
Abstract
1. Introduction
2. Formulation Strategies
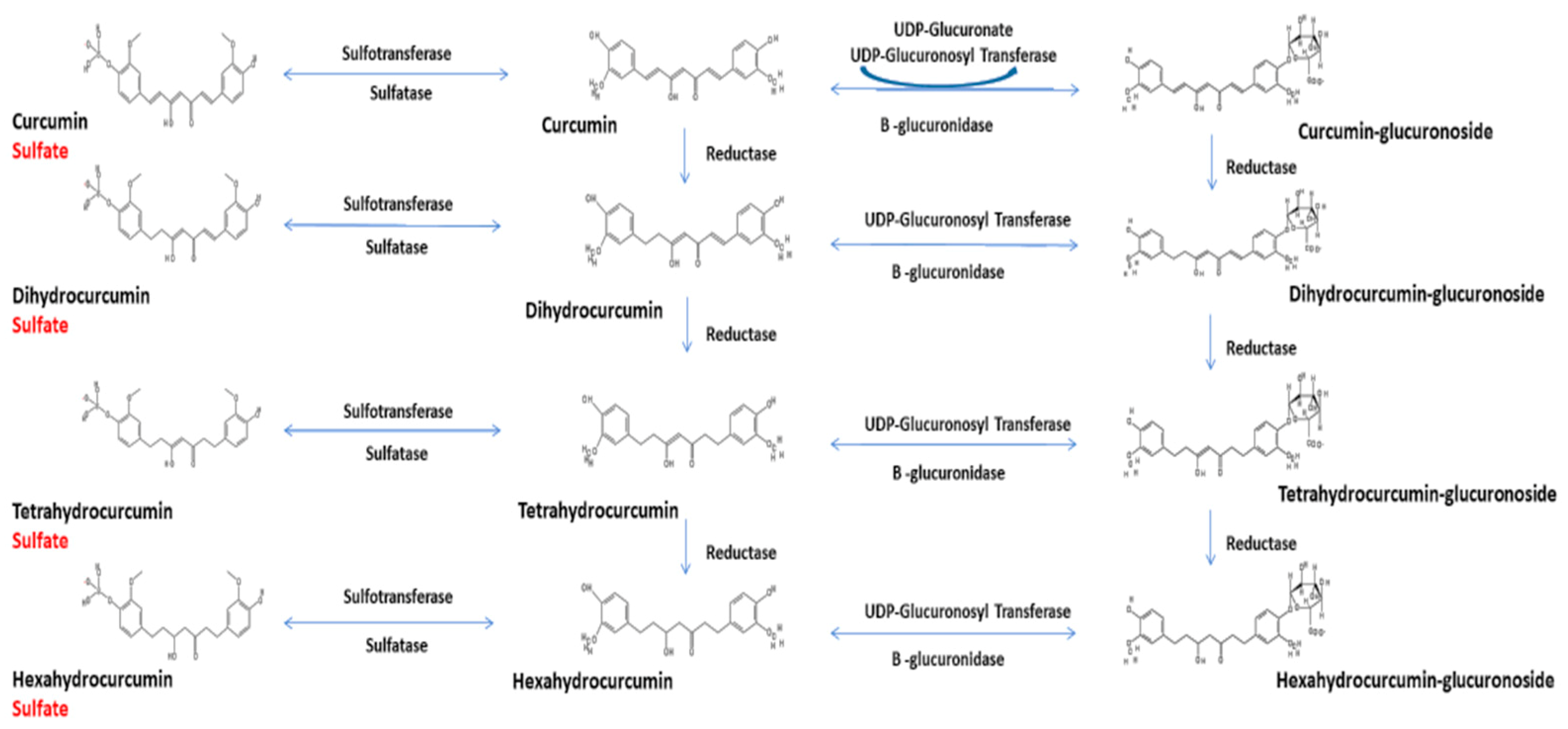
3. Curcumin Chemistry and Metabolism
4. Data Normalization and Inappropriate Hydrolysis of Plasma Samples
5. Health Promotion and Therapeutic Applications
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeung, A.W.K.; Horbańczek, M.; Tzyetkov, N.T.; Mocan, A.; Carradori, S.; Maggi, F.; Marchewka, J.; Sut, S.; Dall’Acqua, S.; Gan, R.Y.; et al. Curcumin: Total-scale analysis of the scientific literature. Molecules 2019, 24, 1393. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other molecules from turmeric and their derivatives—A review. J. Trad. Compl. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Aly, S.M.; Khan, M.A.; Aldebasi, Y.H. Role of curcumin in disease prevention and treatment. Adv. Biomed. Res. 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from the golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, C.M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Huminiecki, L.; Horbanczzuk, J.; Alanasov, A.G. The functional genome studies of curcumin. Semin. Cancer Biol. 2017, 46, 107–118. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, C.; Liu, D.B.; Yan, J.; Liang, H.P. The clinical applications of curcumin: Current state and the future. Curr. Pharm. Des. 2013, 19, 2011–2031. [Google Scholar] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alt. Med. Rev. 2009, 14, 141–1153. [Google Scholar]
- Bulku, E.; Stohs, S.J.; Cicero, L.; Brooks, T.; Halley, H.; and Ray, S.D. Curcumin exposure modulates multiple pro-apoptotic and anti-apoptotic signaling pathways to antagonize acetaminophen-induced toxicity. Curr. Neurovasc. Res. 2012, 9, 58–71. [Google Scholar] [CrossRef]
- Douglass, B.J.; Clouatre, D.L. Beyond -yellow curry: Assessing commercial curcumin absorption techniques. J. Am. Coll. Nutr. 2015, 34, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Ji, J.; Bucci, L.R.; Preuss, R.G. A comparative pharmacokinetic assessment of a novel highly bioavailable curcumin formulation with 95% curcumin: A randomized, double-blind, cross-over study. J. Am. Coll. Nutr. 2018, 37, 51–59. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennartz, K.; Joyal, S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95®CG (Biocurcumax™), a novel bioenhanced preparation of curcumin. Ind. J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Hirano, S.; Katanaska, Y.; Miyazaki, Y.; Funamoto, M.; Ksamura, N.; Hojo, Y.; Sukuki, J.; Doi, O.; Yokoji, T.; et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency: A double-blind, 3-way crossover study. J. Nutr. Sci. Vitaminol. 2015, 61, 37–44. [Google Scholar] [CrossRef]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef]
- Jager, R.; Lowrey, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Klickovic, U.; Doberer, D.; Ghazalch, G.; Aschauer, S.; Weisshaar, S.; Storka, A.; Bilban, M.; Wolzt, M. Human pharmacokinetics of high dose oral curcumin and its effects on heme oxygenase-1 expression in healthy male subjects. Biomed. Res. Intern. 2014, 2014, 458592. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowrey, R.P.; Wilson, J.M.; Mannan, H.; Munch, G.; Razmovski-Maumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Asher, G.N.; Xie, Y.; Moaddel, R.; Sanghvi, M.; Sossou, K.S.S.; Kashuba, A.D.M.; Sandler, R.S.; Hawke, R.L. Randomized pharmacokinetic crossover study comparing 2 curcumin preparations in plasma and rectal tissue of healthy human volunteers. J. Clin. Pharmacol. 2017, 57, 185–191. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelsteder, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Kumar, D.; Della, J.; Subash, P.S.; Maliakkal, A.; Johannah, N.M.; Ramadassan, K.; Balu, M.; Veera, K.; Krishnakumar, I.M. Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following oral administration of a food-grade formulation with fenugreek dietary fibre: A randomized double-blind crossover study. J. Funct. Foods. 2016, 22, 478–587. [Google Scholar] [CrossRef]
- Pawar, Y.B.; Munjal, B.; Arora, S.; Karwa, M.; Kohli, G.; Paliwal, J.K.; Bansal, A.K. Bioavailability of a lipidic formulation of curcumin in healthy human volunteers. Pharmaceutics 2012, 4, 517–530. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigation the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1539. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Katanassaka, Y.; Hiraon, S.; Namiki, M.; Watanabe, Y.; Suzuki, H.; Doi, O.; Suzuki, K.; Yamauchi, M.; et al. Drinkable preparation of Theracurmin exhibits high absorption efficiency—A single-dose, double-blind, 4-way crossover study. Biol. Pharm. Bull. 2013, 36, 1708–1714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madhavi, D.; Kagan, D. Bioavailability of a sustained release formulation of curcumin. Integr. Med. 2014, 13, 24–30. [Google Scholar]
- Briskey, D.; Sax, A.; Mallard, A.R.; Rao, A. Increased bioavailability of curcumin using a novel dispersion technology system (LipiSperse®). Eur. J. Nutr. 2019, 58, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced curcumin bioavailability through nonionic surfactant/caseinate mixed nano-emulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nano-formulations. Am. Assoc. Pharm. Sci. J. 2015, 17, 1341–1356. [Google Scholar]
- Rahimi, H.R.; Nedaeinia, R.; Shamloo, A.S.; Nikdoust, S.; Oskuee, R.K. Novel delivery systems for natural products: Nano-curcumin formulations. Avicenna. J. Phytomed. 2016, 6, 383–398. [Google Scholar]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study delivery by design of curcumin. J. Colloid. Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule-based nano-delivery systems for enhancing the bioavailability of polyphenols. J. Food Drug. Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Dobberpuhl, M.R.; Maher, D.M.; Jaggi, M.; Chauhan, S.C. Design of curcumin loaded cellulose nanoparticles for prostate cancer. Current. Drug Metab. 2012, 13, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.E.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2013, 68, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat.Methodol. 2014, 39, 113–204. [Google Scholar]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2019, 9, 705–714. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.I.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ray, S.D. Issues with human bioavailability determinations of bioactive curcumin. Biomed. J. Sci. Tech. Res. 2019, 12, 9417–9419. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, C.Y.O.; Preuss, H.G.; Ray, S.D.; Bucci, L.R.; Ji, J.; Ruff, K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: A comparative study. BMC Comp. Alt. Med. 2019, 19, 923. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules. 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard, J.W., Jr.; Singh, R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235–245. [Google Scholar]
- Benzer, F.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Caglayan, C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018, 32, e22030. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Ameruoso, A.; Palomba, R.; Palanoe, A.L.; Cervadoro, A.; Lee, A.; DiMascolo, D.; Decuzzi, P. Ameliorating amyloid-β triggered inflammation via curcumin-loaded polymeric nano-constructs. Front. Immunol. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Maiti, P.; Paladuou, L.; Dunbar, G.L. Solid liquid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects that curcumin in the 5xFAD mouse model of Alzheimer’s disease. BNC Neurosci. 2018, 19, 7. [Google Scholar]
- Farkhondeh, T.; Samarghandian, S.; Roshanrayan, B.; Peivasteh-Roudsari, L. Impact of curcumin on traumatic brain injury and involved molecular signaling pathways. Recent Pat. Food Nutr. Agric. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Curcumin for neuropsychiatric disorders: A review of in vitro, animal and human studies. J. Psychopharmacol. 2017, 31, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Onakpova, I.J.; Spencer, E.A.; Perera, B.; Heneghan, C.J. Effectiveness of curcuminoids in the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Int. J. Rheum. Dis. 2017, 20, 420–433. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, E.; Wang, C. Effectiveness of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, J.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomed 2018, 48, 129–140. [Google Scholar] [CrossRef]
- Bulboacð, A.E.; Bolboacð, S.D.; Bulboacð, A.C.; Porfire, A.S.; Tefas, L.R.; Suciu, S.M.; Dogaru, G.; Stănescu, I.C. Liposomal curcumin enhances the effect of naproxen in a rat model of migraine. Med. Sci. Monit. 2019, 25, 5087–5097. [Google Scholar] [CrossRef]
| Technical Approaches | Commercial Products | PK Studies | Hydrolysis | References |
|---|---|---|---|---|
| A. Lipid Additions | ||||
| Turmeric oil | BCM-95®; BioCurcumax® | Yes | Yes | 17–20 |
| Piperine | Curcumin C3 Comples® | Yes | Yes | 21–23 |
| Turmeric oleoresin | Curcugen® | No | NA | No |
| B. Adsorption and Dispersion on Matrices | ||||
| ɣ-Cyclodextrin | Cavacurmin® | Yes | Yes | 24 |
| Microcrystalline cellulose/Lecithin | Meriva® | Yes | Yes | 18–20,24–26 |
| Cellulosic derivatives | CurcuWIN® | Yes | Yes | 20 |
| Silicon dioxide/triacetin/Panodan® | Micronized Curcumin | No | NA | 27 |
| Carbohydrates/protein/oil/Fiber | Cureit®; Acumin® | Yes | No | 19 |
| Whey protein | CurcuminPro™ | No | NA | No |
| Rice flour/ silica/magnesium/Stearate | Curcufresh™ | No | NA | No |
| C. Particle Size Reduction | ||||
| Gelucire®/Polysorbate 20 | BioCurc® | Yes | No | 15 |
| Galactomannan fiber | CurQfen® | Yes | yes | 28 |
| Gelucire®/Labrasol® | No | No | NA | 29 |
| Ghatti gum/glycerin/Lipids/hydroxymethyl | Theracurmin® | Yes | Yes | 30–33 |
| cellulose/sodium alginate | MicroActive Curcumin™ | No | NA | 34 |
| Surfactants/polar lipids Solvents | HydroCurc™ | No | NA | 35 |
| Docosahexaenoic acid/Lecithin/stearic acid | Longvida® | Yes | NO | 36 |
| Sod. Caseinate/Tween 80 | No | No | NA | 37 |
| Proprietary microcapsules Curcushine™ | Curcushine™ | No | NA | No |
| Acacia gum/quillaia/sunflower oil | TurmiPure® | No | NA | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. https://doi.org/10.3390/molecules25061397
Stohs SJ, Chen O, Ray SD, Ji J, Bucci LR, Preuss HG. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules. 2020; 25(6):1397. https://doi.org/10.3390/molecules25061397
Chicago/Turabian StyleStohs, Sidney J., Oliver Chen, Sidhartha D. Ray, Jin Ji, Luke R. Bucci, and Harry G. Preuss. 2020. "Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review" Molecules 25, no. 6: 1397. https://doi.org/10.3390/molecules25061397
APA StyleStohs, S. J., Chen, O., Ray, S. D., Ji, J., Bucci, L. R., & Preuss, H. G. (2020). Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules, 25(6), 1397. https://doi.org/10.3390/molecules25061397






