Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway
Abstract
1. Introduction
2. Results
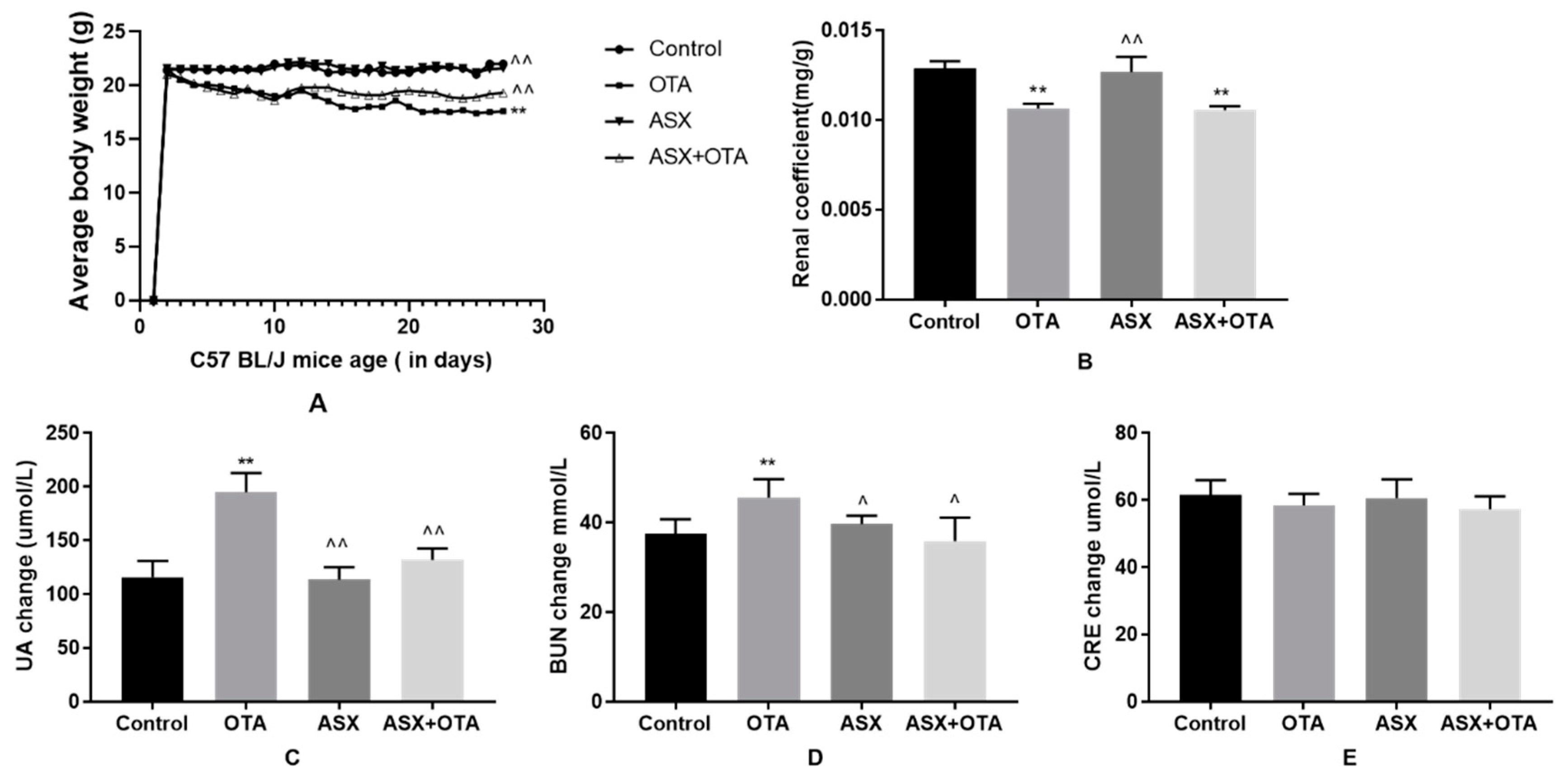
2.1. Average Body Weight, Organ Coefficients, and Changes in Serum Biochemical Indicators
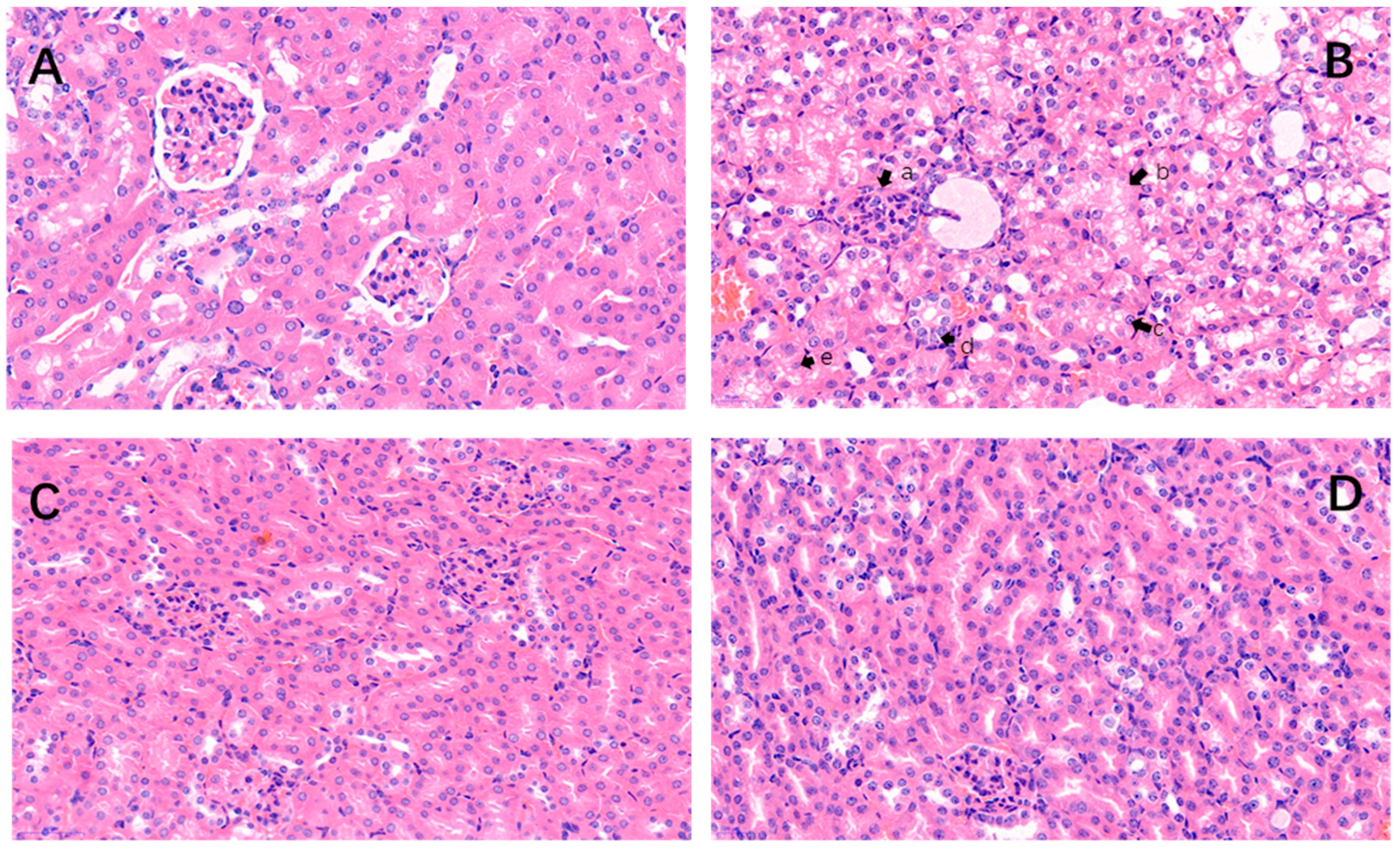
2.2. Histopathological Changes
 ” indicate a pathological injury in the kidney.
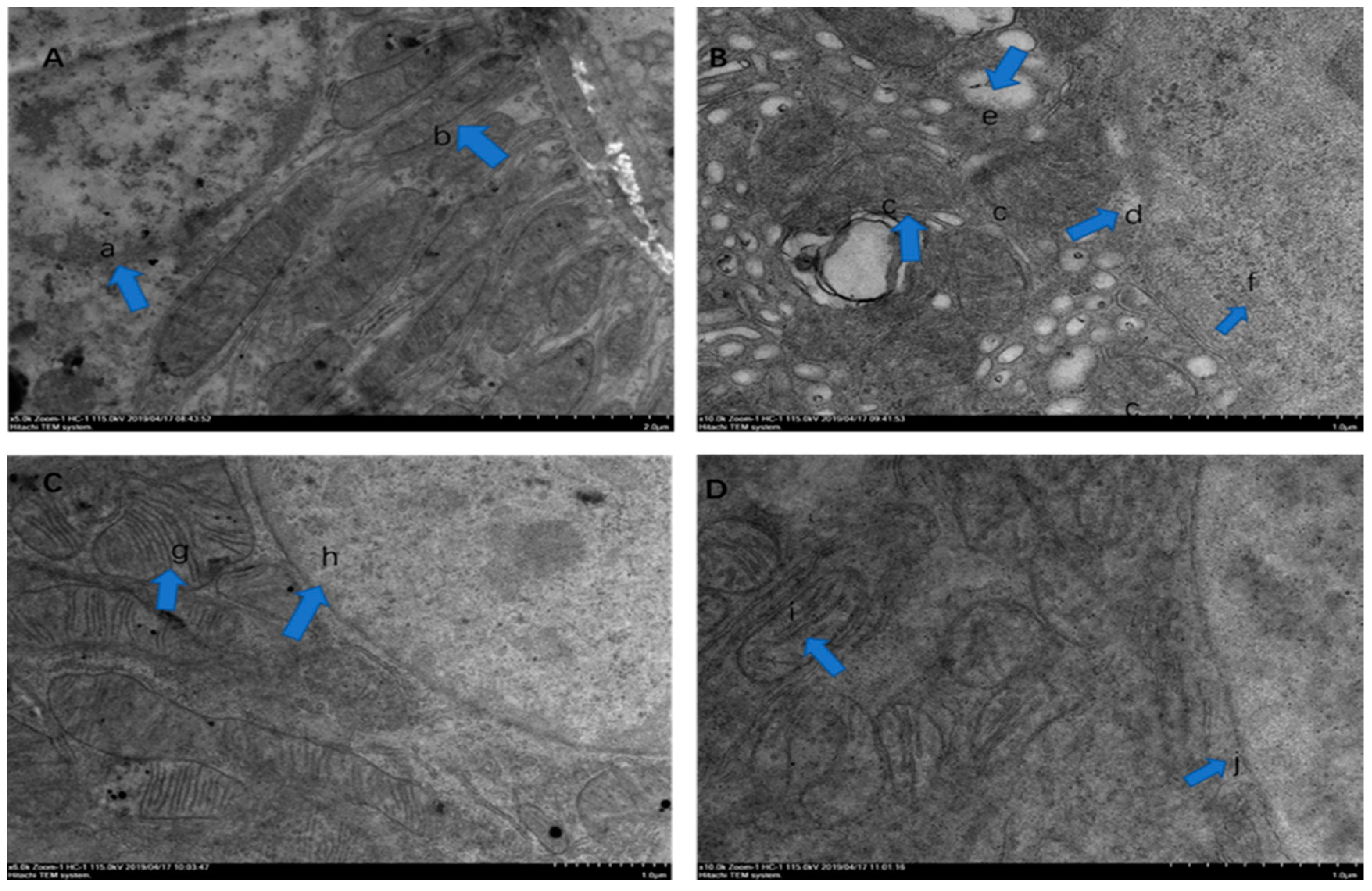
” indicate a pathological injury in the kidney.2.3. Electron Microscopic Observation
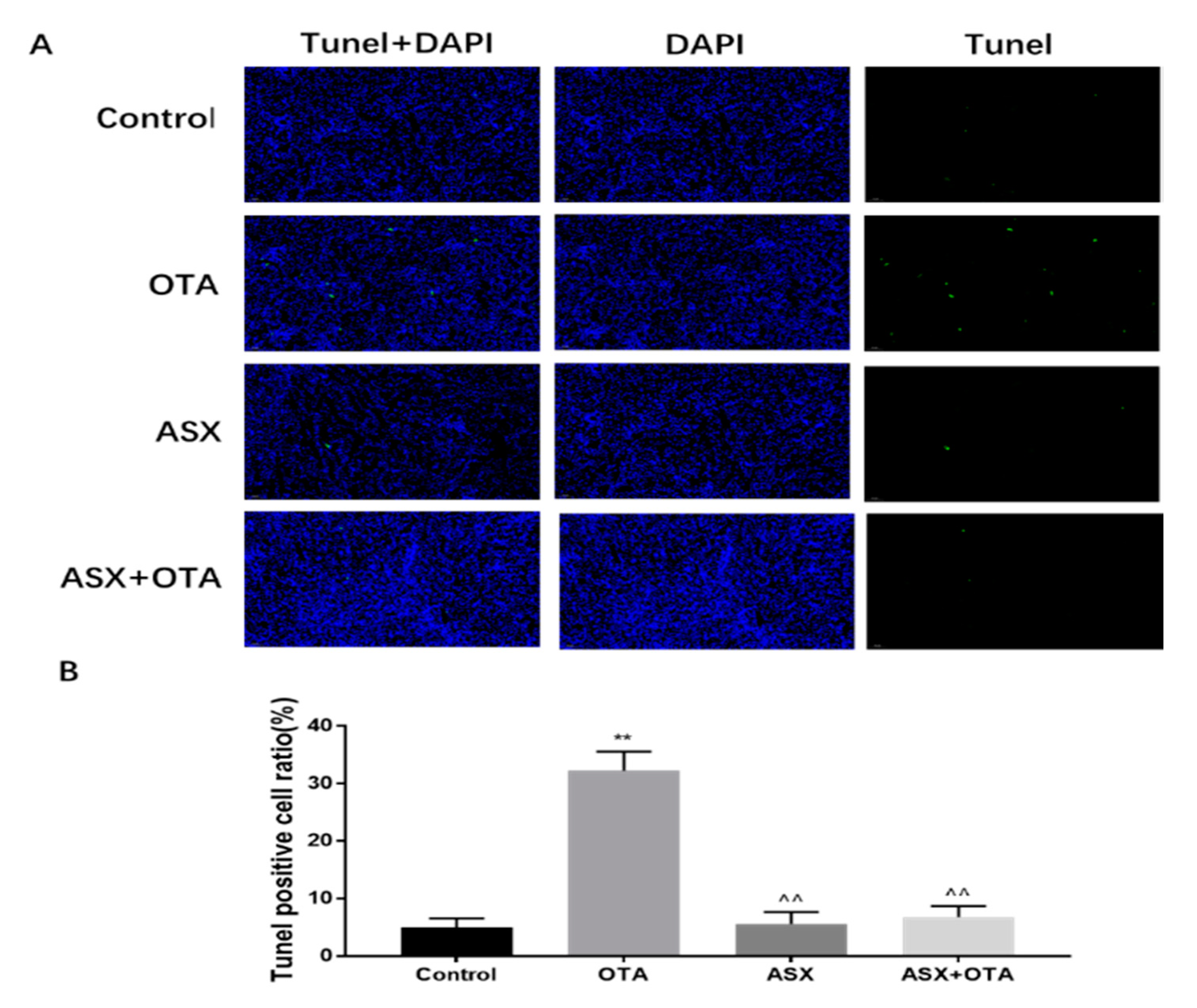
2.4. Apoptotic Analysis
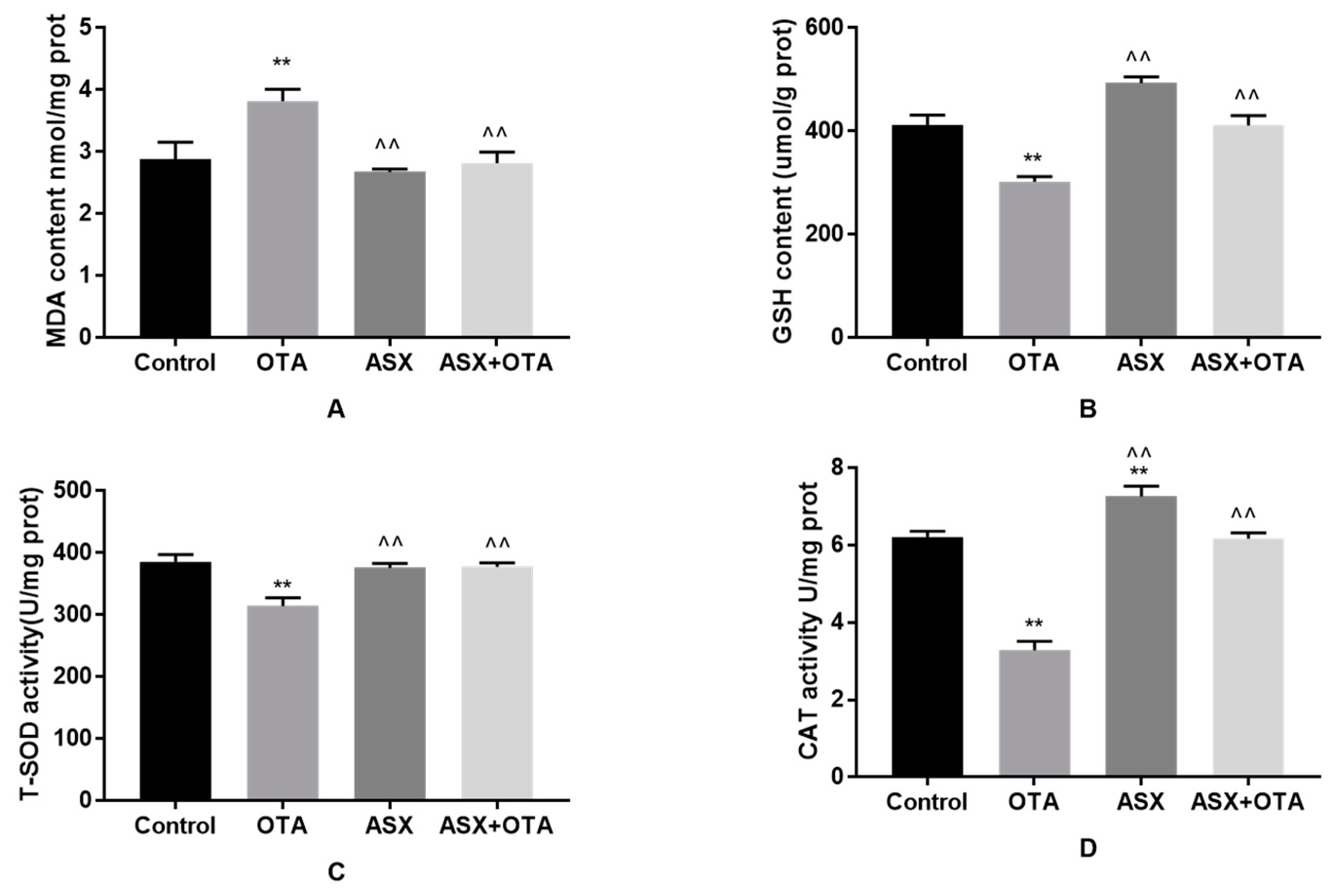
2.5. Antioxidant Status
2.6. Gene Expression Related to the NRF2/KEAP1 Signaling Pathway
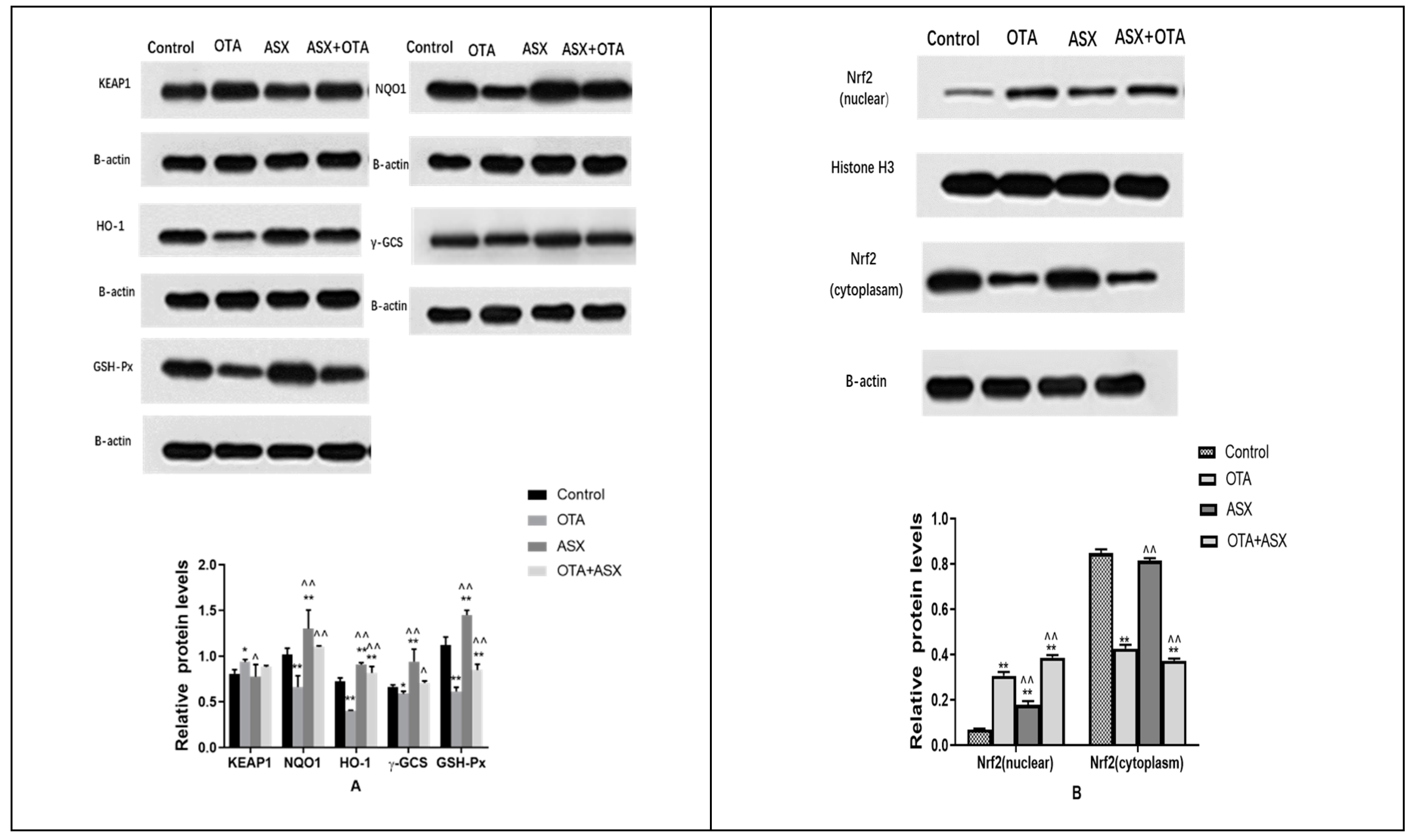
2.7. Protein Levels of Members of the NRF2/KEAP1 Signaling Pathway
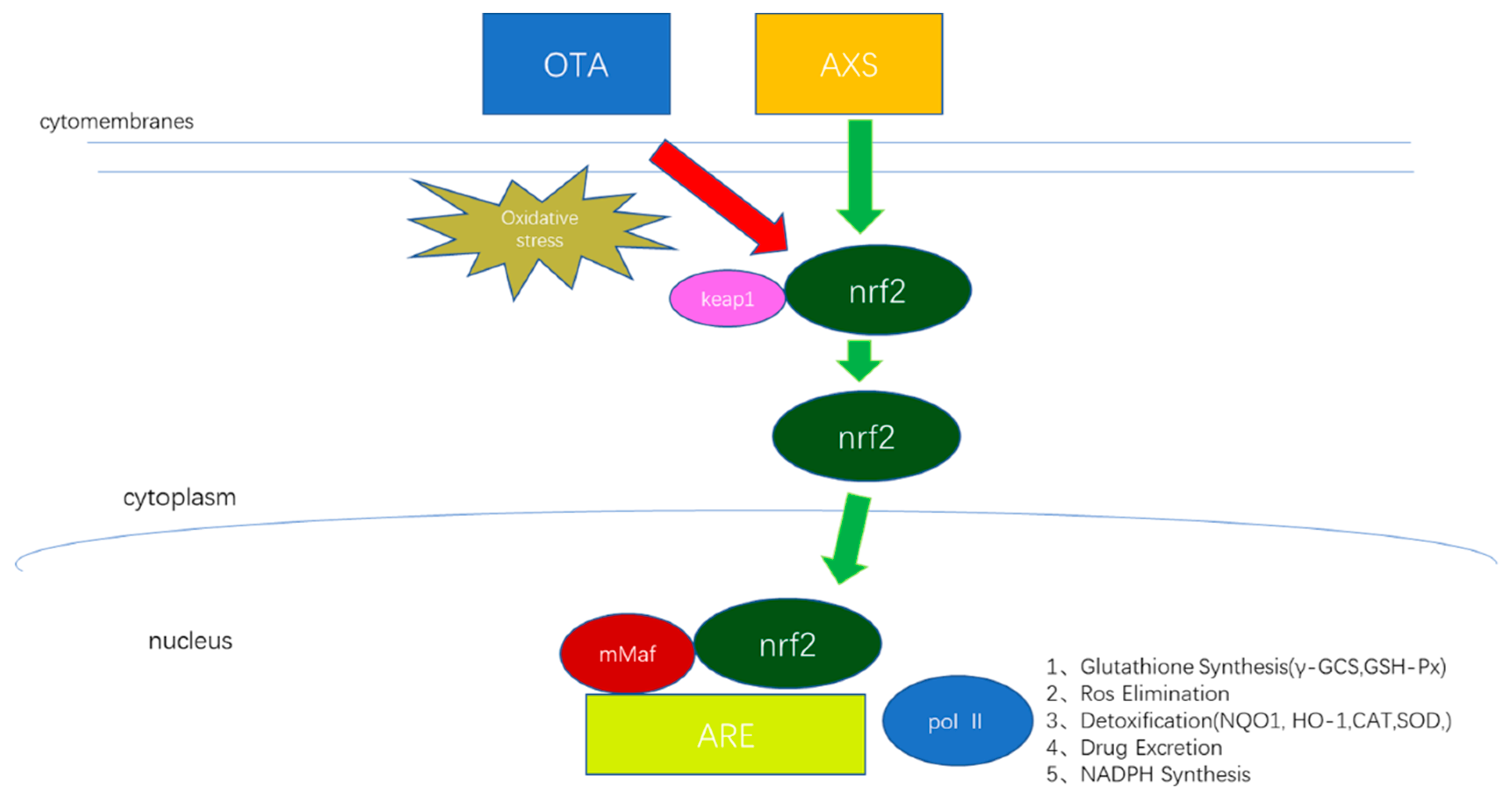
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. Animals
4.3. Experimental Design
4.4. Sample Preparation
4.5. Measurement of Serum Biochemical Indices
4.6. Histopathology of the Kidney
4.7. Electron Microscopy Observation
4.8. Observation of Apoptotic Cells
4.9. Antioxidant Status
4.10. Analysis of Gene Expression
4.11. Western Blotting Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L. Mycotoxins II. The constitution of ochratoxins A, B, and C, metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 1965, 7083–7088. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 181. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- Shotwell, O.L.; Hesseltine, C.W.; Goulden, M.L. Ochratoxin A: Occurrence as natural contaminant of a corn sample. Appl. Microbiol. 1969, 17, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Koszegi, T.; Poor, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Sara, C.C.; Fernandes, J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef]
- O’Brien, E.; Dietrich, D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Toxicol. 2005, 35, 33–60. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A. Ochratoxin A and aristolochic acid involvement in nephropathies and associated urothelial tract tumours. Arhiv za higijenu rada i toksikologiju 2009, 60, 465–483. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Aljawish, A.; Kenawy, A.M.; El-Nekeety, A.A.; Hamed, H.S.; Abdel-Aziem, S.H. Grafting of gallic acid onto chitosan nano particles enhances antioxidant activities in vitro and protects against ochratoxin A toxicity in catfish (Clarias gariepinus). Environ. Toxicol. Pharmacol. 2016, 41, 279–288. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Silva, M.J.; Miranda, J.P.; Castro, M.; Batinic-Haberle, I.; Fernandes, A.S.; Oliveira, N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016, 87, 65–76. [Google Scholar] [CrossRef]
- Gan, F.; Xue, H.; Huang, Y.; Pan, C.; Huang, K. Selenium alleviates porcine nephrotoxicity of ochratoxin A by improving selenoenzyme expression in vitro. PLoS ONE 2015, 10, e0119808. [Google Scholar] [CrossRef]
- Lee, H.J.; Pyo, M.C.; Shin, H.S.; Ryu, D.; Lee, K.W. Renal toxicity through AhR, PXR, and Nrf2 signaling pathway activation of ochratoxin A-induced oxidative stress in kidney cells. Food Chem. Toxicol. 2018, 122, 59–68. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Zhang, B.; Zhou, Z.; Shen, Y.; Liao, X.; Yang, J.; Wang, Y.; Li, X.; Li, Y.; et al. Advances in Biodetoxification of Ochratoxin A-A Review of the Past Five Decades. Front. Microbiol. 2018, 9, 1386. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications--a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Kumar, D.J.; Hegde, M.L.; Rao, K.S. Carotenoids as Novel Therapeutic Molecules Against Neurodegenerative Disorders: Chemistry and Molecular Docking Analysis. Int. J. Mol. Sci. 2019, 20, 5553. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, T.; Yang, L.; Xu, Y.; Ota, T.; Fu, Z. Protective effects of astaxanthin on a combination of D-galactose and jet lag-induced aging model in mice. Endocr. J. 2018, 65, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Fanaee-Danesh, E.; Gali, C.C.; Tadic, J.; Zandl-Lang, M.; Carmen Kober, A.; Agujetas, V.R.; de Dios, C.; Tam-Amersdorfer, C.; Stracke, A.; Albrecher, N.M.; et al. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 2224–2245. [Google Scholar] [CrossRef] [PubMed]
- Igielska-Kalwat, J.; Goscianska, J.; Nowak, I. Carotenoids as natural antioxidants. Postepy Hig. Med. Dosw. (Online) 2015, 69, 418–428. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gonczi, M.; Czirjak, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pinter, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, H.; Zhang, L.; Wu, B.; Zha, Z. Maintenance of mitochondrial function by astaxanthin protects against bisphenol A-induced kidney toxicity in rats. Biomed. Pharmacother. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2019, 19, 22–27. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2019, 4, 1–25. [Google Scholar] [CrossRef]
- Jiang, S.; Tong, S. Advances in astaxanthin biosynthesis in Haematococcus pluvialis. Chin. J. Biotechnol. 2019, 35, 988–997. [Google Scholar]
- Raghubeer, S.; Nagiah, S.; Chuturgoon, A. Ochratoxin A upregulates biomarkers associated with hypoxia and transformation in human kidney cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 57, 211–216. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.W. Ochratoxin A-Induced Hepatotoxicity through Phase I and Phase II Reactions Regulated by AhR in Liver Cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef]
- Gan, F.; Hou, L.; Zhou, Y.; Liu, Y.; Huang, D.; Chen, X.; Huang, K. Effects of ochratoxin A on ER stress, MAPK signaling pathway and autophagy of kidney and spleen in pigs. Environ. Toxicol. 2017, 32, 2277–2286. [Google Scholar] [CrossRef]
- El Cafsi, I.; Bjeoui, S.; Rabeh, I.; Nechi, S.; Chelbi, E.; El Cafsi, M.; Ghram, A. Effects of ochratoxin A on membrane phospholipids of the intestine of broiler chickens, practical consequences. Anim. Int. J. Anim. Biosci. 2019, volume, 1–9. [Google Scholar] [CrossRef]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S.; et al. Astaxanthin Protects OTA-Induced Lung Injury in Mice through the Nrf2/NF-kappaB Pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef]
- Mor, F.; Kilic, M.A.; Ozmen, O.; Yilmaz, M.; Eker, I.; Uran, K. The effects of orchidectomy on toxicological responses to dietary ochratoxin A in Wistar rats. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2014, 66, 267–275. [Google Scholar] [CrossRef]
- Hope, J.H.; Hope, B.E. A review of the diagnosis and treatment of Ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J. Environ. Public Health 2012, 2012, 835059. [Google Scholar] [CrossRef]
- Meucci, V.; Luci, G.; Vanni, M.; Guidi, G.; Perondi, F.; Intorre, L. Serum levels of ochratoxin A in dogs with chronic kidney disease (CKD): A retrospective study. J. Vet. Med Sci. 2017, 79, 440–447. [Google Scholar] [CrossRef]
- Zhang, Z.; Gan, F.; Xue, H.; Liu, Y.; Huang, D.; Khan, A.Z.; Chen, X.; Huang, K. Nephropathy and hepatopathy in weaned piglets provoked by natural ochratoxin A and involved mechanisms. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2016, 68, 205–213. [Google Scholar] [CrossRef]
- Mantle, P.; Kilic, M.A.; Mor, F.; Ozmen, O. Contribution of organ vasculature in rat renal analysis for ochratoxin a: Relevance to toxicology of nephrotoxins. Toxins 2015, 7, 1005–1017. [Google Scholar] [CrossRef]
- El-Haleem, M.R.; Kattaia, A.A.; El-Baset, S.A.; Mostafa Hel, S. Alleviative effect of myricetin on ochratoxin A-induced oxidative stress in rat renal cortex: Histological and biochemical study. Histol. Histopathol. 2016, 31, 441–451. [Google Scholar]
- Yang, X.; Liu, S.; Huang, C.; Wang, H.; Luo, Y.; Xu, W.; Huang, K. Ochratoxin A induced premature senescence in human renal proximal tubular cells. Toxicology 2017, 382, 75–83. [Google Scholar] [CrossRef]
- Enciso, J.M.; Lopez de Cerain, A.; Pastor, L.; Azqueta, A.; Vettorazzi, A. Is oxidative stress involved in the sex-dependent response to ochratoxin A renal toxicity? Food Chem. Toxicol. An Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 116, 379–387. [Google Scholar] [CrossRef]
- Rasic, D.; Micek, V.; Klaric, M.S.; Peraica, M. Oxidative stress as a mechanism of combined OTA and CTN toxicity in rat plasma, liver and kidney. Hum. Exp. Toxicol. 2019, 38, 434–445. [Google Scholar] [CrossRef]
- Rasic, D.; Mladinic, M.; Zeljezic, D.; Pizent, A.; Stefanovic, S.; Milicevic, D.; Konjevoda, P.; Peraica, M. Effects of combined treatment with ochratoxin A and citrinin on oxidative damage in kidneys and liver of rats. Toxicon Off. J. Int. Soc. Toxinology 2018, 146, 99–105. [Google Scholar] [CrossRef]
- Iskender, H.; Yenice, G.; Dokumacioglu, E.; Hayirli, A.; Sevim, C.; Dokumacioglu, A.; Terim Kapakin, K.A. Astaxanthin alleviates renal damage of rats on high fructose diet through modulating NFkappaB/SIRT1 pathway and mitigating oxidative stress. Arch. Physiol. Biochem. 2018, 26, 1–5. [Google Scholar]
- Zuluaga, M.; Gueguen, V.; Letourneur, D.; Pavon-Djavid, G. Astaxanthin-antioxidant impact on excessive Reactive Oxygen Species generation induced by ischemia and reperfusion injury. Chem. Biol. Interact. 2018, 279, 145–158. [Google Scholar] [CrossRef]
- Spezia, R.; Knecht, S.; Mennucci, B. Excited state characterization of carbonyl containing carotenoids: A comparison between single and multireference descriptions. Phys. Chem. Chem. Phys. PCCP 2017, 19, 17156–17166. [Google Scholar] [CrossRef]
- Turpaev, K.T. Keap1-Nrf2 signaling pathway: Mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochem. Biokhimiia 2013, 78, 111–126. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef]
- Han, H.; Wang, H.; Du, Y.; Gao, L. Grape Seed Procyanidins Attenuates Cisplatin-induced Human Embryonic Renal Cell Cytotoxicity by Modulating Heme Oxygenase-1 in Vitro. Cell Biochem. Biophys. 2019, 77, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Kim, G.Y. Anthocyanins from Hibiscus syriacus L. Inhibit Oxidative Stress-Mediated Apoptosis by Activating the Nrf2/HO-1 Signaling Pathway. Antioxid 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, D.; Yao, W.; Zhu, Q.; Liu, Y.; Huang, F.; Feng, J.; Chen, X.; Huang, Y.; Chi, X.; et al. Hyperglycemia Aggravates Hepatic Ischemia Reperfusion Injury by Inducing Chronic Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 3919627. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Sun, Q.; Xia, Z.Y.; Meng, Q.T.; Lei, S.Q.; Zhao, B.; Tang, L.H.; Xue, R.; Chen, R. Overexpression of DJ-1 reduces oxidative stress and attenuates hypoxia/reoxygenation injury in NRK-52E cells exposed to high glucose. Int. J. Mol. Med. 2016, 38, 729–736. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective Effects of Quercetin on Mitochondrial Biogenesis in Experimental Traumatic Brain Injury via the Nrf2 Signaling Pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef]
- Patil, R.D.; Dwivedi, P.; Sharma, A.K. Critical period and minimum single oral dose of ochratoxin A for inducing developmental toxicity in pregnant Wistar rats. Reprod. Toxicol. 2006, 22, 679–687. [Google Scholar] [CrossRef]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef]
- Hibi, D.; Kijima, A.; Suzuki, Y.; Ishii, Y.; Jin, M.; Sugita-Konishi, Y.; Yanai, T.; Nishikawa, A.; Umemura, T. Effects of p53 knockout on ochratoxin A-induced genotoxicity in p53-deficient gpt delta mice. Toxicology 2013, 304, 92–99. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, M.; Zhang, J.; Li, L.; Zhu, X.; Zhang, L.; Wang, C.; Gao, M. Astaxanthin ameliorates renal interstitial fibrosis and peritubular capillary rarefaction in unilateral ureteral obstruction. Mol. Med. Rep. 2019, 19, 3168–3178. [Google Scholar] [CrossRef]
- Carbone, L.; Carbone, E.T.; Yi, E.M.; Bauer, D.B.; Lindstrom, K.A.; Parker, J.M.; Austin, J.A.; Seo, Y.; Gandhi, A.D.; Wilkerson, J.D. Assessing cervical dislocation as a humane euthanasia method in mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2012, 51, 352–356. [Google Scholar]
- He, L.; Li, P.; Yu, L.H.; Li, L.; Zhang, Y.; Guo, Y.; Long, M.; He, J.B.; Yang, S.H. Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ. Toxicol. Pharmacol. 2018, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Primer | Sequence (5′-3′) | Melting Temperature (°C) | Size (bp) |
|---|---|---|---|
| M-Nrf2-F | CCTATGCGTGAATCCCAAT | 58.7 | 120 |
| M-Nrf2-R | TGTGAGATGAGCCTCTAAGCG | 58.1 | |
| M-Keap1-F | ATGTTGACACGGAGGATTGG | 57.7 | 133 |
| M-Keap1-R | TCATCCGCCACTCATTCCT | 58.2 | |
| M-Nqo1-F | GCGAGAAGAGCCCTGATTGT | 59.2 | 177 |
| M-Nqo1-R | CTTCAGCTCACCTGTGATGTCAT | 59.2 | |
| M-Ho1-F | CAAGCCGAGAATGCTGAGTT | 57.9 | 105 |
| M-Ho1-R | CAGGGCCGTGTAGATATGGTA | 57.8 | 172 |
| M-Gclc-F | TCGCCTCCGATTGAAGATG | 59.1 | |
| M-Gclc-R | TACTATTGGGTTTTACCTGTGCC | 58.3 | |
| M-Gpx1-F | AGGAGAATGGCAAGAATGAAGA | 58.3 | 136 |
| M-Gpx1-R | AGGAAGGTAAAGAGCGGGTG | 58.6 | 174 |
| M-b-actin-F | GTGCTATGTTGCTCTAGACTTCG | 56.8 | |
| M-b-actin-R | ATGCCACAGGATTCCATACC | 56.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, L.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. https://doi.org/10.3390/molecules25061386
Li L, Chen Y, Jiao D, Yang S, Li L, Li P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules. 2020; 25(6):1386. https://doi.org/10.3390/molecules25061386
Chicago/Turabian StyleLi, Lin, Yueli Chen, Danyang Jiao, Shuhua Yang, Lin Li, and Peng Li. 2020. "Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway" Molecules 25, no. 6: 1386. https://doi.org/10.3390/molecules25061386
APA StyleLi, L., Chen, Y., Jiao, D., Yang, S., Li, L., & Li, P. (2020). Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules, 25(6), 1386. https://doi.org/10.3390/molecules25061386





