Natural and Biomimetic Antitumor Pyrazoles, A Perspective
Abstract
1. Introduction
2. Antitumor Pyrazoles from Natural Sources
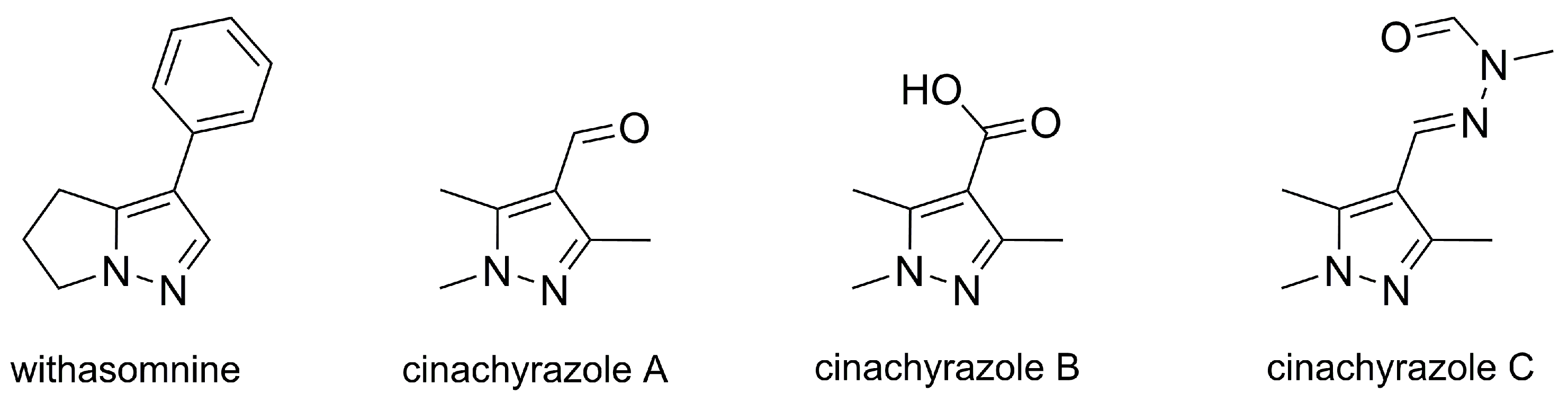
2.1. Pyrazole Alkaloids Found in Watermelon Seeds
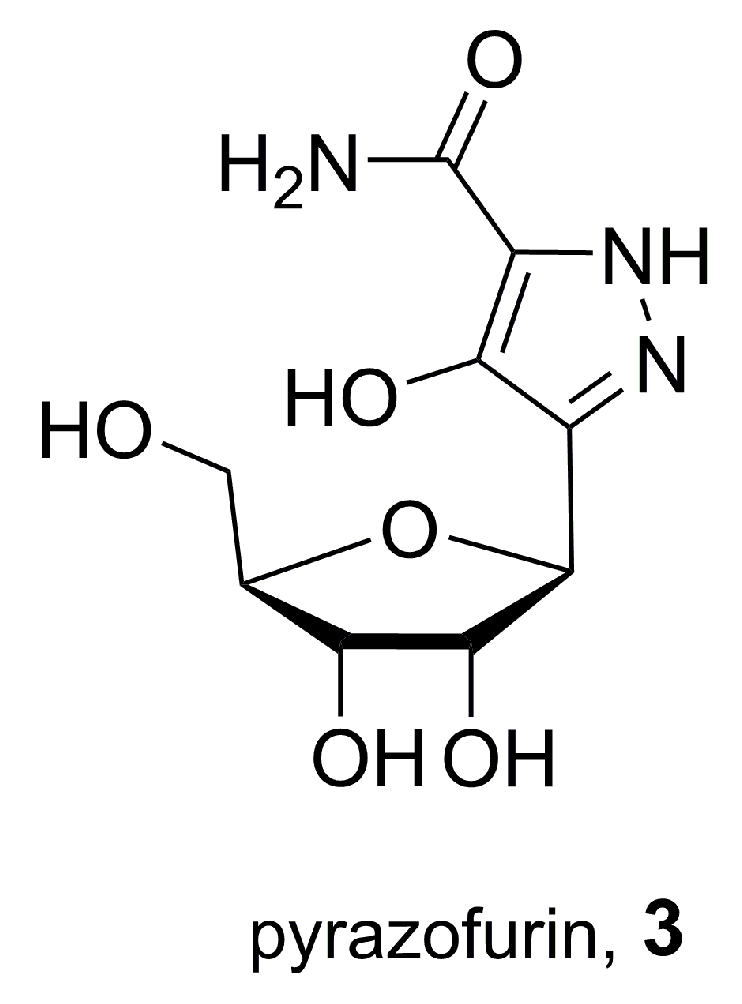
2.2. Pyrazofurin, A Natural C-Nucleoside
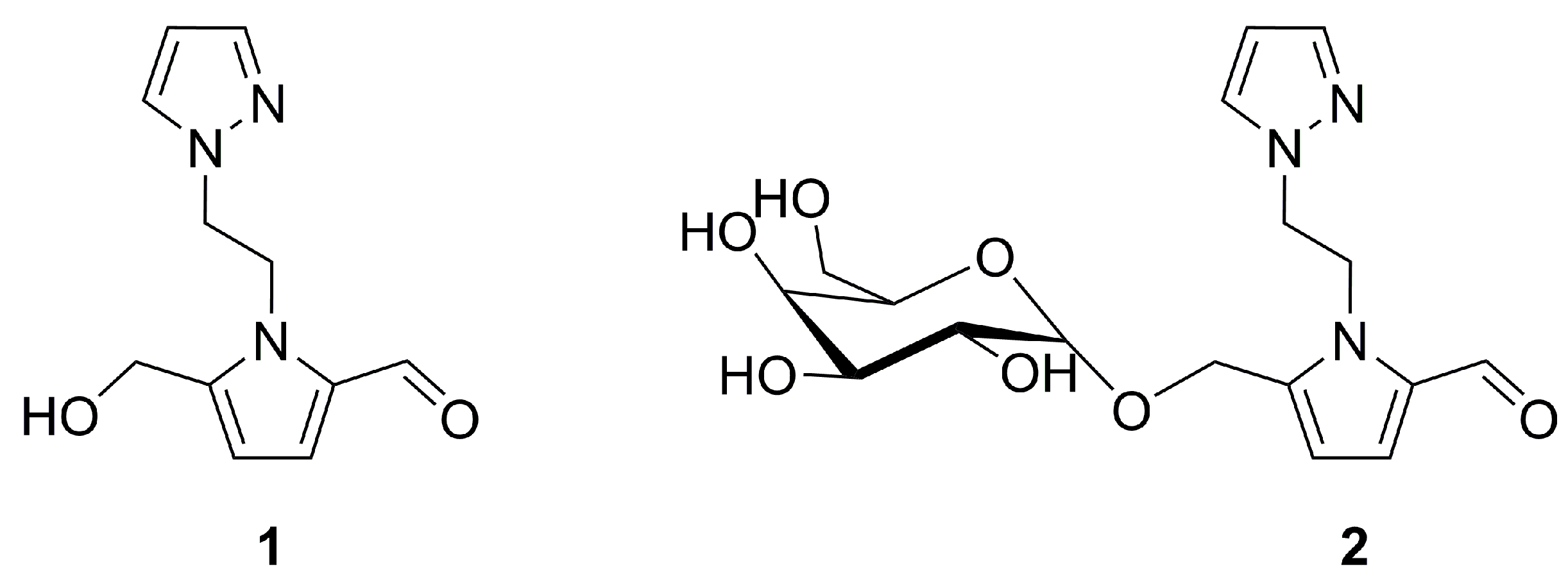
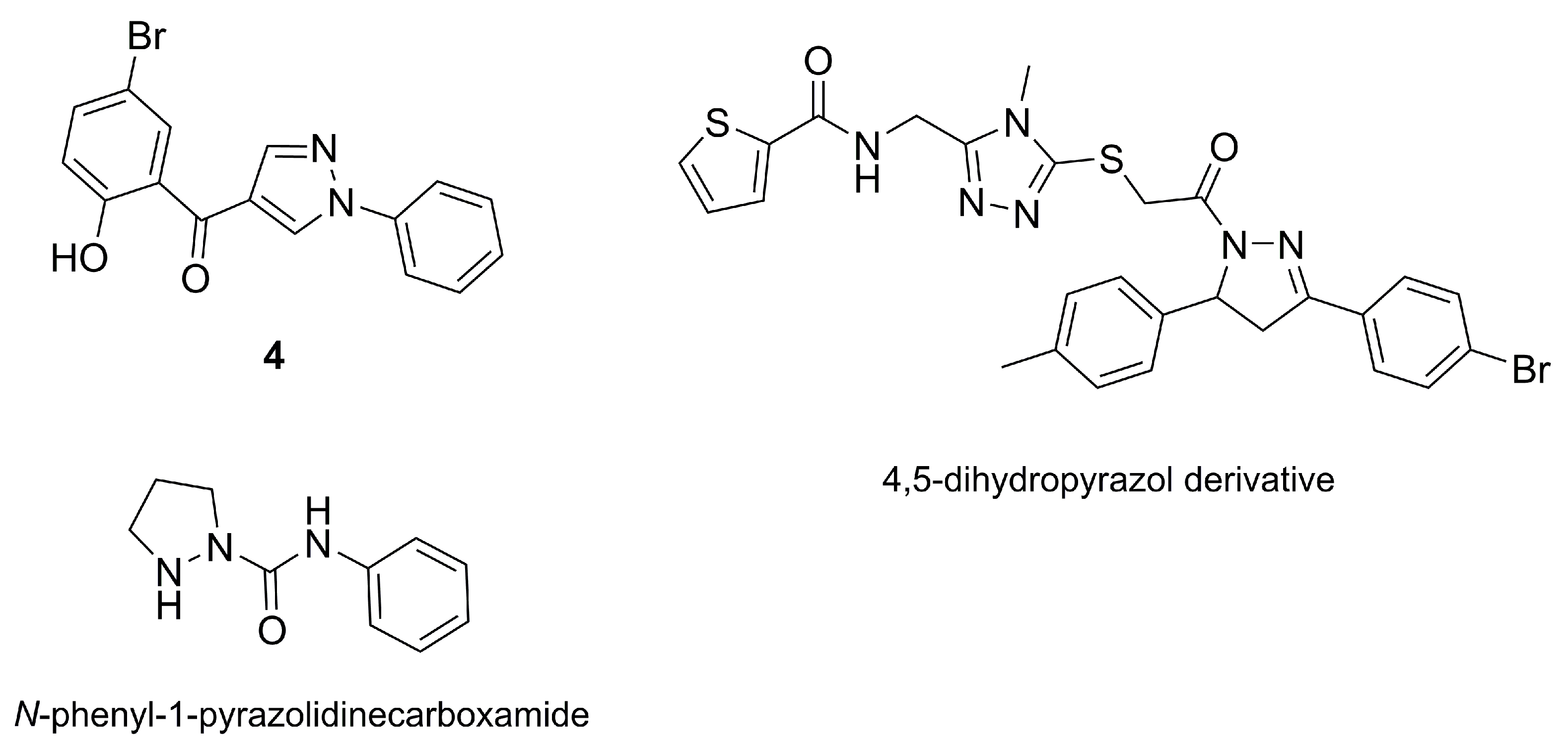
2.3. Pyrazole Derivatives from the Tall-stilted Mangrove Tree
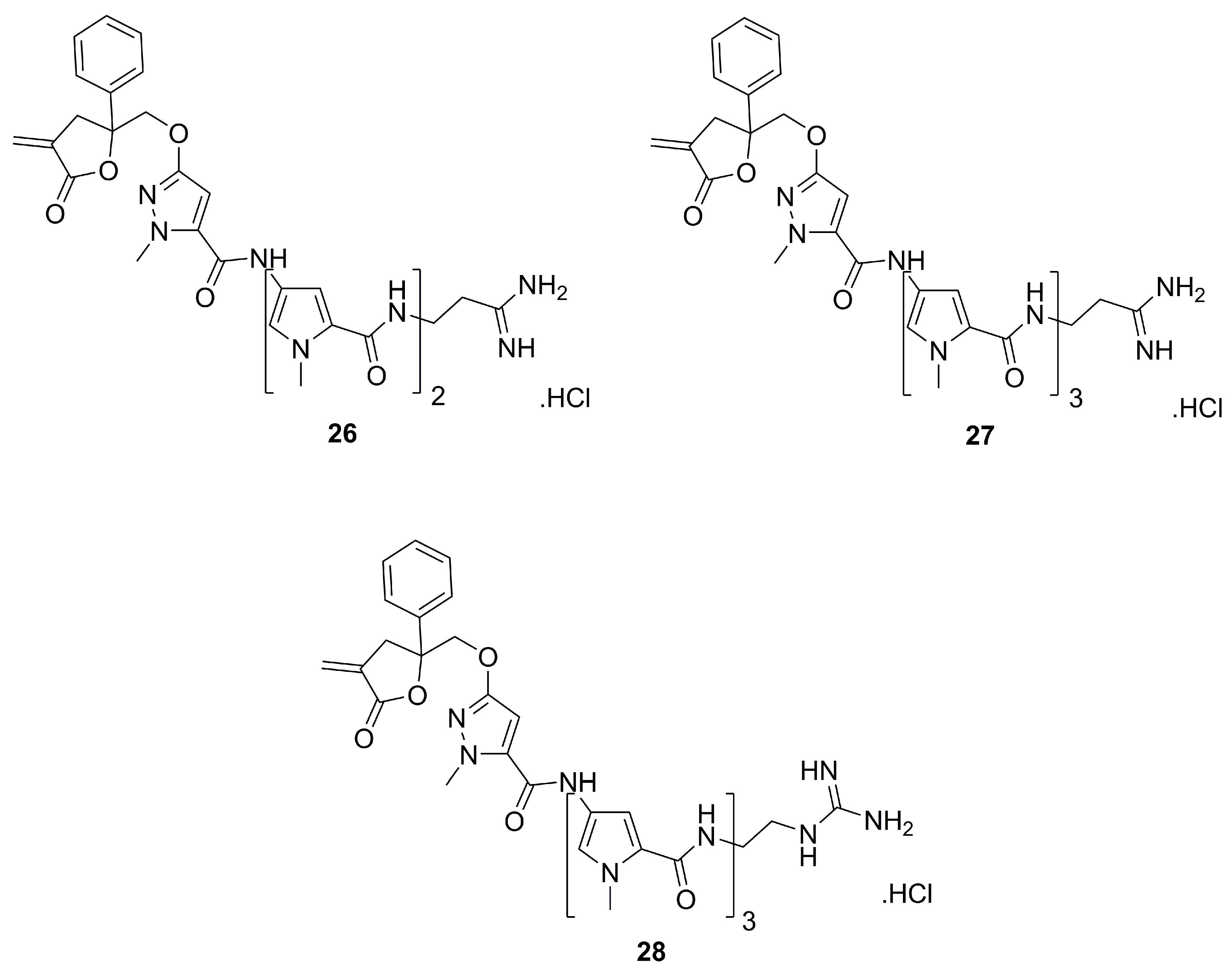
3. Pyrazole Analogues of Natural Products
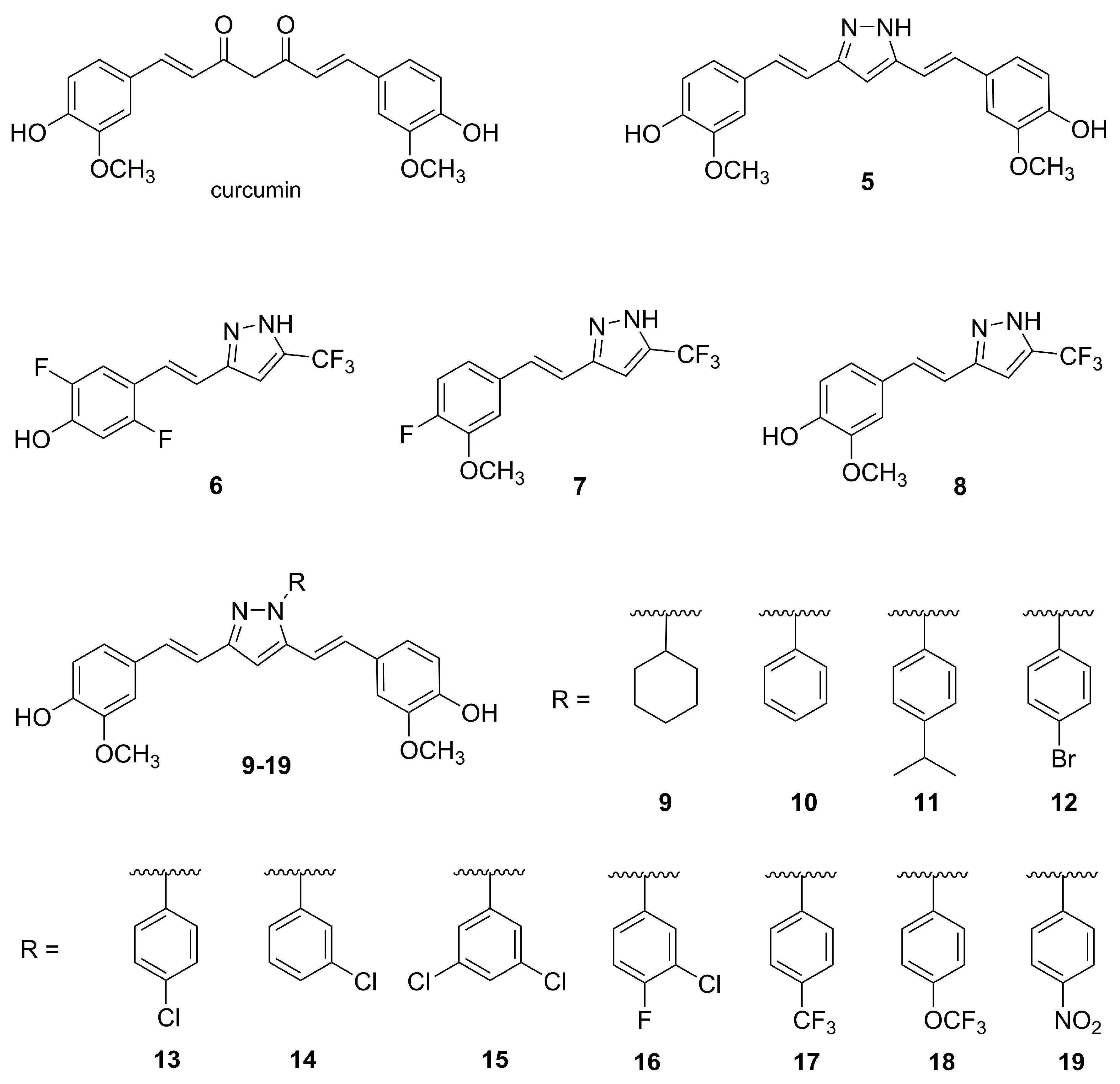
3.1. Curcuminoid Pyrazoles for Telomerase Inhibition
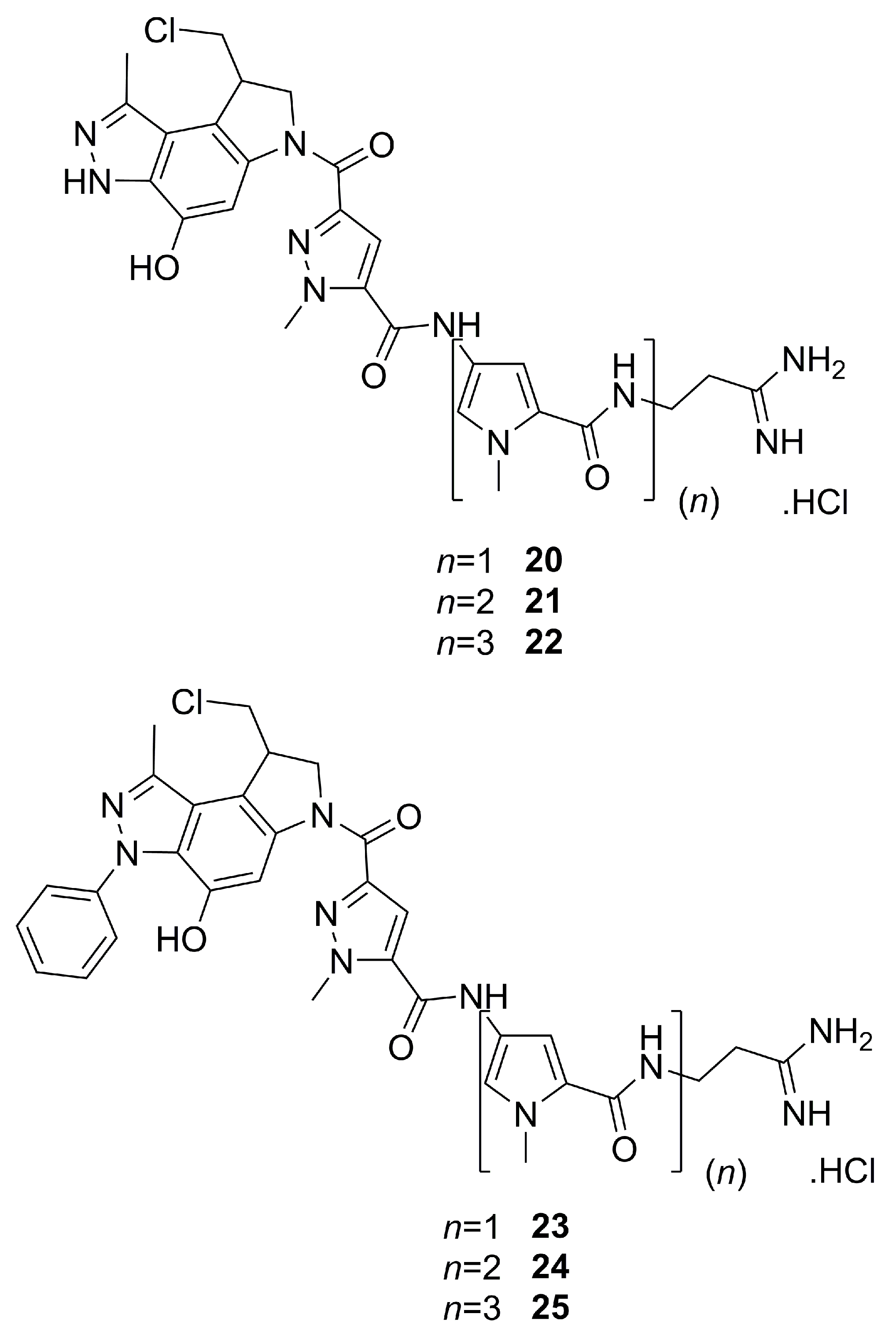
3.2. Pyrazole-bearing Dystamycin Analogues as DNA Alkylating Agents
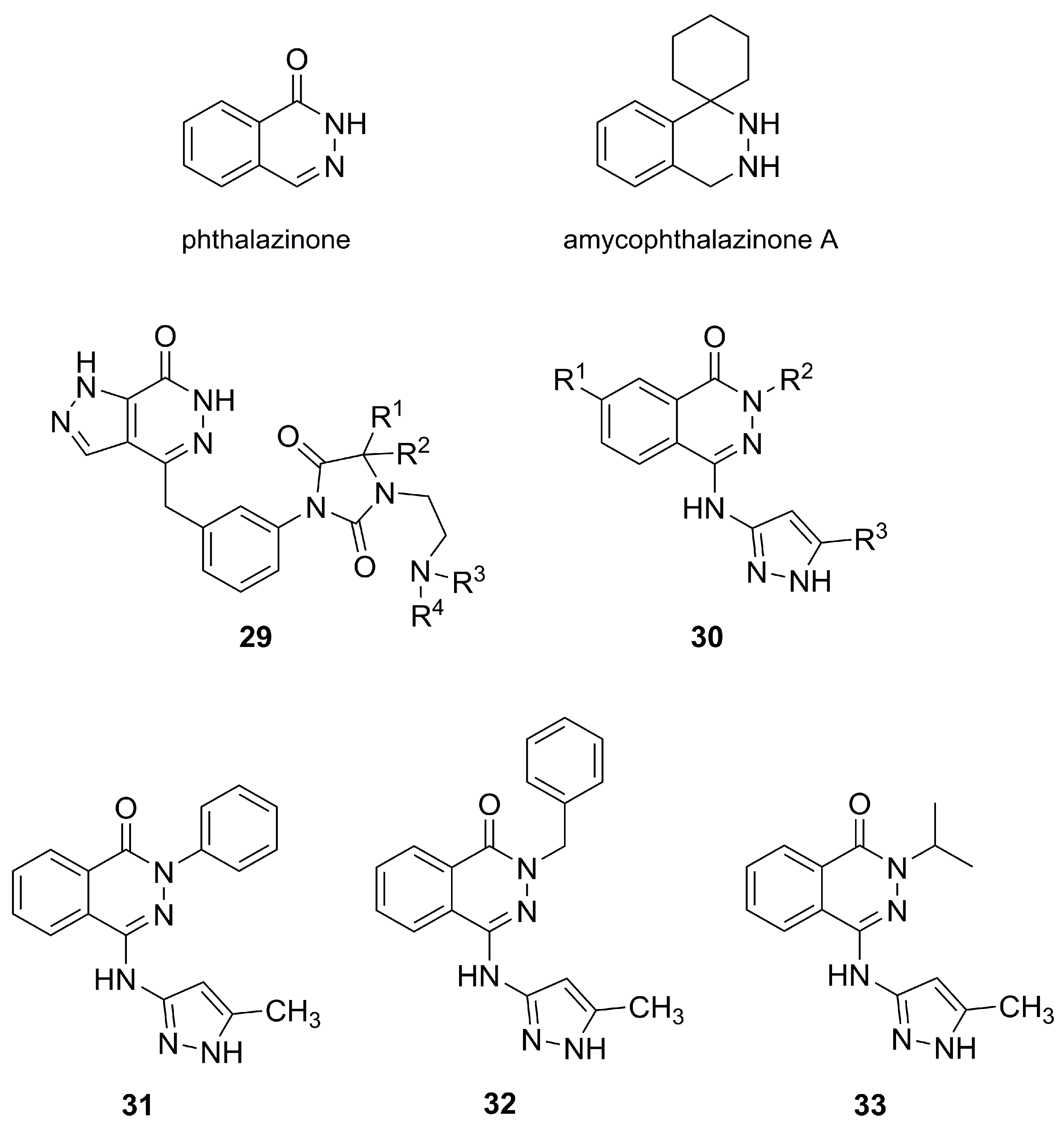
3.3. Phthalazinone-derived Pyrazoles for Aurora Kinase Inhibition
4. Conclusions
Funding
Conflicts of Interest
References
- De Furia, M.D. Paclitaxel (Taxol®): A new natural product with major anticancer activity. Phytomed 1997, 4, 273–282. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Costa-Lotufo, L.V. Marine drugs for cancer: Surfacing biotechnological innovations from the oceans. Clinics (Sao Paulo) 2018, 73, 482. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Sankhala, K.K.; Chawla, N.; Chawla, S.P. Trabectedin for soft tissue sarcoma: Current status and future perspectives. Adv. Ther. 2016, 33, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Webster, K.A.; Gustafson, T.A.; Kedes, L. The anti-cancer agent distamycin A displaces essential transcription factors and selectively inhibits myogenic differentiation. Mol. Cell Biochem. 1997, 169, 61–72. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Preti, D.; Fruttarolo, F.; Tabrizi, M.A.; Romagnoli, R. Hybrid molecules between distamycin A and active moieties of antitumor agents. Bioorg. Med. Chem. 2007, 15, 17–35. [Google Scholar] [CrossRef]
- Kehraus, S.; König, G.M.; Wright, A.D.; Woerheide, G. Leucamide A: A New Cytotoxic Heptapeptide from the Australian Sponge Leucetta microraphis. J. Org. Chem. 2002, 67, 4989–4992. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Asari, T.; Harada, T.; Katada, J.; Muramatsu, M.; Kawashima, H.; Sekiya, H.; Uno, I. (-)-Phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg. Med. Chem. Lett. 1997, 22, 2847–2852. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Katada, J.; Hayashi, Y.; Muramatsu, M.; Uno, I. Antitumor Activity of Phenylahistin in Vitro and in Vivo. Biosci. Biotech. Biochem. 1999, 63, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Burres, N.S.; Barber, D.A.; Gunasekera, S.P.; Shen, L.L.; Clement, J.J. Antitumor activity and biochemical effects of topsentin. Biochem. Pharmacol. 1991, 42, 745–751. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Schröter, H.-B.; Neumann, D.; Katritzky, A.R.; Swinbourne, F.J. Withasomnine. A pyrazole alkaloid from Withania somnifera Dun. Tetrahedron 1966, 22, 2895–2897. [Google Scholar] [CrossRef]
- Aladesanmi, A.J.; Rene, N.; Nahrstedt, A. New Pyrazole Alkaloids from the Root Bark of Newbouldia laevis. Planta Med. 1998, 64, 90–91. [Google Scholar] [CrossRef]
- Ravikant, V.; Ramesh, P.; Diwan, P.V.; Venkateswarlu, Y. Pyrazole alkaloids from Elytraria acaulis. Biochem. System. Ecol. 2001, 29, 753–754. [Google Scholar] [CrossRef]
- Mokhlesi, A.; Hartmann, R.; Kurtán, T.; Weber, H.; Lin, W.; Chaidir, C.; Müller, W.E.G.; Daletos, G.; Proksch, P. New 2-Methoxy Acetylenic Acids and Pyrazole Alkaloids from the Marine Sponge Cinachyrella sp. Mar. Drugs 2017, 15, 356. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Kuruvilla, C.A.; Guruvayoorappan, C. Potentiating Effect of 1, 2-diazole a plant alkaloid on carrageenan and formalin induced paw edema in experimental mice. Int. J. Pharm. Pharm. Sci. 2012, 4, 380–383. [Google Scholar]
- Prabhu, V.V.; Kannan, K.; Guruvayoorappan, C. 1,2-Diazole prevents cisplatin-induced nephrotoxicity in experimental rats. Pharmacol. Rep. 2013, 65, 980–990. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Elangovan, P.; Devaraj, S.N.; Sakthivel, K.M. Targeting apoptosis by 1,2-diazole through regulation of EGFR, Bcl-2 and CDK-2 mediated signaling pathway in human nonsmall cell lung carcinoma A549 cells. Gene 2018, 679, 352–359. [Google Scholar] [CrossRef]
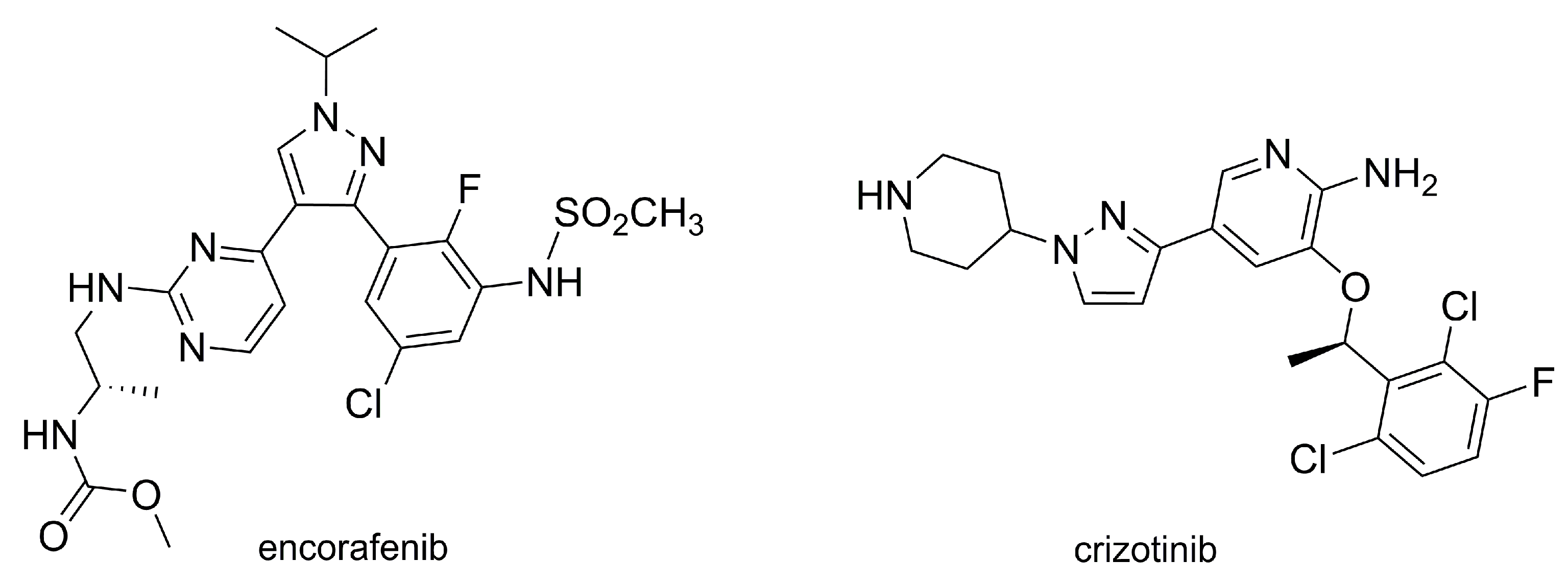
- FDA Approves Encorafenib and Binimetinib in Combination for Unresectable or Metastatic Melanoma with BRAF Mutations. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-and-binimetinib-combination-unresectable-or-metastatic-melanoma-braf (accessed on 7 February 2020).
- Meneguzzo, A.; Lazzarotto, A.; Alaibac, M. Eruptive melanocytic nevi secondary to Encorafenib for BRAF mutant metastatic colorectal cancer. In Vivo 2020, 34, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Li, X.F. Encorafenib inhibits migration, induces cell cycle arrest and apoptosis in colorectal cancer cells. Mol. Cell. Biochem. 2019, 459, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, C.H.; Ching, G.; Du, L.L.; Saporito, R.C.; Bansal, V.; Pence, L.J.; Liang, R.; Tsai, K.Y. Comparative profiles of BRAF inhibitors: The paradox index as a predictor of clinical toxicity. Oncotarget 2016, 7, 30453–30460. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Crizotinib Capsules. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-capsules (accessed on 12 February 2020).
- Kosaka, T.; Yajima, T.; Yamaki, E.; Nakazawa, S.; Tomizawa, K.; Onozato, R.; Yamazaki, R.; Hirato, J.; Yatabe, Y.; Shimizu, K.; et al. Long-term complete response in a patient with postoperative recurrent ALK-rearranged lung adenocarcinoma treated with crizotinib: A case report. Mol. Clin. Oncol. 2019, 11, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer. 2013, 2, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Ikedaya, A.; Toda, A.; Ikushima, K.; Yamakawa, T.; Okada, R.; Yamada, T.; Tanaka, R. Pyrazole alkaloids from watermelon (Citrullus lanatus) seeds. Phytochem. Lett. 2015, 12, 94–97. [Google Scholar] [CrossRef]
- Zhao, G.; Yao, S.; Rothchild, K.W.; Liu, T.; Liu, Y.; Lian, J.; He, H.-Y.; Ryan, K.S.; Du, Y.-L. The biosynthetic gene cluster of pyrazomycin—A C-nucleoside antibiotic with a rare pyrazole moiety. ChemBioChem 2020. [Google Scholar] [CrossRef]
- Williams, R.; Hoehn, M. Pyrazomycin and Process for Production Thereof. Patent US3802999A, 9 April 1974. [Google Scholar]
- Olah, E.; Lui, M.S.; Tzeng, D.Y.; Weber, G. Phase and cell cycle specificity of pyrazofurin action. Cancer Res. 1980, 40, 2869–2875. [Google Scholar]
- Sweeney, M.J.; Davis, F.A.; Gutowski, G.E.; Marnili, R.L.; Hoffman, D.H.; Poore, G.A. Experimental Antitumor Activity of Pyrazomycin. Cancer Res. 1973, 33, 2619–2623. [Google Scholar]
- Salem, P.A.; Bodey, G.P.; Burgess, M.A.; Murphy, W.K.; Freireich, E.M. A phase I study of pyrazofurin. Cancer 1977, 40, 2806–2809. [Google Scholar] [CrossRef]
- De Clercq, E. C-Nucleosides to be revisited. J. Med. Chem. 2016, 59, 2301–2311. [Google Scholar] [CrossRef]
- Duke, N.C. Rhizophora apiculata, R. mucronata, R. stylosa, R. × annamalai, R. × lamarckii (Indo–West Pacific stilt mangrove). In Traditional Trees of Pacific Islands: The Culture, Environment and Use; Elevitch, C.R., Ed.; Permanent Agriculture Resources: Holualoha, HI, USA, 2006. [Google Scholar]
- Prabhu, V.V.; Guruvayoorappan, C. Anti-inflammatory and anti-tumor activity of the marine mangrove Rhizophora apiculata. J. Immunotox. 2012, 9, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.A.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, A.; Lin, J.; Zheng, Z.; Shi, X.; Di, W.; Qi, W.; Zhu, Y.; Zhou, G.; Fang, Y. Telomerase: A target for therapeutic effects of curcumin and a curcumin derivative in a β1-42 insult in vitro. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef]
- Flynn, D.L.; Belliotti, T.R.; Boctor, A.M.; Connor, D.T.; Kostlan, C.R.; Nies, D.E.; Ortwine, D.F.; Schrier, D.J.; Sircar, J.C. Styrylpyrazoles, styrylisoxazoles, and styrylisothiazoles. Novel 5-lipooxygenase and cyclooxygenase inhibitors. J. Med. Chem. 1991, 34, 518–525. [Google Scholar] [CrossRef]
- R. Martí-Centelles, R.; Falomir, E.; Carda, M.; Nieto, C.I.; Cornago, M.P.; Claramunt, R.M. Effects of curcuminoid pyrazoles on cancer cells and on the expression of telomerase related genes. Arch. Pharm. Chem. Life Sci. 2016, 349, 532–538. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.H.; Wei, X. Synthesis and Cytotoxic Activity of Novel Tetrahydrocurcumin Derivatives Bearing Pyrazole Moiety. Nat. Prod. Bioprospect. 2017, 7, 461–469. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Balboni, G.; Pavani, M.G.; Spalluto, G.; Tabrizi, M.A.; De Clercq, E.; Balzarini, J.; Bando, T.; Sugiyama, H.; Romagnoli, R. Design, synthesis, DNA binding, and biological evaluation of water-soluble hybrid molecules containing two pyrazole analogues of the alkylating cyclopropylpyrroloindole (CPI) subunit of the antitumor agent CC-1065 and polypyrrole minor groove binders. J. Med. Chem. 2001, 44, 2536–2543. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Nunez, M.C.; Tabrizi, M.A.; De Clercq, E.; Balzarini, J.; Bermejo, J.; Estévez, F.; Romagnoli, R. Design, synthesis, and biological evaluation of hybrid molecules containing α-methylene-γ-butyrolactones and polypyrrole minor groove binders. J. Med. Chem. 2004, 47, 2877–2886. [Google Scholar] [CrossRef]
- Sutherland, J.B.; Freeman, J.P.; Williams, A.J.; Deck, J. Biotransformation of phthalazine by Fusarium moniliforme and Cunninghamella elegans. Mycologia 1999, 91, 114–116. [Google Scholar] [CrossRef]
- Zheng, K.X.; Jiang, Y.; Jiang, J.X.; Huang, R.; He, J.; Wua, S.H. A new phthalazinone derivative and a new isoflavonoid glycoside from lichen-associated Amycolatopsis sp. Fitoterapia 2019, 135, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Menear, K.A.; Hummersone, M.G.; Gomez, S.; Javaid, M.H.; Martin, N.M.B. Phthalazinone Derivatives and Their Use as Medicament to Treat Cancer. Patent WO2008122810A1, 16 October 2008. [Google Scholar]
- Boyd, E.; Brookfield, F.; Courtney, S.; Georges, G.; Goller, B.; Limberg, A.; Prime, M.; Rueger, P.; Rueth, M. Phthalazinone Pyrazole Derivatives, Their Manufacture and Use as Pharmaceutical Agents. Patent WO2007107298A1, 17 September 2007. [Google Scholar]
- Prime, M.E.; Courtney, S.M.; Brookfield, F.A.; Marston, R.W.; Walker, V.; Warne, J.; Boyd, A.E.; Kairies, N.A.; Saal, W.; Limberg, A.; et al. Phthalazinone Pyrazoles as Potent, Selective, and Orally Bioavailable Inhibitors of Aurora-A Kinase. J. Med. Chem. 2011, 54, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.D.; Grina, J.; Newhouse, B.; Welch, M.; Topalov, G.; Littman, N.; Callejo, M.; Gloor, S.; Martinson, M.; Laird, E.; et al. Potent and selective pyrazole-based inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett. 2008, 18, 4692–4695. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.E.; Carreira, A.R.F.; Silva, V.L.M.; Braga, S.S. Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules 2020, 25, 1364. https://doi.org/10.3390/molecules25061364
Santos NE, Carreira ARF, Silva VLM, Braga SS. Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules. 2020; 25(6):1364. https://doi.org/10.3390/molecules25061364
Chicago/Turabian StyleSantos, Nádia E., Ana R.F. Carreira, Vera L. M. Silva, and Susana Santos Braga. 2020. "Natural and Biomimetic Antitumor Pyrazoles, A Perspective" Molecules 25, no. 6: 1364. https://doi.org/10.3390/molecules25061364
APA StyleSantos, N. E., Carreira, A. R. F., Silva, V. L. M., & Braga, S. S. (2020). Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules, 25(6), 1364. https://doi.org/10.3390/molecules25061364