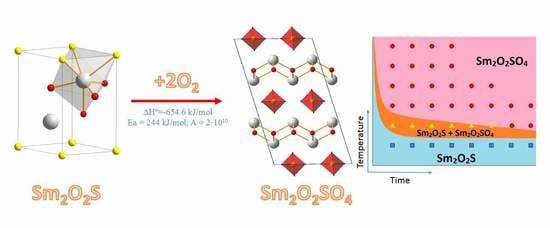
Synthesis of Samarium OxysulfateSm2O2SO4 in the High-Temperature Oxidation Reaction and Its Structural, Thermal and Luminescent Properties
Abstract
1. Introduction
2. Results and Discussion
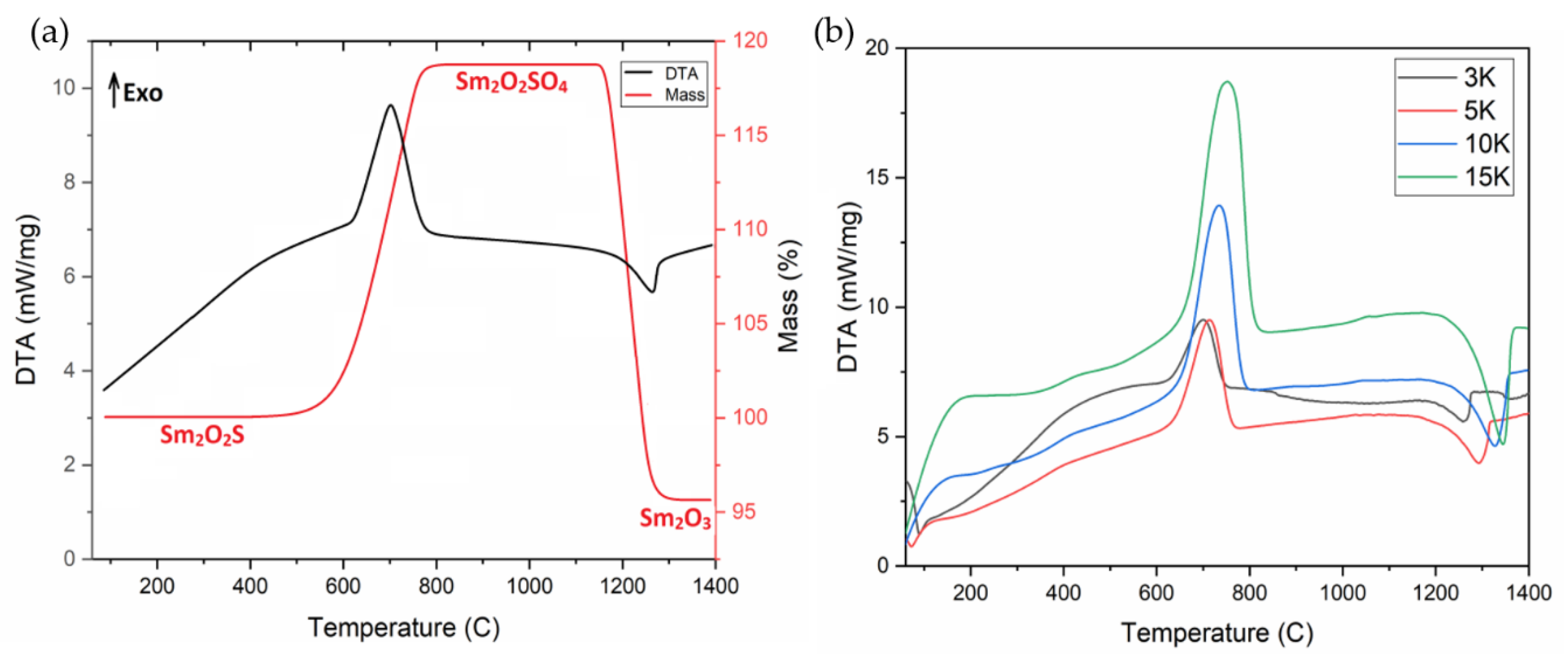
2.1. Dynamic Oxidation of Sm2O2S and Thermal Stability of Sm2O2SO4
2.2. Isothermal Oxidation of Sm2O2S
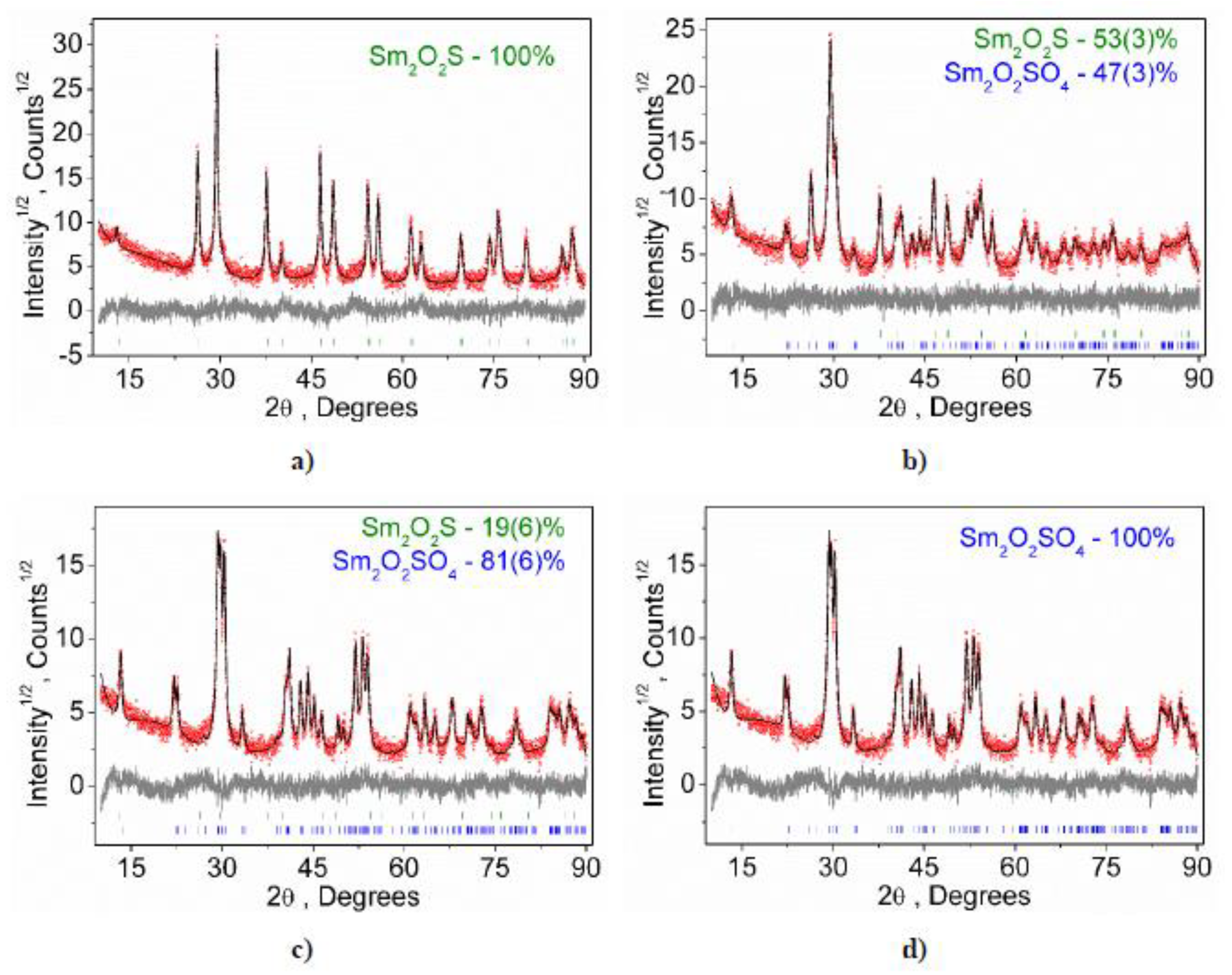
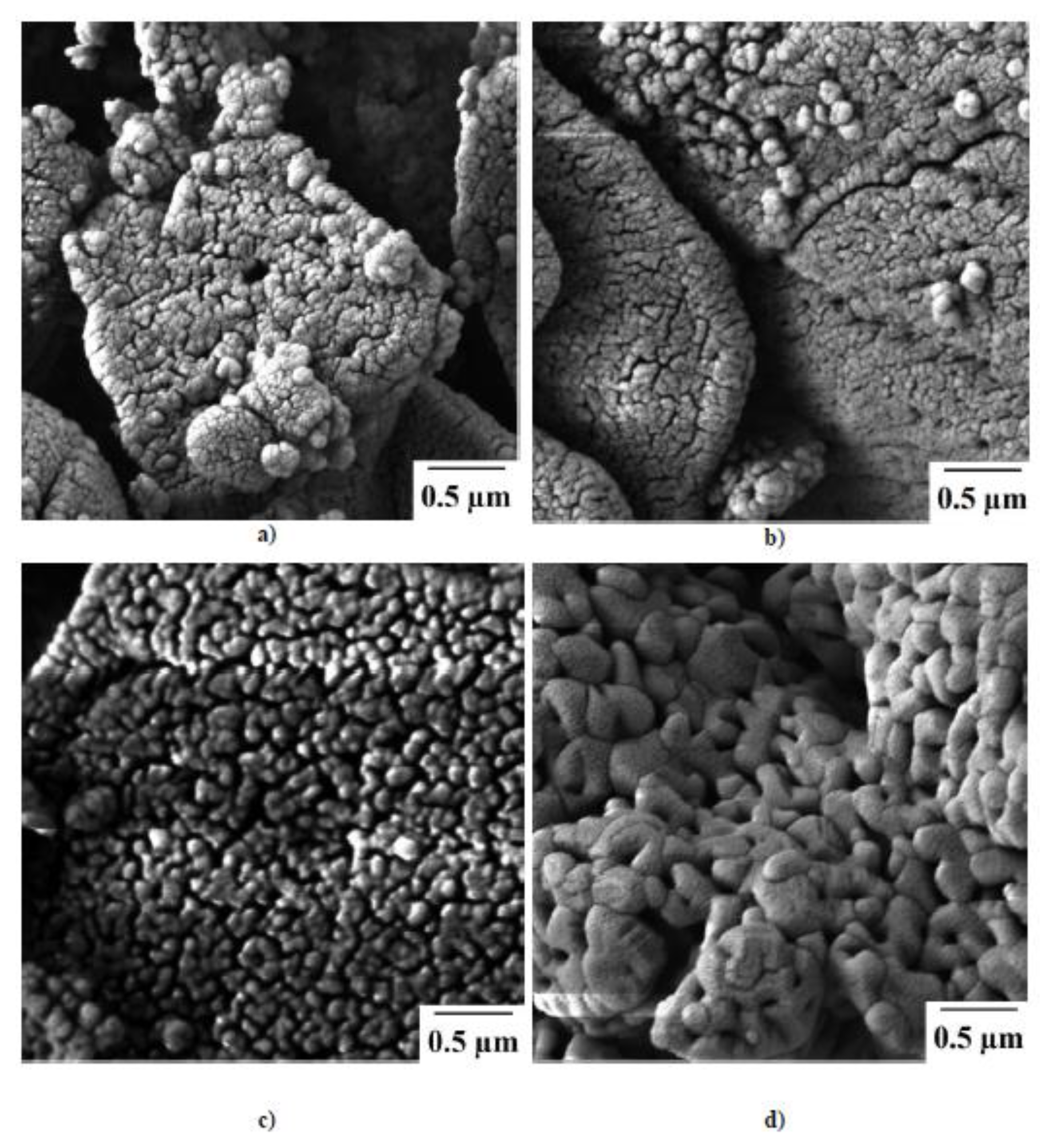
2.3. Structural Properties of Sm2O2SO4
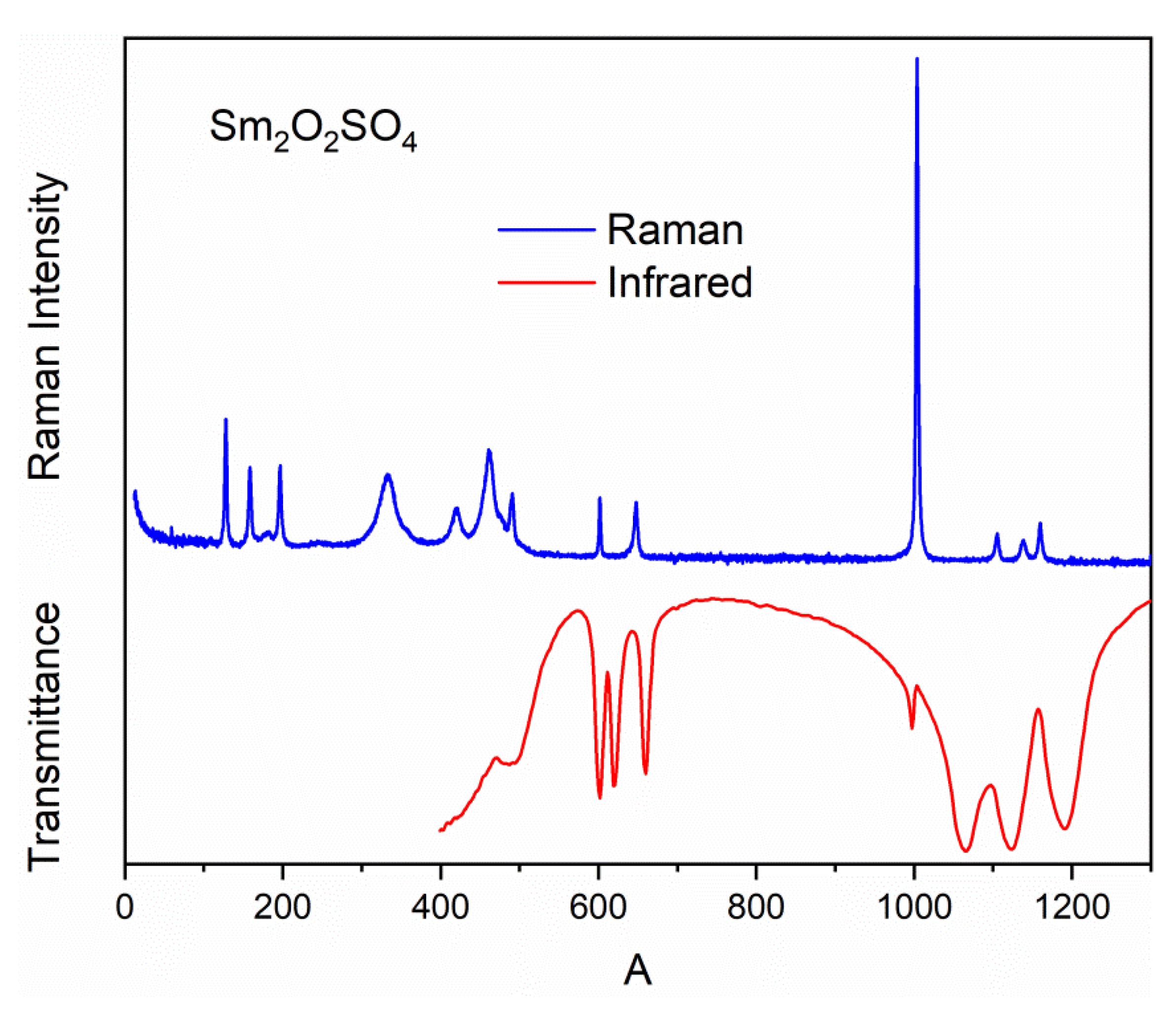
2.4. Vibrational Spectra of Sm2O2SO4
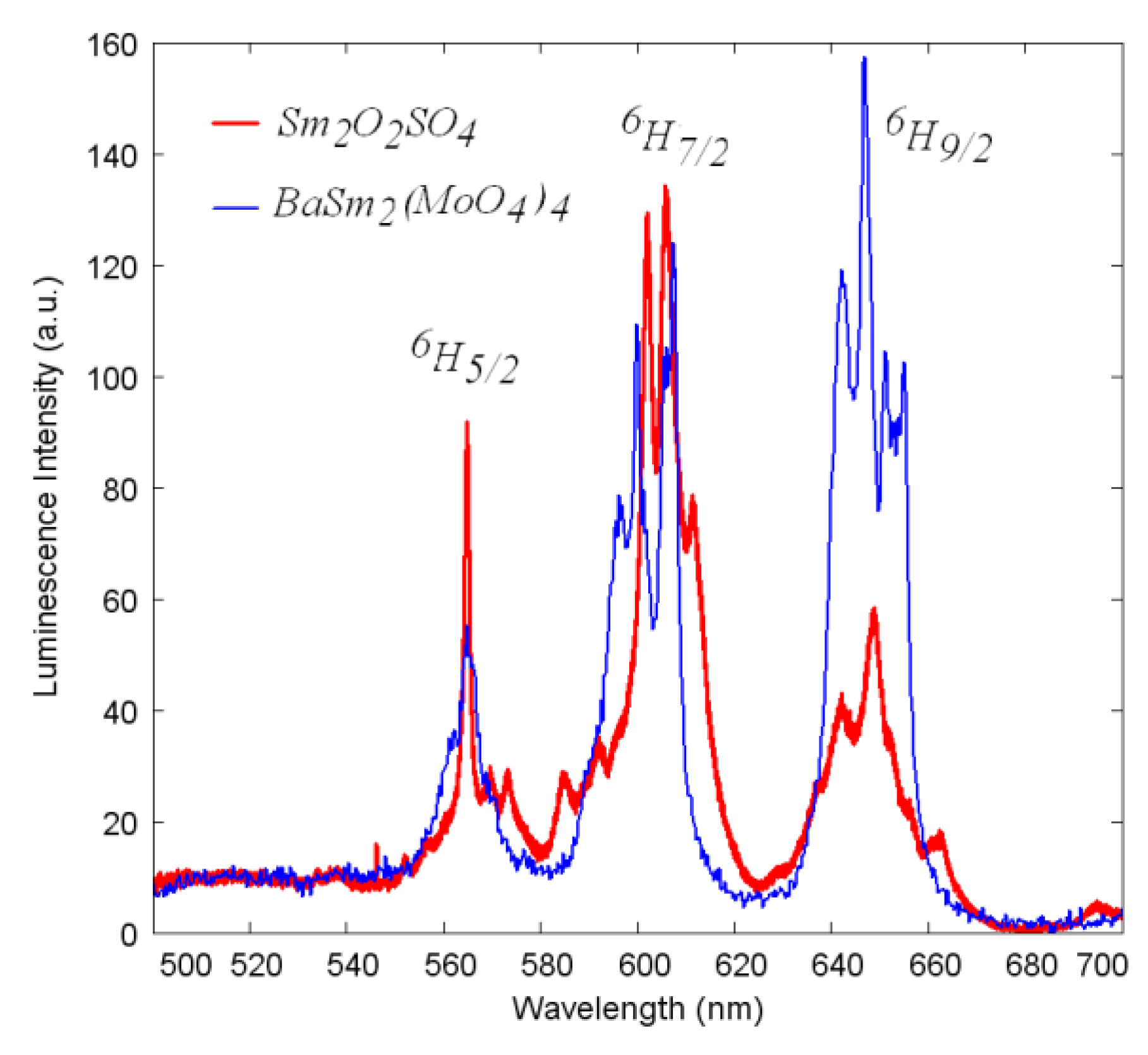
2.5. Luminescent Properties of Sm2O2SO4
3. Materials and Methods
3.1. Synthesis Methods
3.2. Methods of Physico-Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreev, P.O.; Sal’nikova, E.I.; Kislitsyn, A.A. Kinetics of the transformation of Ln2O2SO4 into Ln2O2S (Ln = La, Pr, Nd, and Sm) in a hydrogen flow. Russ. J. Phys. Chem. A 2013, 87, 1482–1487. [Google Scholar] [CrossRef]
- Sal’nikova, E.I.; Andreev, P.O.; Antonov, S.M. Kinetic diagrams of Ln2O2SO4 phase transformations in a H2 flow (Ln = La, Pr, Nd, Sm). Russ. J. Phys. Chem. A 2013, 87, 1280–1283. [Google Scholar] [CrossRef]
- Sal’nikova, E.I.; Kaliev, D.I.; Andreev, P.O. Kinetics of phase formation upon the treatment of La2(SO4)3 and La2O2SO4 in a hydrogen flow. Russ. J. Phys. Chem. A 2011, 85, 2121–2125. [Google Scholar] [CrossRef]
- Machado, L.C.; de Azeredo, M.T.D.; Corrêa, H.P.S.; do Rosário Matos, J.; Mazali, Í.O. Formation of oxysulfide LnO2S2 and oxysulfate LnO2SO4 phases in the thermal decomposition process of lanthanide sulfonates (Ln = La, Sm). J. Therm. Anal. Calorim. 2012, 107, 305–311. [Google Scholar] [CrossRef]
- Kim, S.; Masui, T.; Imanaka, N. Synthesis of red-emitting phosphors based on gadolinium oxysulfate by a flux method. Electrochemistry 2009, 77, 611–613. [Google Scholar] [CrossRef]
- Kijima, T.; Shinbori, T.; Sekita, M.; Uota, M.; Sakai, G. Abnormally enhanced Eu3+ emission in Y2O2SO4: Eu3+ inherited from their precursory dodecylsulfate-templated concentric-layered nanostructure. J. Lumin. 2008, 128, 311–316. [Google Scholar] [CrossRef]
- Kijima, T.; Isayama, T.; Sekita, M.; Uota, M.; Sakai, G. Emission properties of Tb3+ in Y2O2SO4 derived from their precursory dodecylsulfate-templated concentric- and straight-layered nanostructures. J. Alloys Compd. 2009, 485, 730–733. [Google Scholar] [CrossRef]
- Machida, M.; Kawamura, K.; Ito, K.; Ikeue, K. Large-capacity oxygen storage by lanthanide oxysulfate/oxysulfide systems. Chem. Mater. 2005, 17, 1487–1492. [Google Scholar] [CrossRef]
- Machida, M.; Kawamura, K.; Kawano, T.; Zhang, D.; Ikeue, K. Layered Pr-dodecyl sulfate mesophases as precursors of Pr2O2SO4 having a large oxygen-storage capacity. J. Mater. Chem. 2006, 16, 3084–3090. [Google Scholar] [CrossRef]
- Machida, M.; Kawano, T.; Eto, M.; Zhang, D.; Ikeue, K. Ln dependence of the large-capacity oxygen storage/release property of Ln oxysulfate/oxysulfide systems. Chem. Mater. 2007, 19, 954–960. [Google Scholar] [CrossRef]
- Zhang, D.; Yoshioka, F.; Ikeue, K.; Machida, M. synthesis and oxygen release/storage properties of Ce-substituted La-oxysulfates, (La1−xCex)2O2SO4. Chem. Mater. 2008, 20, 6697–6703. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Aleksandrovsky, A.S.; Atuchin, V.V.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Andreev, O.V. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Ind. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef]
- Hartenbach, I.; Schleid, T. Serendipitous Formation of Single-Crystalline Eu2O2[SO4]. Z. Anorg. Allg. Chem. 2002, 628, 2171. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Khritokhin, N.A.; Andreev, O.V.; Basova, S.A.; Sal’nikova, E.I.; Polkovnikov, A.A. Thermal decomposition of europium sulfates Eu2(SO4)3·8H2O and EuSO4. J. Solid State Chem. 2017, 255, 219–224. [Google Scholar] [CrossRef]
- Poston, J.A.; Siriwardane, R.V.; Fisher, E.P.; Miltz, A.L. Thermal decomposition of the rare earth sulfates of cerium (III), cerium (IV), lanthanum (III) and samarium (III). Appl. Surf. Sci. 2003, 214, 83–102. [Google Scholar] [CrossRef]
- Liang, J.; Ma, R.; Geng, F.; Ebina, Y.; Sasaki, T. Ln2(OH)4SO4·nH2O (Ln = Pr to Tb; n ∼ 2): A new family of layered rare-earth hydroxides rigidly pillared by sulfate ions. Chem. Mater. 2010, 22, 6001–6007. [Google Scholar] [CrossRef]
- Chen, F.; Chen, G.; Liu, T.; Zhang, N.; Liu, X.; Luo, H.; Li, J.; Chen, L.; Ma, R.; Qiu, G. Controllable fabrication and optical properties of uniform gadolinium oxysulfate hollow spheres. Sci. Rep. 2016, 5, 17934. [Google Scholar] [CrossRef]
- Zhang, D.; Kawada, T.; Yoshioka, F.; Machida, M. Oxygen gateway effect of CeO2/La2O2SO4 composite oxygen storage materials. ACS Omega 2016, 1, 789–798. [Google Scholar] [CrossRef]
- Xing, T.H.; Song, L.X.; Xiong, J.; Cao, H.B.; Du, P.F. Preparation and luminescent properties of Tb3+ doped Y2O2SO4microflakes. Adv. Appl. Ceram. 2013, 112, 455–459. [Google Scholar] [CrossRef]
- Sal’nikova, E.I.; Denisenko, Y.G.; Aleksandrovsky, A.S.; Kolesnikov, I.E.; Lähderanta, E.; Andreev, P.O.; Azarapin, N.O.; Andreev, O.V.; Basova, S.A.; Matigorov, A.V. Synthesis and optical properties RE2O2S: Ln (RE= La, Y.; Ln= Ce, Eu, Dy, Er). J. Solid State Chem. 2019, 279, 120964. [Google Scholar]
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Motto Ros, V. Imaging rare-earth elements in minerals by laser-induced plasma spectroscopy: Molecular emission and plasma-induced luminescence. Spectrochim. Acta Part B At. Spectrosc. 2019, 151, 12–19. [Google Scholar] [CrossRef]
- Yu, M.; Xu, H.; Li, Y.; Dai, Q.; Wang, G.; Qin, W. Morphology luminescence and photovoltaic performance of lanthanide-doped CaWO4 nanocrystals. J. Colloid Interface Sci. 2020, 559, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Zhai, Z.; Chen, H.; Zhang, Y. Optical temperature sensing using upconversion luminescence in rare-earth ions doped Ca2Gd8(SiO4)6O2 phosphors. J. Alloys Compd. 2019, 771, 838–846. [Google Scholar] [CrossRef]
- Pejchal, J.; Barta, J.; Trojek, T.; Kucerkova, R.; Beitlerova, A.; Nikl, M. Luminescence and scintillation properties of rare-earth-doped LaAlO3 single crystals. Radiat. Meas. 2019, 121, 26–31. [Google Scholar] [CrossRef]
- Kichanov, S.E.; Gorshkova, Y.E.; Rachkovskaya, G.E.; Kozlenko, D.P.; Zakharevich, G.B.; Savenko, B.N. Structural evolution of luminescence nanoparticles with rare-earth ions in the oxyfluoride glass ceramics. Mater. Chem. Phys. 2019, 237, 121830. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Bazarov, B.G.; Bazarova, J.G. Synthesis and spectroscopic properties of monoclinic α-Eu2(MoO4)3. J. Phys. Chem. C 2014, 118, 15404–15411. [Google Scholar] [CrossRef]
- Lim, C.S.; Atuchin, V.V.; Aleksandrovsky, A.S.; Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S. Fabrication of microcrystalline NaPbLa(WO4)3:Yb3+/Ho3+ phosphors and their upconversionphotoluminescent characteristics. Korean J. Mater. Res. 2019, 29, 741–746. [Google Scholar] [CrossRef]
- Osseni, S.A.; Denisenko, Y.G.; Fatombi, J.K.; Sal’nikova, E.I.; Andreev, O.V. Synthesis and characterization of Ln2O2SO4 (Ln = Gd, Ho, Dy and Lu) nanoparticles obtained by coprecipitation method and study of their reduction reaction under H2 flow. J. Nanostructure Chem. 2017, 7, 337–343. [Google Scholar] [CrossRef]
- Osseni, S.A.; Lechevallier, S.; Verelst, M.; Dujardin, C.; Dexpert-Ghys, J.; Neumeyer, D.; Leclercq, M.; Baaziz, H.; Cussac, D.; Santran, V.; et al. New nanoplatform based on Gd2O2S:Eu3+ core: Synthesis, characterization and use for in vitro bio-labelling. J. Mater. Chem. 2011, 21, 18365–18372. [Google Scholar] [CrossRef]
- Osseni, S.A.; Lechevallier, S.; Verelst, M.; Perriat, P.; Dexpert-Ghys, J.; Neumeyer, D.; Garcia, R.; Mayer, F.; Djanashvili, K.; Peters, J.A.; et al. Gadolinium oxysulfide nanoparticles as multimodal imaging agents for T2-weighted MR, X-ray tomography and photoluminescence. Nanoscale 2014, 6, 555–564. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Kurth, D.G.; Müller-Buschbaum, K. Two series of lanthanide coordination polymers and complexes with 4′-phenylterpyridine and their luminescence properties. Eur. J. Inorg. Chem. 2019, 2019, 4564–4571. [Google Scholar] [CrossRef]
- Ribbeck, T.; Kerpen, C.; Löw, D.; Sedykh, A.E.; Müller-Buschbaum, K.; Ignat’ev, N.V.; Finze, M. Lanthanide trifluoromethyltricyanoborates: Synthesis, crystal structures and thermal properties. J. Fluor. Chem. 2019, 219, 70–78. [Google Scholar] [CrossRef]
- Stangl, J.M.; Dietrich, D.; Sedykh, A.E.; Janiak, C.; Müller-Buschbaum, K. Luminescent MOF polymer mixed matrix membranes for humidity sensing in real status analysis. J. Mater. Chem. C 2018, 6, 9248–9257. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Chimitova, O.D.; Denisenko, Y.G.; Gavrilova, T.A.; Krylov, A.S.; Maximovskiy, E.A.; Molokeev, M.S.; et al. Exploration of structural, vibrational and spectroscopic properties of self-activated orthorhombic double molybdateRbEu(MoO4)2 with isolated MoO4 units. J. Alloys Compd. 2019, 785, 692–697. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Atuchin, V.V.; Azarapin, N.O.; Plyusnin, P.E.; Sal’nikova, E.I.; Andreev, O.V. High-Temperature Oxidation of Europium (II) Sulfide. J. Ind. Eng. Chem. 2019, 79, 62–70. [Google Scholar] [CrossRef]
- Andrrev, O.V.; Razumkova, I.A.; Boiko, A.N. Synthesis and thermal stability of rare earth compounds REF3, REF3·nH2O and (H3O)RE3F10·nH2O (RE= Tb−Lu, Y), obtained from sulphide precursors. J. Fluor. Chem. 2018, 207, 77–83. [Google Scholar] [CrossRef]
- Razumkova, I.A.; Boiko, A.N.; Andreev, O.V.; Basova, S.A. Synthesis of [(H3O)Tm3F10]·nH2O, ErF3, and TmF3 powders and their physicochemical properties. Russ. J. Inorg. Chem. 2017, 62, 418–422. [Google Scholar]
- Razumkova, I.A. Synthesis of NaYF4 compounds from sulfide precursors. J. Fluor. Chem. 2018, 205, 1–4. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S.; Krylov, A.S.; Oreshonkov, A.S.; Zhou, D. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. J. Alloys Compd. 2017, 729, 843–849. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Diao, C.P.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Krylov, A.S.; Bazarov, B.G.; Bazarova, J.G.; et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalt. Trans. 2015, 44, 1805–1815. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalt. Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef] [PubMed]
- Biondo, V.; Sarvezuk, P.W.C.; Ivashita, F.F.; Silva, K.L.; Paesano, A.; Isnard, O. Geometric magnetic frustration in RE2O2S oxysulfides (RE= Sm, Eu and Gd). Mater. Res. Bull. 2014, 54, 41–47. [Google Scholar] [CrossRef]
- Yu, C.; Yu, M.; Li, C.; Liu, X.; Yang, J.; Yang, P.; Lin, J. Facile sonochemical synthesis and photoluminescent properties of lanthanide orthophosphate nanoparticles. J. Solid State Chem. 2009, 182, 339–347. [Google Scholar] [CrossRef]
- Cascales, C.; Lor, B.G.; Puebla, E.G.; Iglesias, M.; Monge, M.A.; Valero, C.R.; Snejko, N. Catalytic behavior of rare-earth sulfates: Applications in organic hydrogenation and oxidation reactions. Chem. Mater. 2004, 16, 4144–4149. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Hou, Z.; Peng, C.; Lin, J. Morphological control and luminescence properties of lanthanide orthovanadate LnVO4 (Ln= La to Lu) nano-/microcrystals via hydrothermal process. Cryst. Eng. Comm. 2011, 13, 474–482. [Google Scholar] [CrossRef]
- Baker, F.B.; Fitzgibbon, G.C.; Pavone, D.; Holley, C.E.; Hansen, L.D.; Lewis, E.A. Enthalpies of formation of Sm2O3 (monoclinic) and Sm2O3 (cubic). J. Chem. Thermodyn. 1972, 4, 621–636. [Google Scholar] [CrossRef]
- Eckman, J.R.; Rossini, F.D. The heat of formation of sulphur dioxide. Bur. Stand. J. Res. 1929, 3, 597. [Google Scholar] [CrossRef]
- Suponitskiy, Y.L. Thermal Chemistry of Oxygen-Containing Compounds of REE Elements and Elements of Group VI. Thesis of Doctor of Science in Chemistry, D. Mendeleev University of Chemical Technology of Russia, Moscow, Russia, 2002. [Google Scholar]
- Bruker AXS TOPAS V4; Bruker: Karlsruhe, Germany, 2008.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Volkova, S.S.; Andreev, O.V. Structure, thermal stability, and spectroscopic properties of triclinic double sulfate AgEu(SO4)2 with isolated SO4 groups. Inorg. Chem. 2018, 57, 13279–13288. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Bukovec, N.; Bukovec, P.; Šiftar, J. Kinetics of the thermal decomposition of Pr2(SO4)3 to Pr2O2SO4. Thermochim. Acta 1980, 35, 85–91. [Google Scholar] [CrossRef]
- Lyadov, A.S.; Kurilkin, V.V. Reduction specifics of rare-earth orthovanadates (REE= La, Nd, Sm, Dy, Ho, Er, Tm, Yb, and Lu). Russ. J. Inorg. Chem. 2016, 61, 86–92. [Google Scholar] [CrossRef]
- Llópiz, J.; Romero, M.M.; Jerez, A.; Laureiro, Y. Kinetic analysis of thermogravimetric data. Thermochim. Acta 1995, 256, 205–211. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Sm2O2SO4 are available from the authors. |

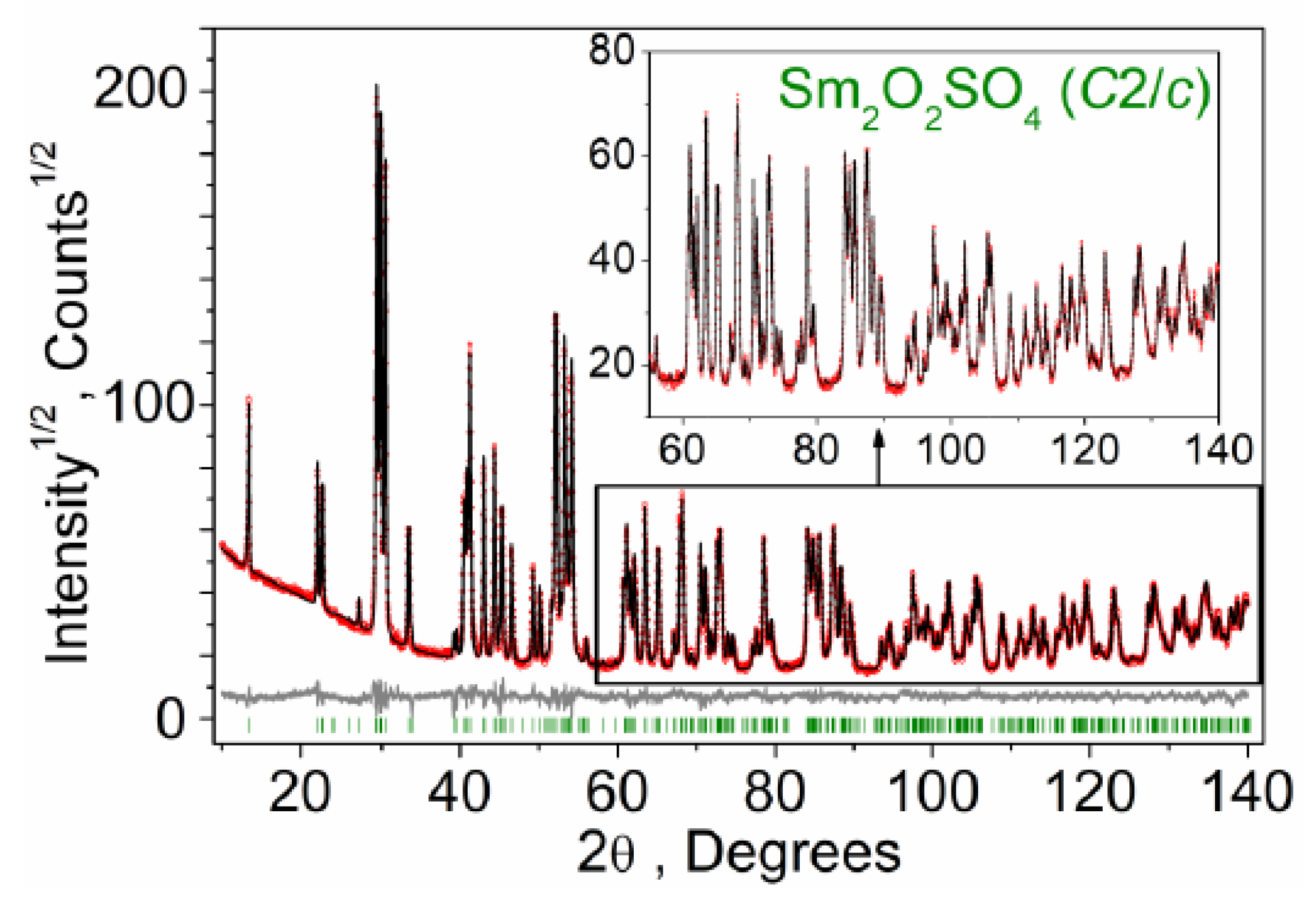
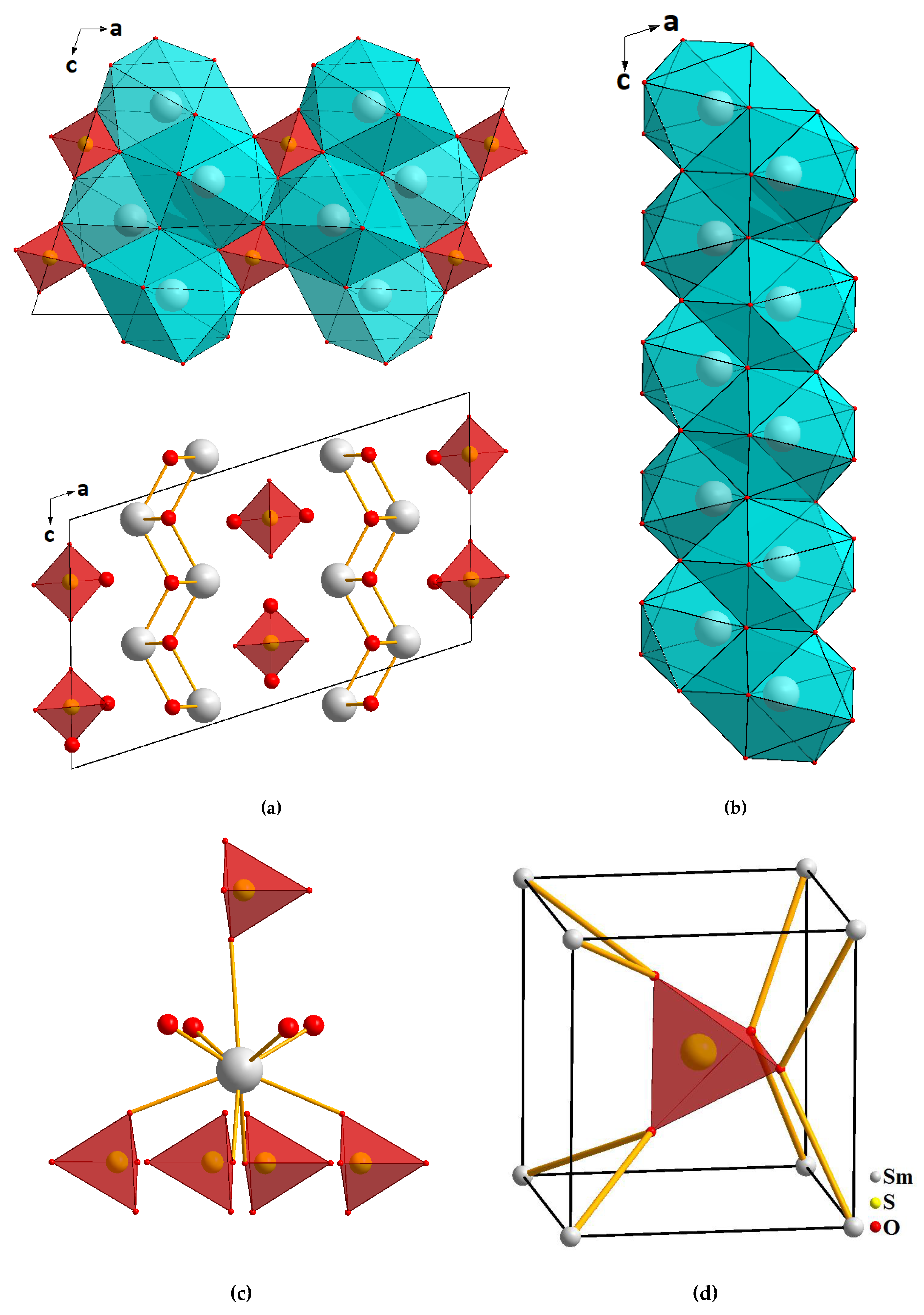
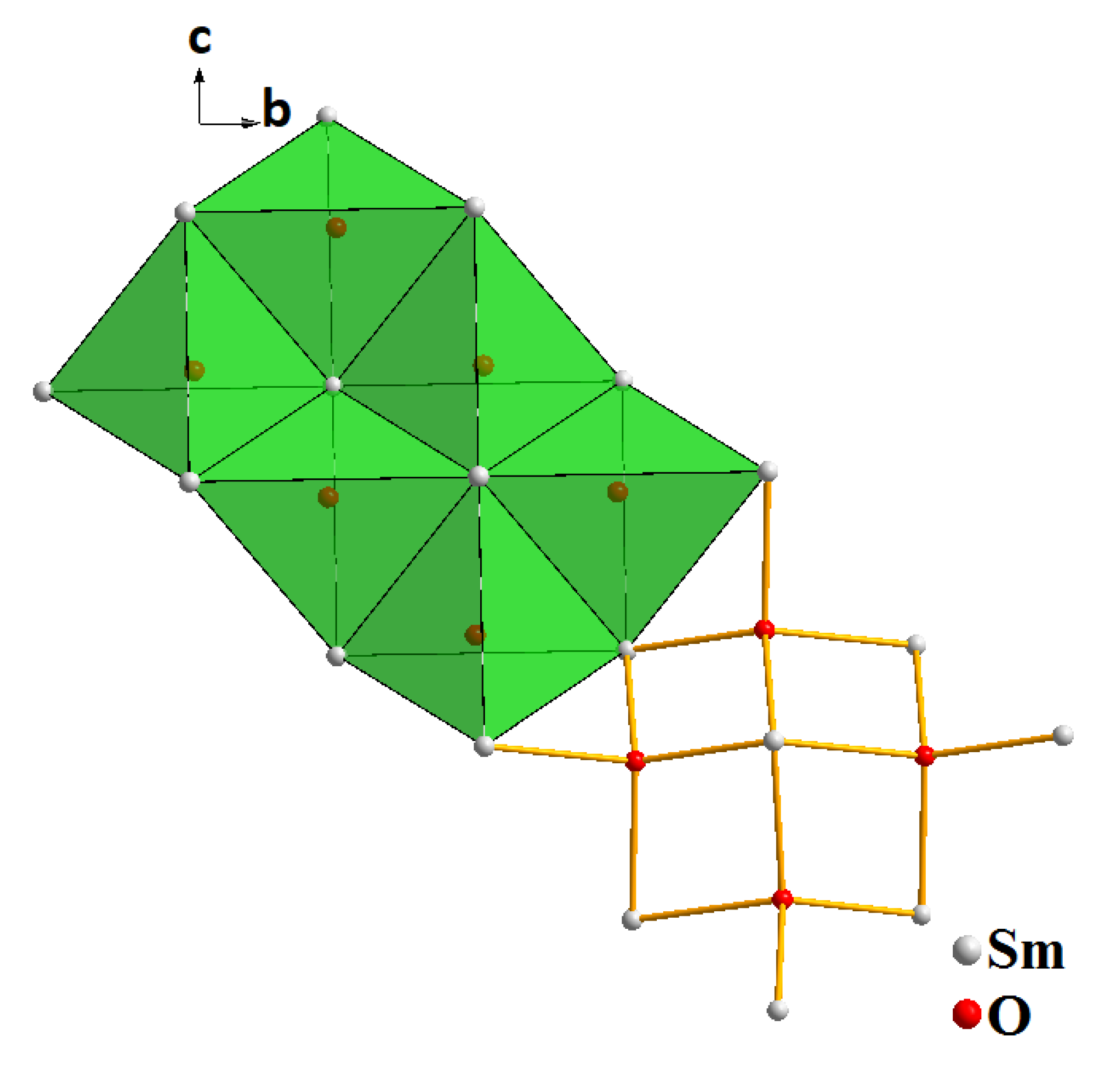
| Compound | Sm2O2SO4 |
|---|---|
| Space group | C2/c |
| a, Å | 13.7442 (2) |
| b, Å | 4.20178 (4) |
| c, Å | 8.16711 (8) |
| β, º | 107.224 (1) |
| V, Å3 | 450.498 (9) |
| Z | 4 |
| 2θ-interval, º | 10–140 |
| Rwp, % | 6.16 |
| Rp, % | 4.52 |
| Rexp, % | 2.78 |
| χ2 | 2.22 |
| RB, % | 1.70 |
| x | y | z | Biso | |
|---|---|---|---|---|
| Sm1 | 0.16930 (3) | 0.5015 (4) | 0.0850 (3) | 0.45 (2) |
| S1 | 0 | 0.0339 (15) | 0.25 | 1.63 (8) |
| O3 | 0.0904 (4) | 0.8717 (12) | 0.2840 (19) | 0.75 (7) |
| O2 | 0.9996 (8) | 0.2711 (12) | 0.0985 (8) | 0.75 (7) |
| O1 | 0.2474 (3) | 0.022 (2) | 0.120 (3) | 0.75 (7) |
| Sm1-O3 | 2.698 (9) | Sm1-O1 v | 2.417 (10) |
| Sm1-O3 i | 2.846 (13) | Sm1-O1 vi | 2.291 (14) |
| Sm1-O3 ii | 3.202 (3) | Sm1-O1 vii | 2.346 (19) |
| Sm1-O2 iii | 2.558 (9) | S1-O3 viii | 1.372 (5) |
| Sm1-O2 iv | 2.547 (8) | S1-O2 iii | 1.588 (7) |
| Sm1-O1 | 2.259 (9) |
| Wavenumber (cm−1) [51] | Td Point Group | C2 Site Symmetry | C2h Factor Group Symmetry |
|---|---|---|---|
| 983 | A1 (ν1) | A | Ag+ Au |
| 450 | E (ν2) | 2A | 2Ag + 2Au |
| 1105 | E (ν3) | A + 2B | Ag + Au+ 2Bg+ 2Bu |
| 611 | E (ν4) | A+ 2B | Ag+ Au+ 2Bg+ 2Bu |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisenko, Y.G.; Sal’nikova, E.I.; Basova, S.A.; Molokeev, M.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Atuchin, V.V.; Volkova, S.S.; Khritokhin, N.A.; et al. Synthesis of Samarium OxysulfateSm2O2SO4 in the High-Temperature Oxidation Reaction and Its Structural, Thermal and Luminescent Properties. Molecules 2020, 25, 1330. https://doi.org/10.3390/molecules25061330
Denisenko YG, Sal’nikova EI, Basova SA, Molokeev MS, Krylov AS, Aleksandrovsky AS, Oreshonkov AS, Atuchin VV, Volkova SS, Khritokhin NA, et al. Synthesis of Samarium OxysulfateSm2O2SO4 in the High-Temperature Oxidation Reaction and Its Structural, Thermal and Luminescent Properties. Molecules. 2020; 25(6):1330. https://doi.org/10.3390/molecules25061330
Chicago/Turabian StyleDenisenko, Yu. G., E. I. Sal’nikova, S. A. Basova, M. S. Molokeev, A. S. Krylov, A. S. Aleksandrovsky, A. S. Oreshonkov, V. V. Atuchin, S. S. Volkova, N. A. Khritokhin, and et al. 2020. "Synthesis of Samarium OxysulfateSm2O2SO4 in the High-Temperature Oxidation Reaction and Its Structural, Thermal and Luminescent Properties" Molecules 25, no. 6: 1330. https://doi.org/10.3390/molecules25061330
APA StyleDenisenko, Y. G., Sal’nikova, E. I., Basova, S. A., Molokeev, M. S., Krylov, A. S., Aleksandrovsky, A. S., Oreshonkov, A. S., Atuchin, V. V., Volkova, S. S., Khritokhin, N. A., & Andreev, O. V. (2020). Synthesis of Samarium OxysulfateSm2O2SO4 in the High-Temperature Oxidation Reaction and Its Structural, Thermal and Luminescent Properties. Molecules, 25(6), 1330. https://doi.org/10.3390/molecules25061330