Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus
Abstract
1. Introduction
2. Results
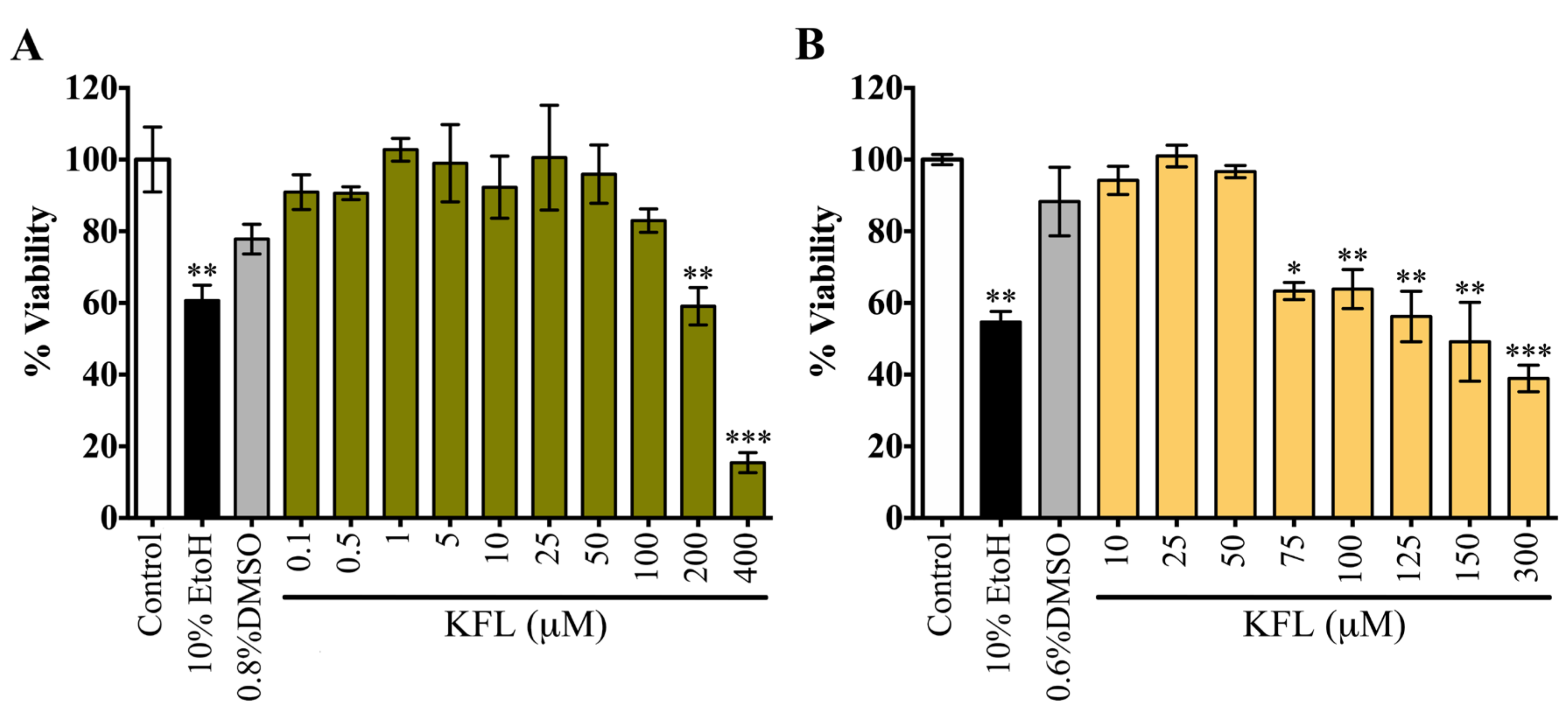
2.1. Evaluation of the Cytotoxicity of Kaempferol
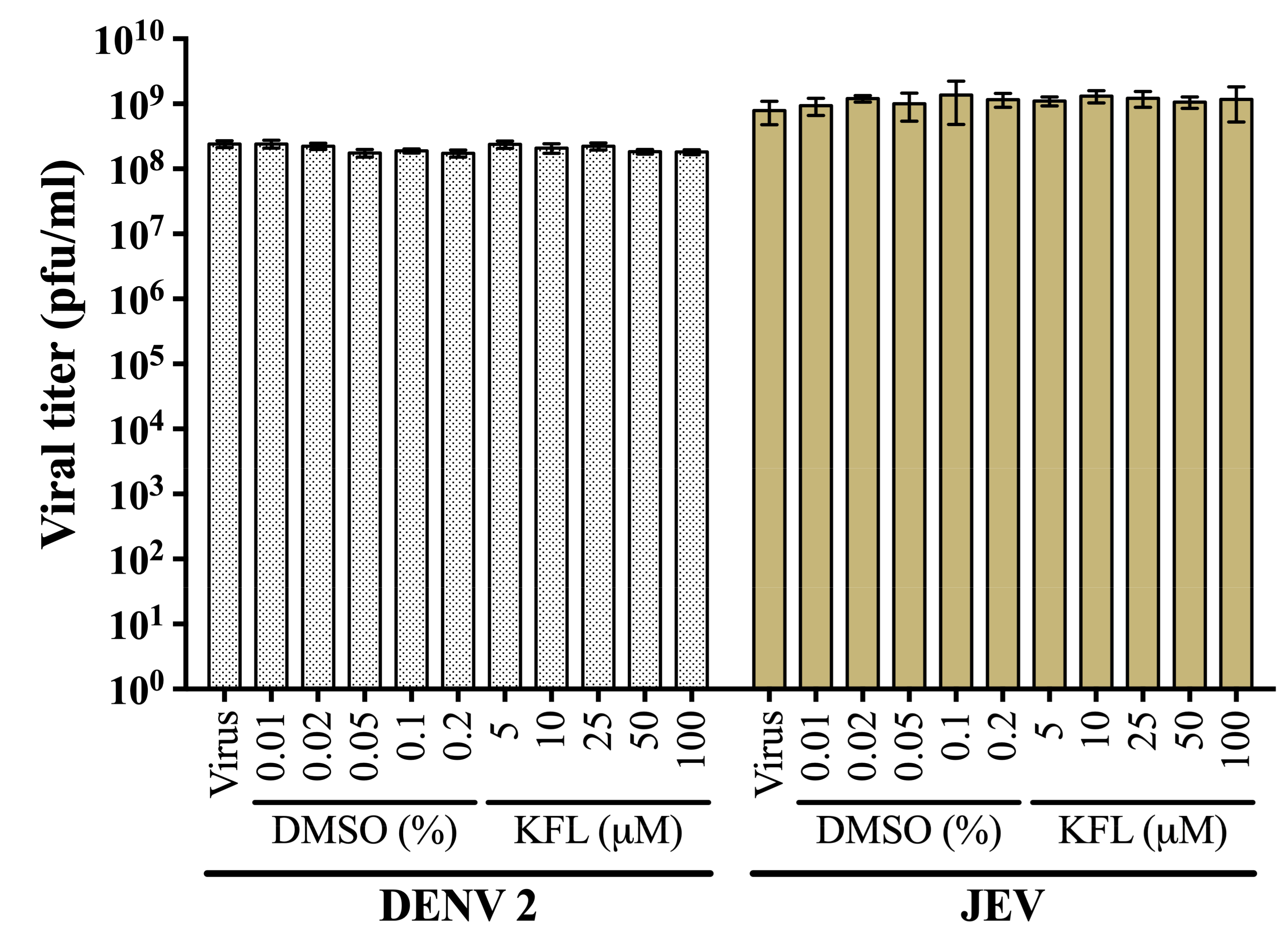
2.2. Evaluation of Virucidal Activity of Kaempferol
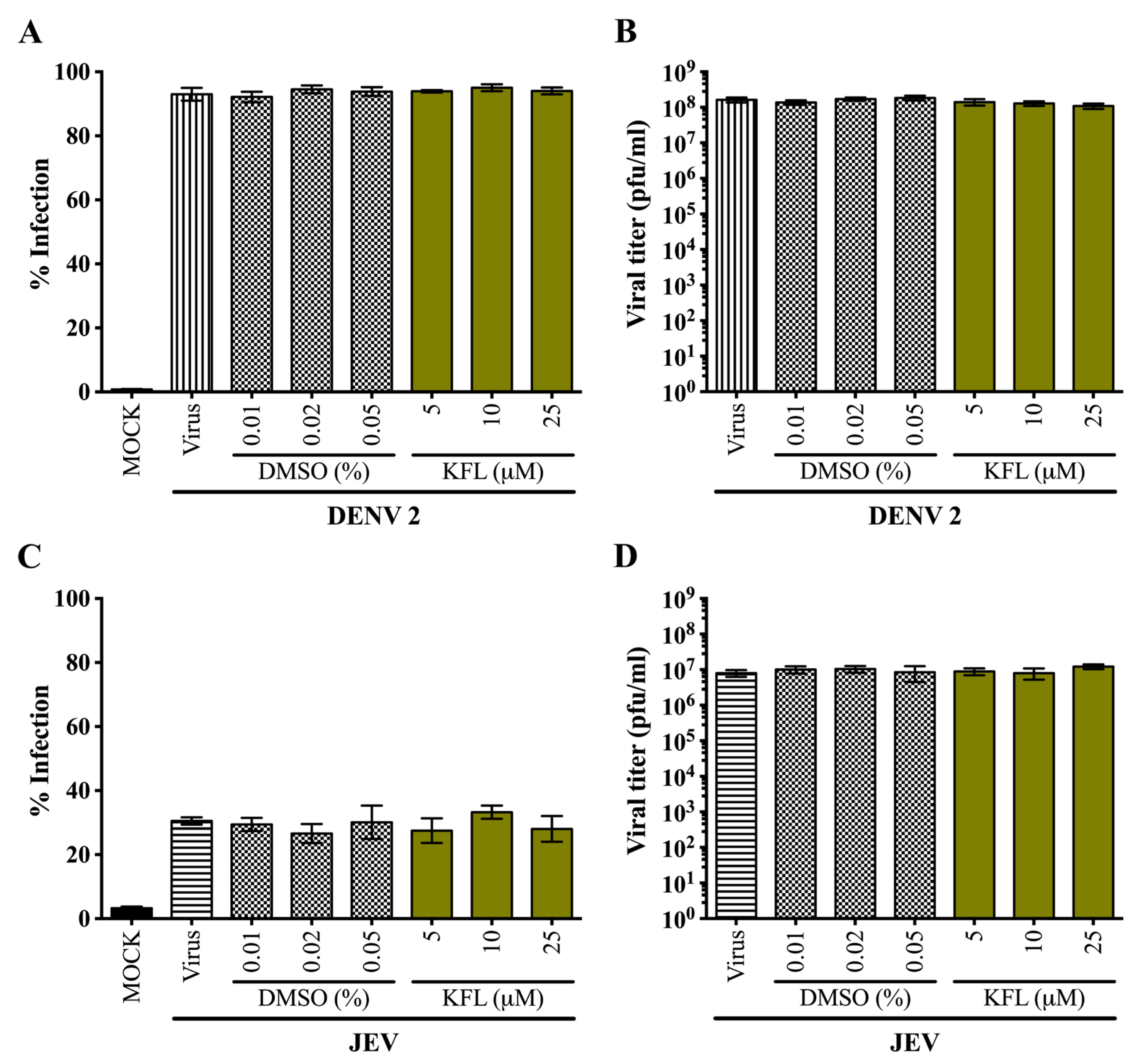
2.3. Effects of Kaempferol on DENV 2 and JEV in HEK293T/17 Cells
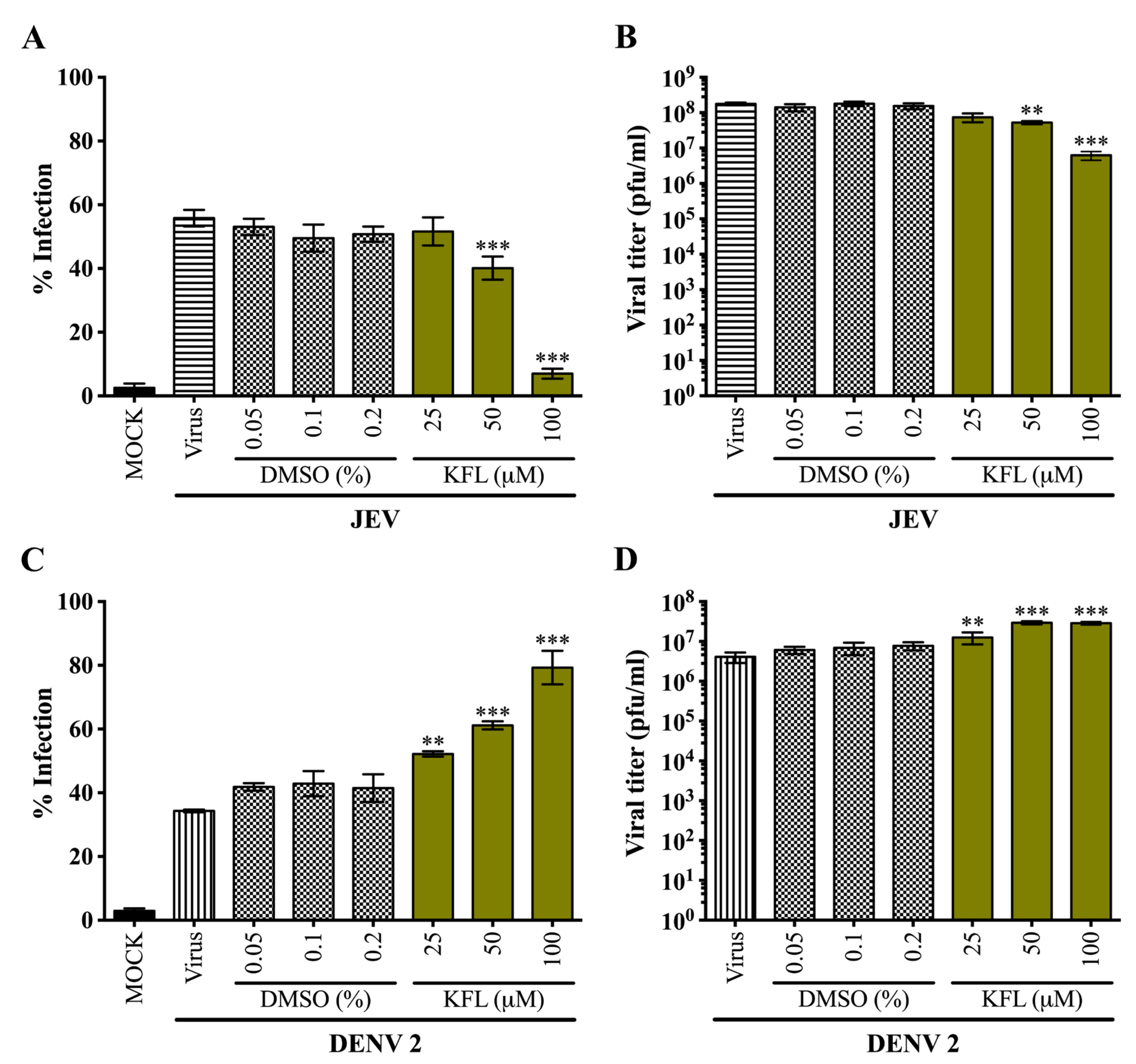
2.4. Effects of Kaempferol on DENV 2 and JEV in BHK-21 Cells
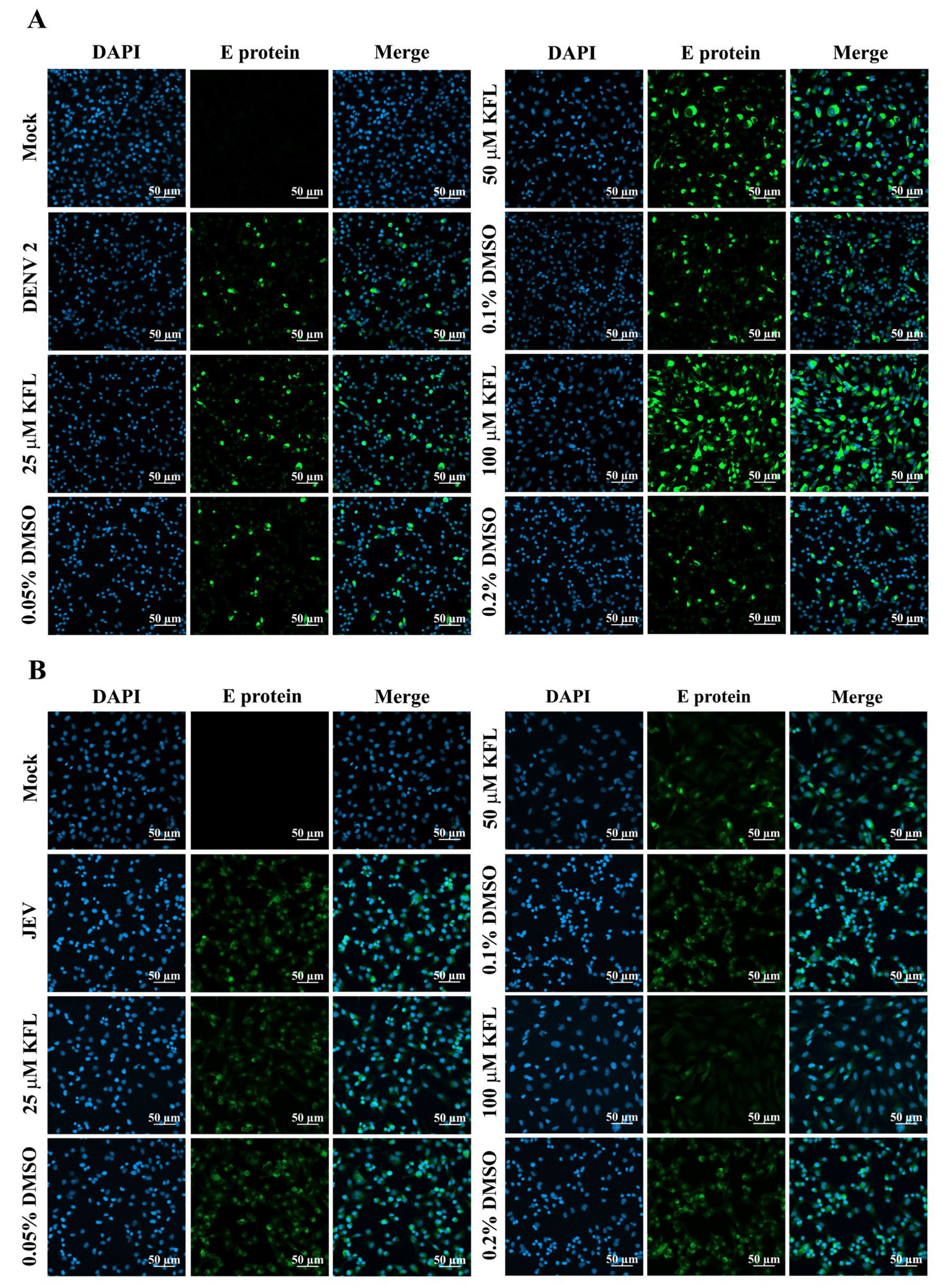
2.5. Immunofluorescence Assay Analysis of Kaempferol on Infections
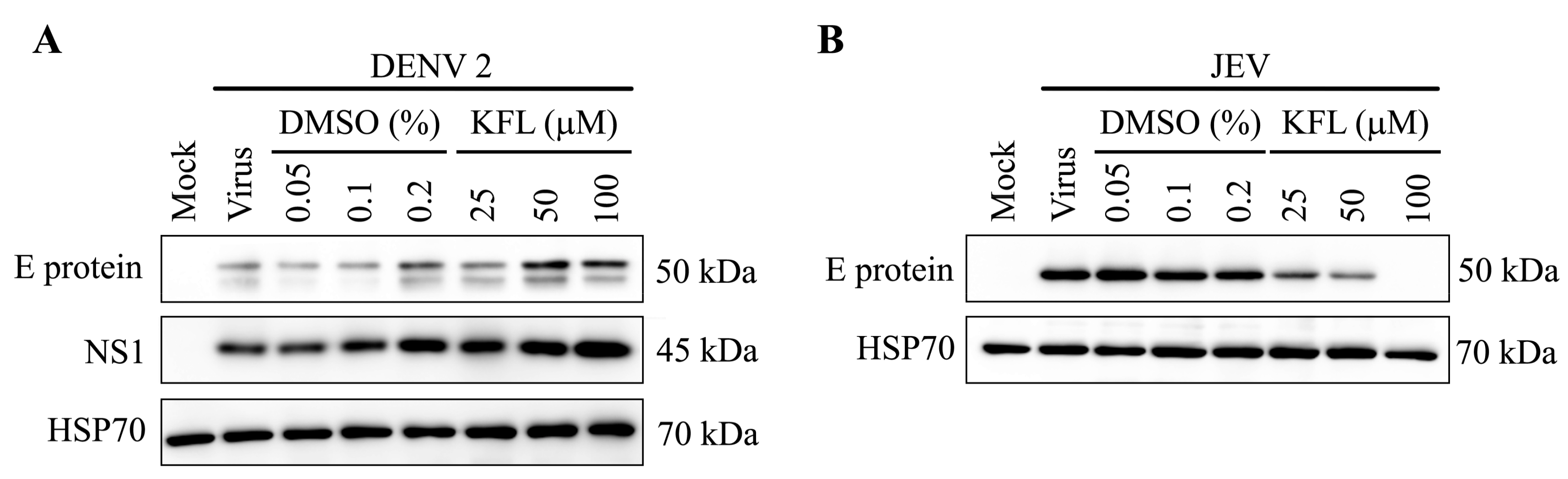
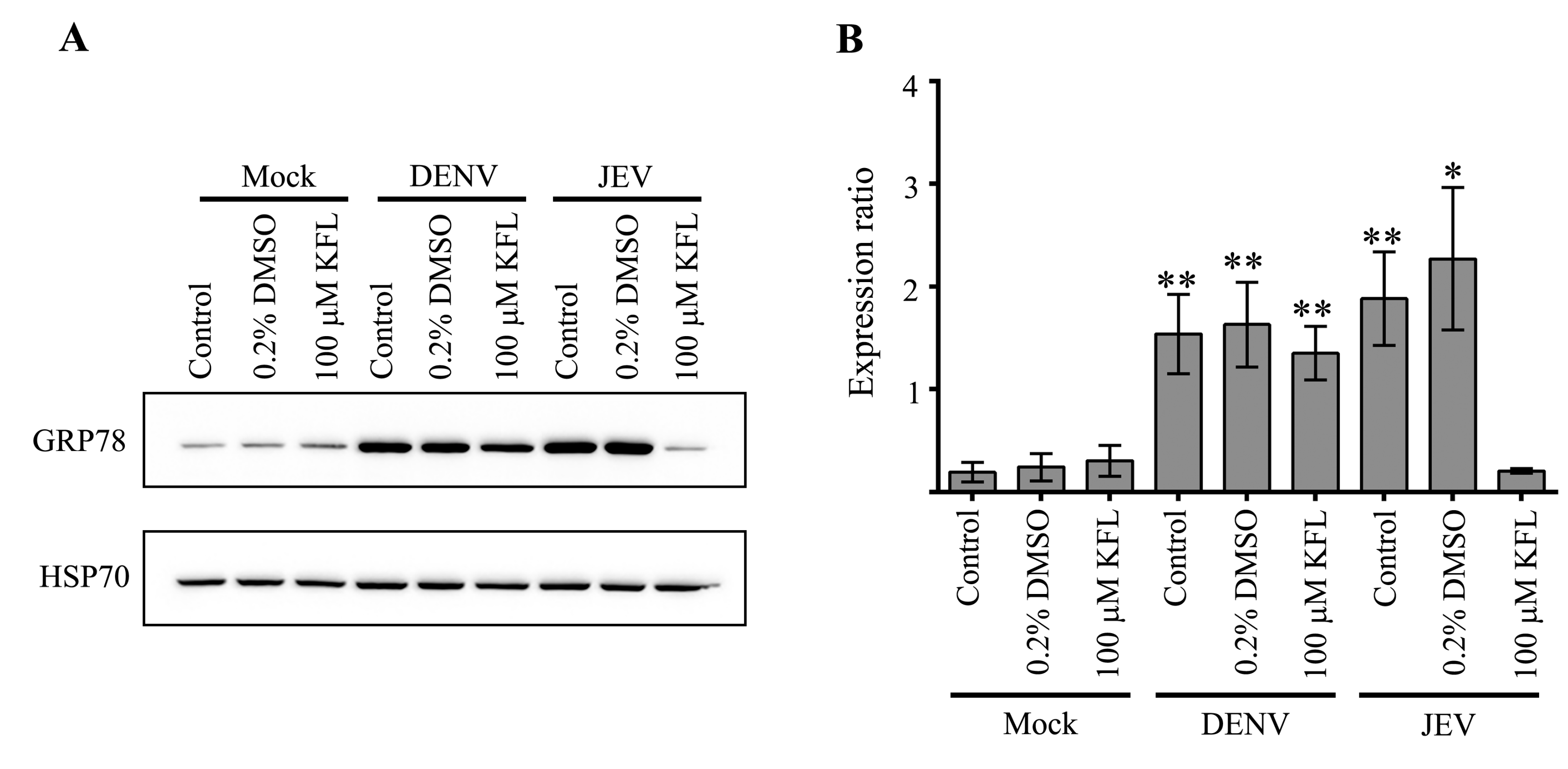
2.6. Effect of Kaempferol on viral Protein Expression
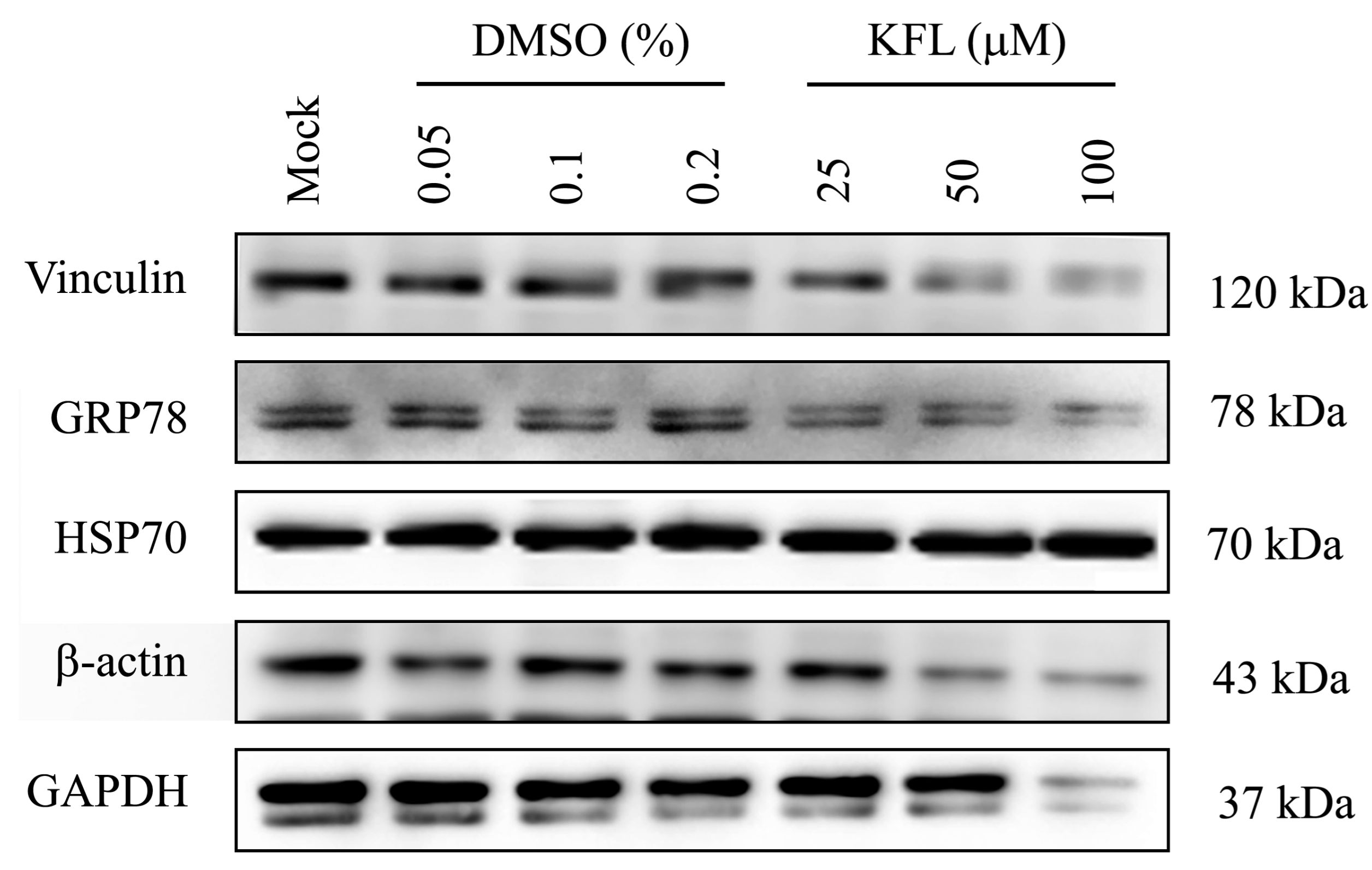
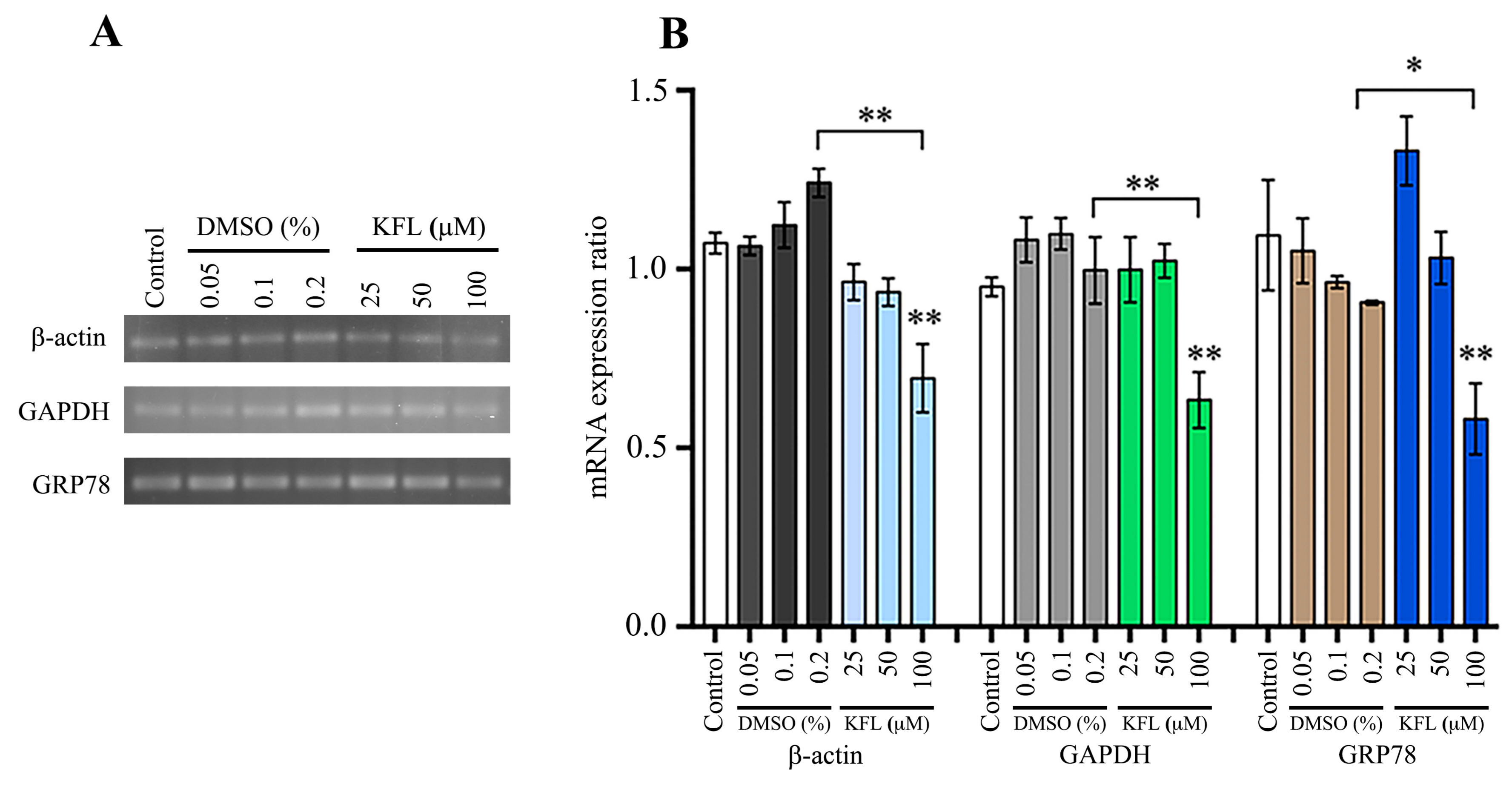
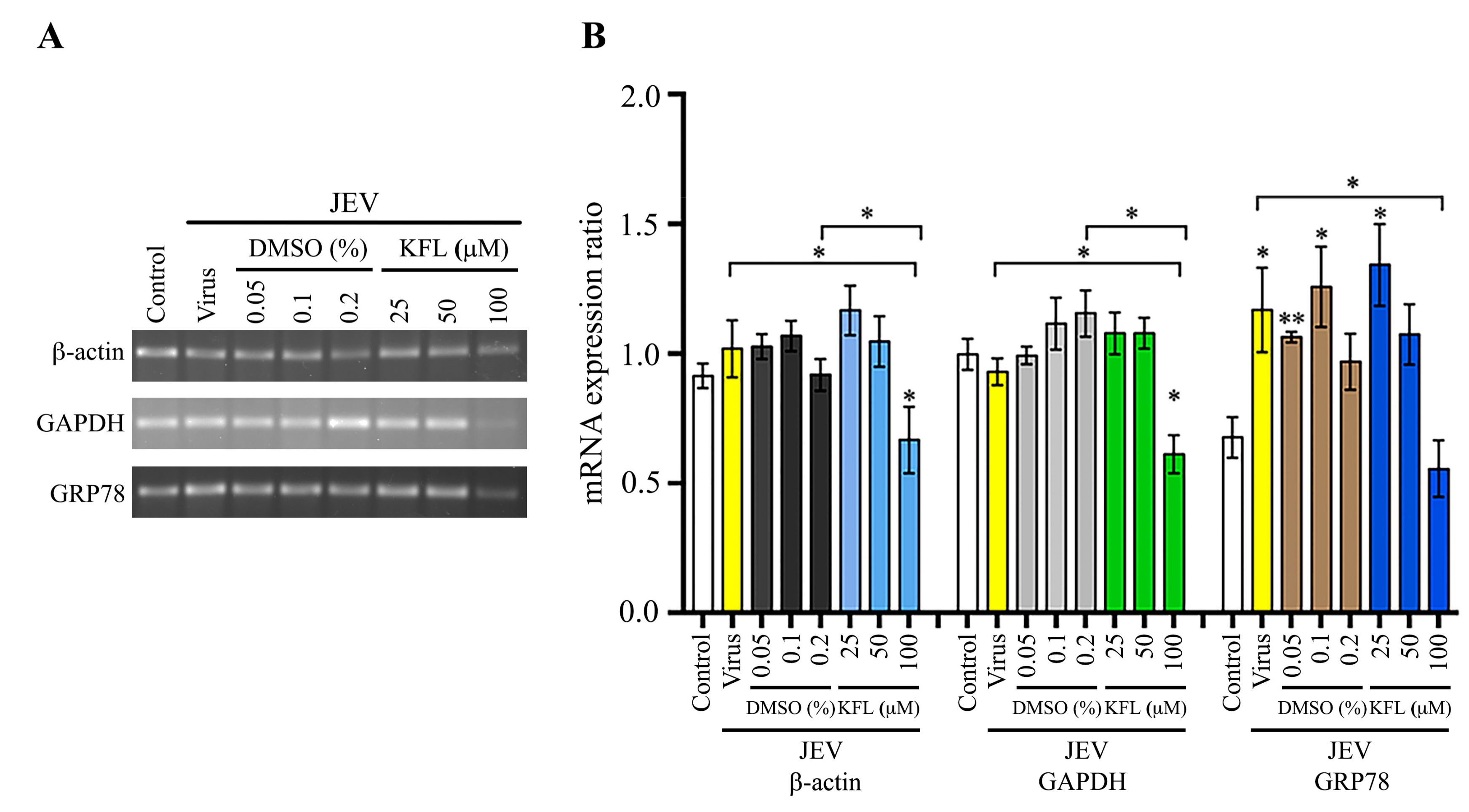
2.7. Effect of Kaempferol on Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Kaempferol
4.3. Cytotoxicity Assays
4.4. Effect of Kaempferol on Cell Morphology
4.5. Virucidal Assay
4.6. Standard Infection
4.7. Flow Cytometry
4.8. Indirect Immunofluorescence Assay
4.9. Western Blot Analysis
4.10. Semiquantitative PCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B. ICTV virus taxonomy profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Acheson, N.H. Introduction to Virology, 2nd ed.; Kaye Pace: John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; p. 500. [Google Scholar]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70 (Pt 1), 37–43. [Google Scholar] [CrossRef]
- Gould, E.A.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Hadinegoro, S.R. The revised WHO dengue case classification: does the system need to be modified? Paediatr Int Child. Health 2012, 32 (Suppl. 1), 33–38. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organ. 2011, 89, 766–774A–774E. [Google Scholar] [CrossRef]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: the virus and vaccines. Hum. Vaccin Immunother 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Supawat, K.; Campbell, A.P.; Anantapreecha, S.; Liamsuwan, S.; Tunlayadechanont, S.; Visudtibhan, A.; Lupthikulthum, S.; Dhiravibulya, K.; Viriyavejakul, A.; et al. Japanese encephalitis virus remains an important cause of encephalitis in Thailand. Int J. Infect. Dis 2010, 14, e888–892. [Google Scholar] [CrossRef] [PubMed]
- Pongpairoj, S.; Choakouychai, B.; Boonrueng, C.; Kutirakan, P.; Ahandrik, S.; Leelasiri, K.; Phanthumachinda, B. A test production of inactivated mouse brain JE vaccine in Thailand. The Southeast. Asian J. Trop Med. Public Health 1989, 20, 647–652. [Google Scholar] [PubMed]
- Smith, D.R. Waiting in the wings: The potential of mosquito transmitted flaviviruses to emerge. Crit Rev. Microbiol 2016, 1–18. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Verri, W.A.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.V.; Casagrande, R. Flavonoids as anti-inflammatory and analgesic drugs: Mechanisms of action and perspectives in the development of pharmaceutical forms. Studies Nat. Prof. Chem 2012, 36, 297–330. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: an overview. J. Nutr Sci 2016, 5, e47. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-Oglucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014, 9, 463–469. [Google Scholar]
- Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C.; et al. Antiviral efficacy of flavonoids against enterovirus 71 infection in vitro and in newborn mice. Viruses 2019, 11. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS One 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.T. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine 2017, 35, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Mitrocotsa, D.; Mitaku, S.; Axarlis, S.; Harvala, C.; Malamas, M. Evaluation of the antiviral activity of kaempferol and its glycosides against human cytomegalovirus. Planta Medica 2000, 66, 377–379. [Google Scholar] [CrossRef]
- Mokrejs, M.; Vopalensky, V.; Kolenaty, O.; Masek, T.; Feketova, Z.; Sekyrova, P.; Skaloudova, B.; Kriz, V.; Pospisek, M. IRESite: the database of experimentally verified IRES structures (www.iresite.org). Nucleic Acids Res. 2006, 34, D125–D130, (Database issue). [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. IRES-mediated cap-independent translation, a path leading to hidden proteome. J. Mol. Cell Biol. 2019, 11, 911–919. [Google Scholar] [CrossRef]
- Jang, S.K.; Krausslich, H.G.; Nicklin, M.J.; Duke, G.M.; Palmenberg, A.C.; Wimmer, E. A segment of the 5’ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol 1988, 62, 2636–2643. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Song, Y.; Mugavero, J.; Stauft, C.B.; Wimmer, E. Dengue and Zika virus 5’ untranslated regions harbor internal ribosomal entry site functions. mBio 2019, 10. [Google Scholar] [CrossRef]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem 2011, 128, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.R.; Sarnow, P. Enterovirus 71 contains a type I IRES element that functions when eukaryotic initiation factor eIF4G is cleaved. Virology 2003, 315, 259–266. [Google Scholar] [CrossRef]
- Wati, S.; Soo, M.L.; Zilm, P.; Li, P.; Paton, A.W.; Burrell, C.J.; Beard, M.; Carr, J.M. Dengue virus infection induces upregulation of GRP78, which acts to chaperone viral antigen production. J. Virol. 2009, 83, 12871–12880. [Google Scholar] [CrossRef] [PubMed]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 is an important host factor for Japanese encephalitis virus entry and replication in mammalian cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Chandrika, B.B.; Maney, S.K.; Lekshmi, S.U.; Joseph, J.; Seervi, M.; Praveen, K.S.; Santhoshkumar, T.R. Bax deficiency mediated drug resistance can be reversed by endoplasmic reticulum stress induced death signaling. Biochem Pharmacol 2010, 79, 1589–1599. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef]
- Abdullah, A.; Ravanan, P. Kaempferol mitigates endoplasmic reticulum stress induced cell death by targeting caspase 3/7. Sci Rep. 2018, 8, 2189. [Google Scholar] [CrossRef]
- Kim, D.S.; Ha, K.C.; Kwon, D.Y.; Kim, M.S.; Kim, H.R.; Chae, S.W.; Chae, H.J. Kaempferol protects ischemia/reperfusion-induced cardiac damage through the regulation of endoplasmic reticulum stress. Immunopharmacol. Immunotoxicol. 2008, 30, 257–270. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, C.C.; Lin, Y.S.; Chang, P.C.; Lu, Z.Y.; Lin, C.F.; Chen, C.L.; Chang, C.P. AR-12 suppresses dengue virus replication by down-regulation of PI3K/AKT and GRP78. Antiviral Res. 2017, 142, 158–168. [Google Scholar] [CrossRef]
- Diwaker, D.; Mishra, K.P.; Ganju, L. Effect of modulation of unfolded protein response pathway on dengue virus infection. Acta Biochim Biophys Sin. (Shanghai) 2015, 47, 960–968. [Google Scholar] [CrossRef]
- Jitobaom, K.; Tongluan, N.; Smith, D.R. Involvement of voltage-dependent anion channel (VDAC) in dengue infection. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, H.R.; Park, S.Y.; Kim, J.Y.; Jeong, Y.S. Constant up-regulation of BiP/GRP78 expression prevents virus-induced apoptosis in BHK-21 cells with Japanese encephalitis virus persistent infection. Virol J. 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Singh, N.; Sengupta, N.; Fatima, M.; Seth, P.; Mahadevan, A.; Shankar, S.K.; Bhattacharyya, A.; Basu, A. Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis 2017, 8, e2556. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Chang, C.M.; Hung, C.Y.; Tsai, M.C.; Schuyler, S.C.; Wang, R.Y. Japanese encephalitis virus co-opts the ER-stress response protein GRP78 for viral infectivity. Virol. J. 2011, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: coping with stress. Trends Cell Biol 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, P.; Luo, S.; Skarnes, W.C.; Sui, G.; Seto, E.; Shi, Y.; Lee, A.S. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol. Cell Biol. 2005, 25, 4529–4540. [Google Scholar] [CrossRef]
- Reddy, R.K.; Mao, C.; Baumeister, P.; Austin, R.C.; Kaufman, R.J.; Lee, A.S. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 2003, 278, 20915–20924. [Google Scholar] [CrossRef]
- Scheuner, D.; Song, B.; McEwen, E.; Liu, C.; Laybutt, R.; Gillespie, P.; Saunders, T.; Bonner-Weir, S.; Kaufman, R.J. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 2001, 7, 1165–1176. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Sithisarn, P.; Suksanpaisan, L.; Thepparit, C.; Smith, D.R. Behavior of the dengue virus in solution. J. Med. Virol. 2003, 71, 532–539. [Google Scholar] [CrossRef]
- Boonsanay, V.; Smith, D.R. Entry into and production of the Japanese encephalitis virus from C6/36 cells. Intervirology 2007, 50, 85–92. [Google Scholar] [CrossRef]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef]
- Henchal, E.A.; Gentry, M.K.; McCown, J.M.; Brandt, W.E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop Med. Hyg. 1982, 31, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Vargas, M.H. ED50plus v1.0. Instituto Nacional de Enfermedades Respiratorias [Computer software]. Mexico, DF, Mexico. 2000. Available online: http://sciencegateway.org/protocols/cellbio/drug/data/ed50v10.xls (accessed on 10 March 2020).
Sample Availability: Samples of the compound are commercially available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Care, C.; Sornjai, W.; Jaratsittisin, J.; Hitakarun, A.; Wikan, N.; Triwitayakorn, K.; Smith, D.R. Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus. Molecules 2020, 25, 1246. https://doi.org/10.3390/molecules25051246
Care C, Sornjai W, Jaratsittisin J, Hitakarun A, Wikan N, Triwitayakorn K, Smith DR. Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus. Molecules. 2020; 25(5):1246. https://doi.org/10.3390/molecules25051246
Chicago/Turabian StyleCare, Chit, Wannapa Sornjai, Janejira Jaratsittisin, Atitaya Hitakarun, Nitwara Wikan, Kanokporn Triwitayakorn, and Duncan R. Smith. 2020. "Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus" Molecules 25, no. 5: 1246. https://doi.org/10.3390/molecules25051246
APA StyleCare, C., Sornjai, W., Jaratsittisin, J., Hitakarun, A., Wikan, N., Triwitayakorn, K., & Smith, D. R. (2020). Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus. Molecules, 25(5), 1246. https://doi.org/10.3390/molecules25051246




