A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials
Abstract
1. Introduction
2. Mechanisms of Shape Change in LCPs
3. Strategy for Preparation of Monodomain LCPs
4. Shape Change of LCPs Triggered by Different Stimuli
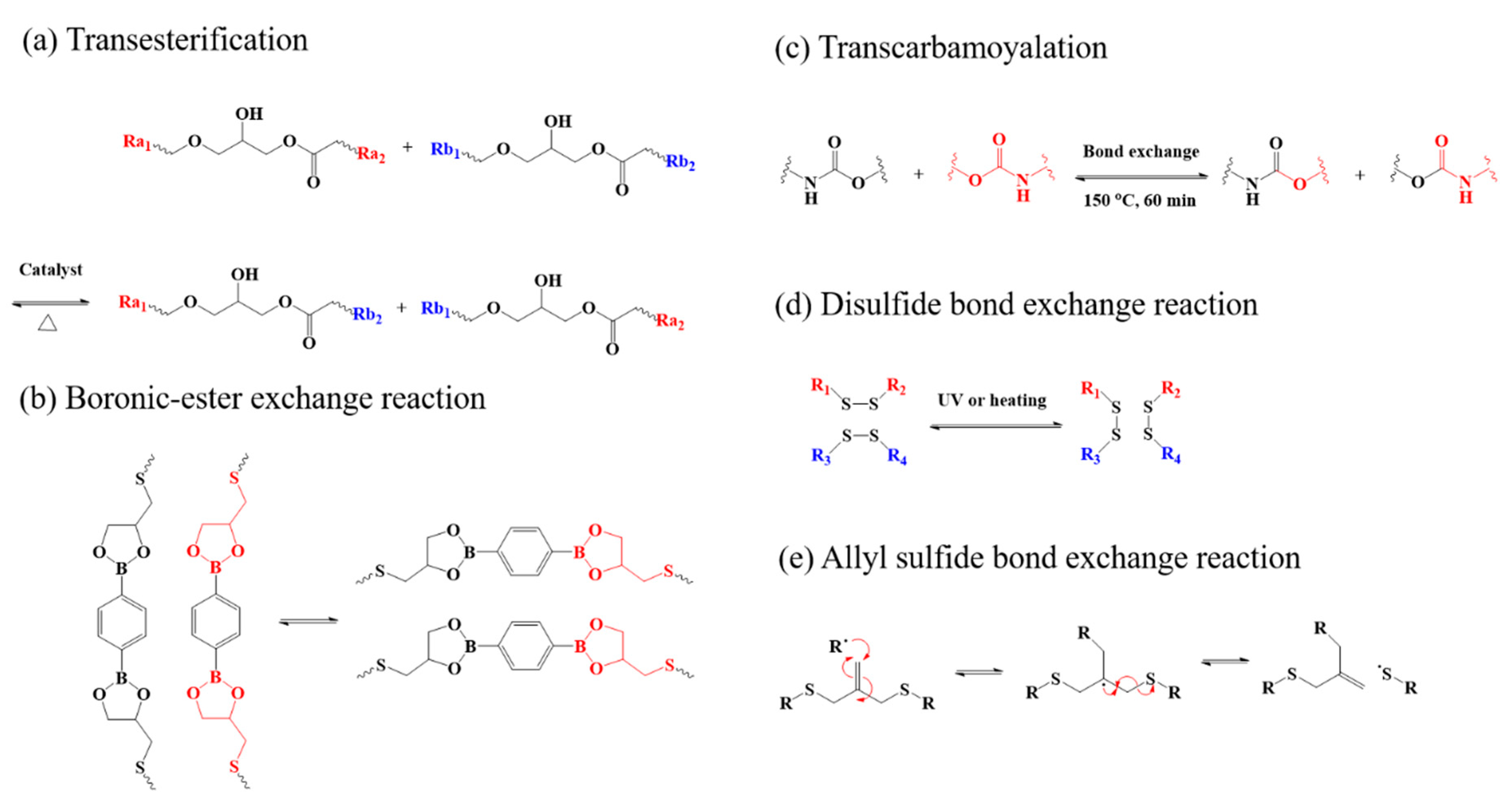
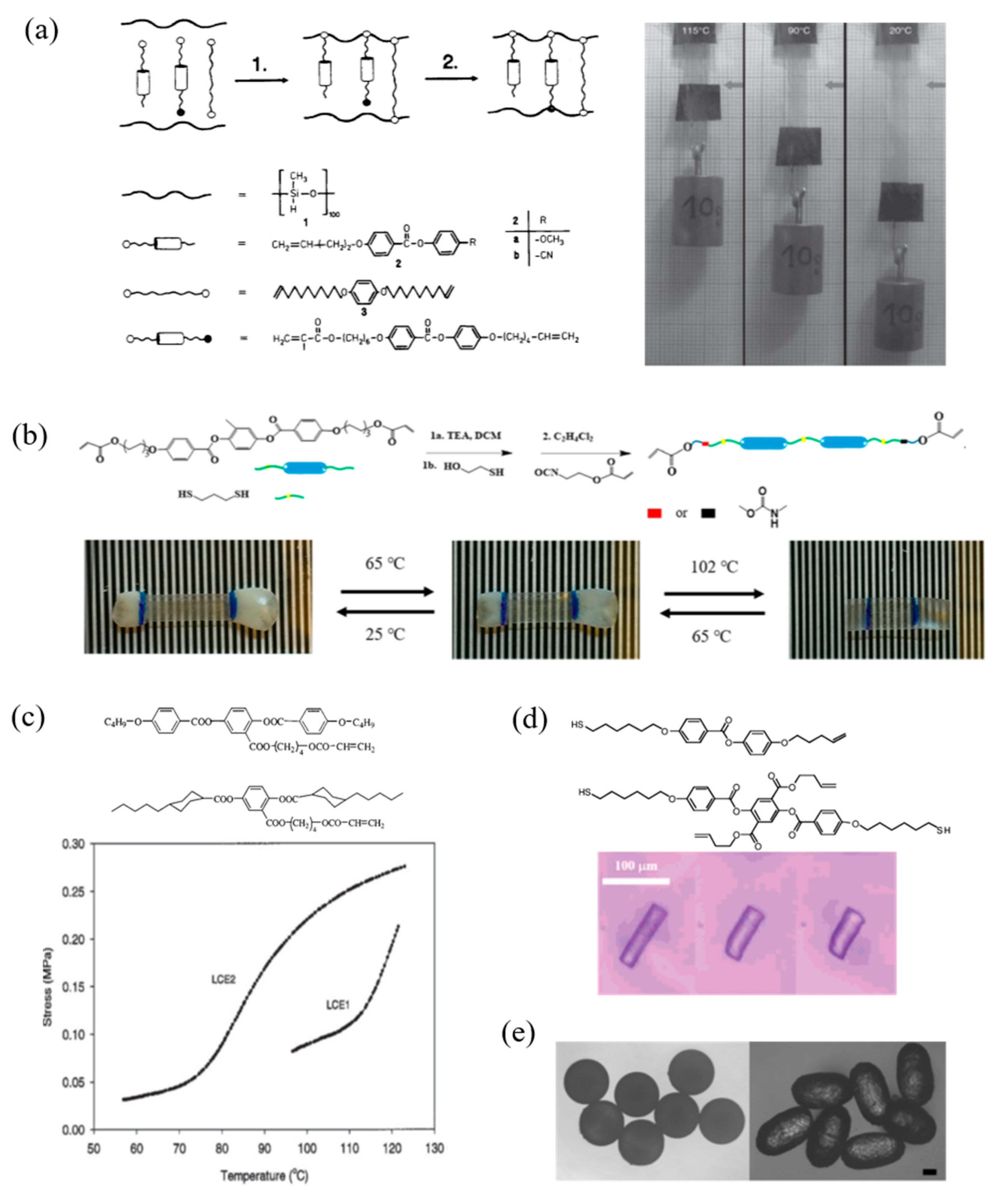
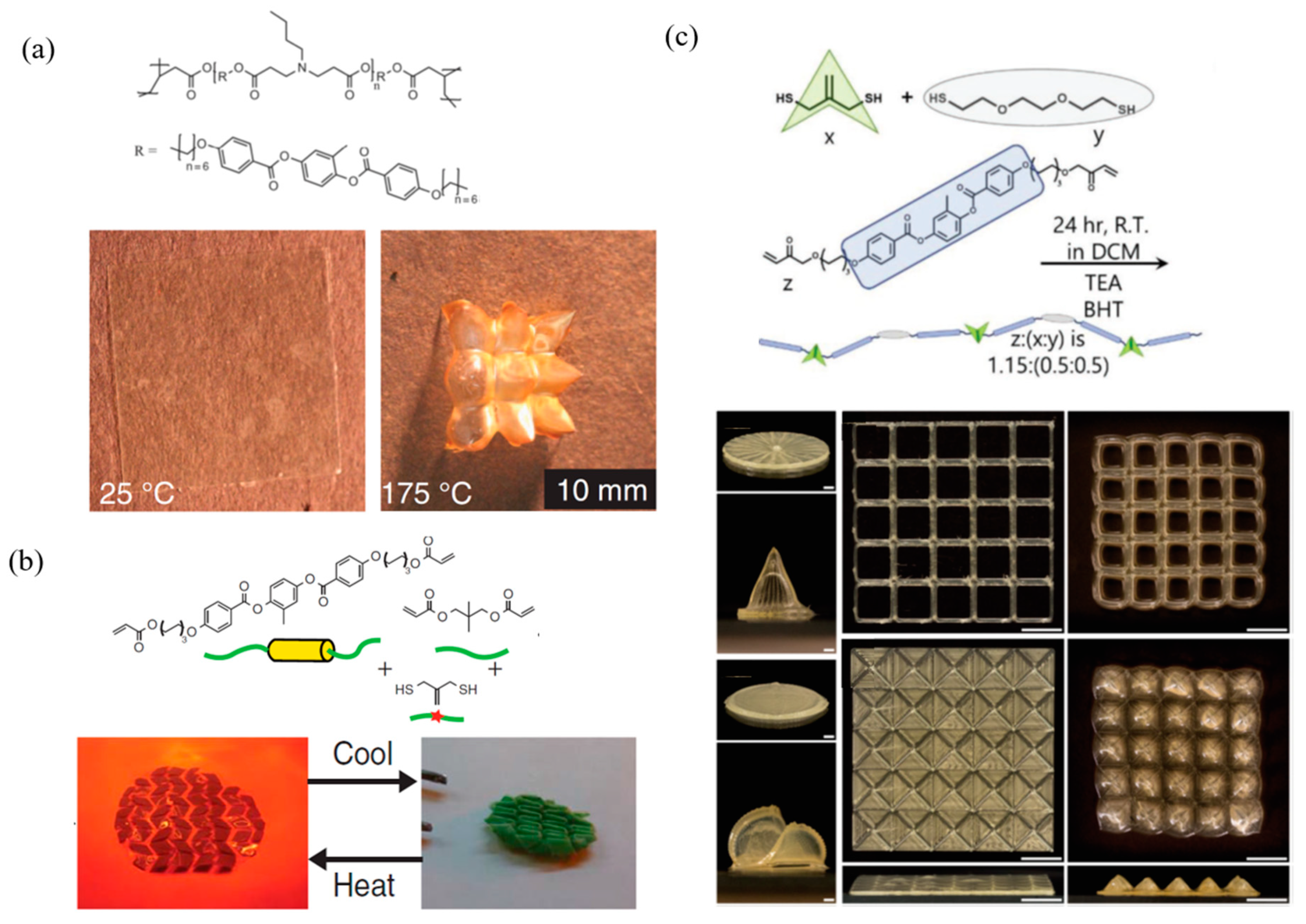
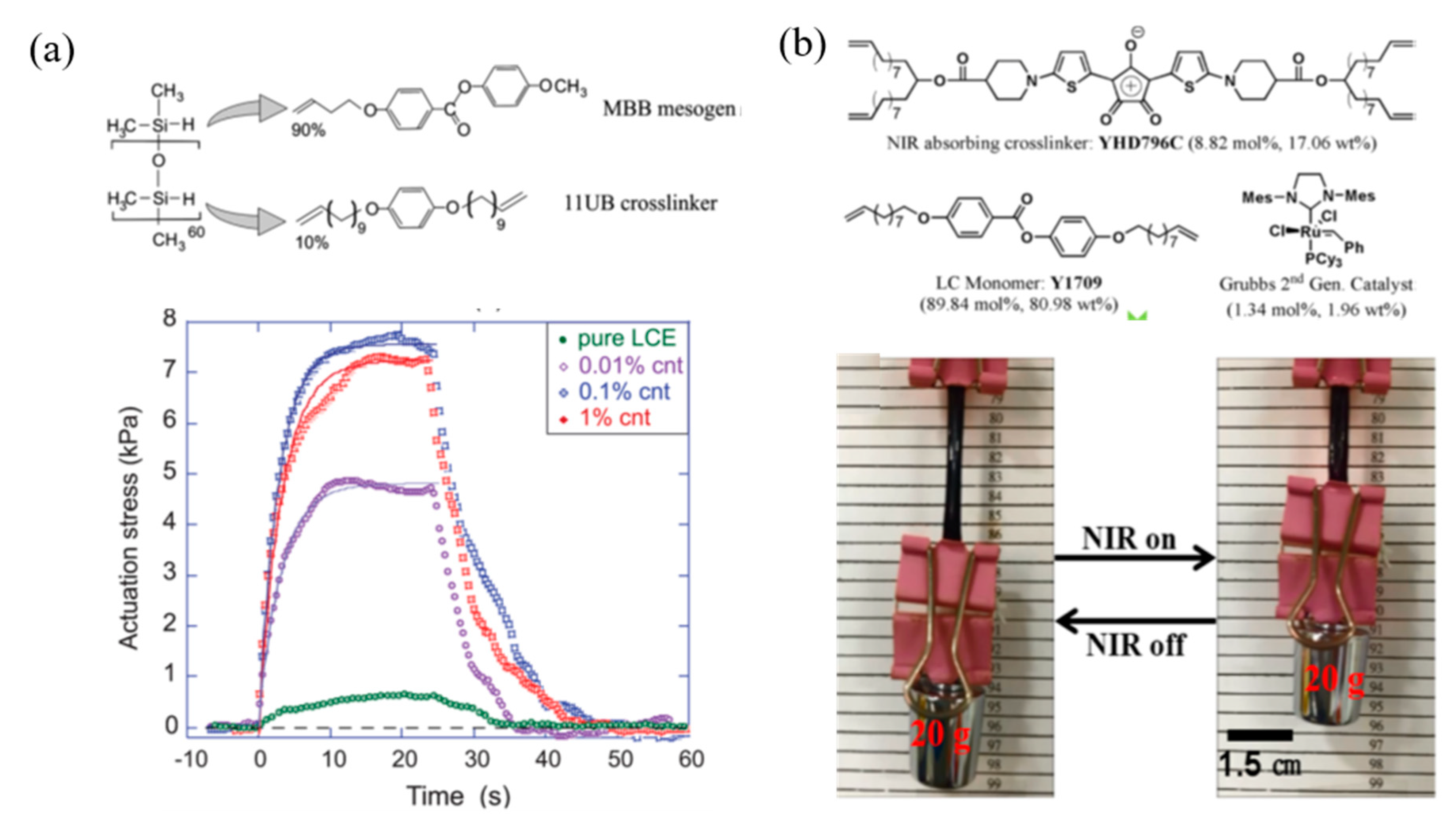
4.1. Thermal Responsive LCPs
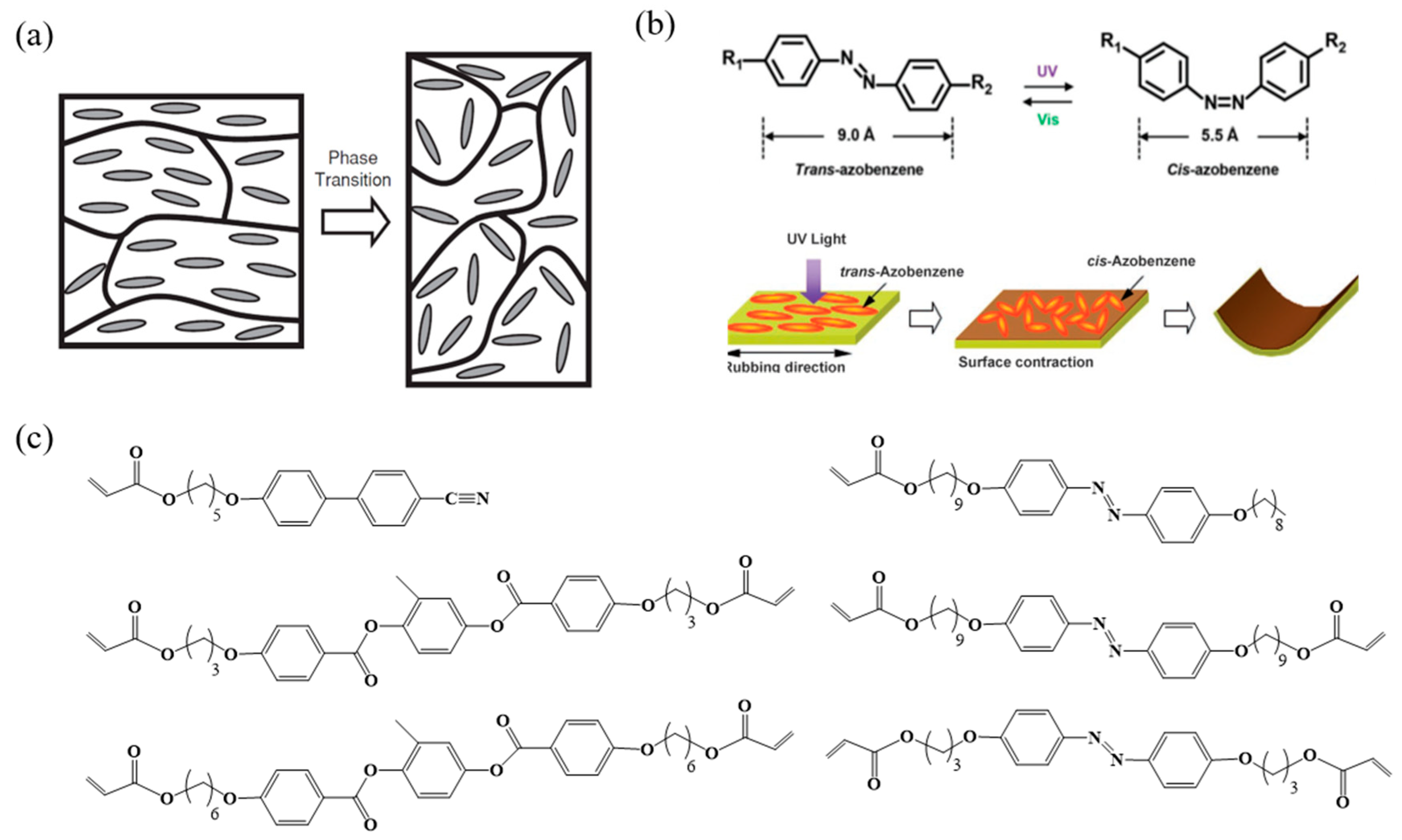
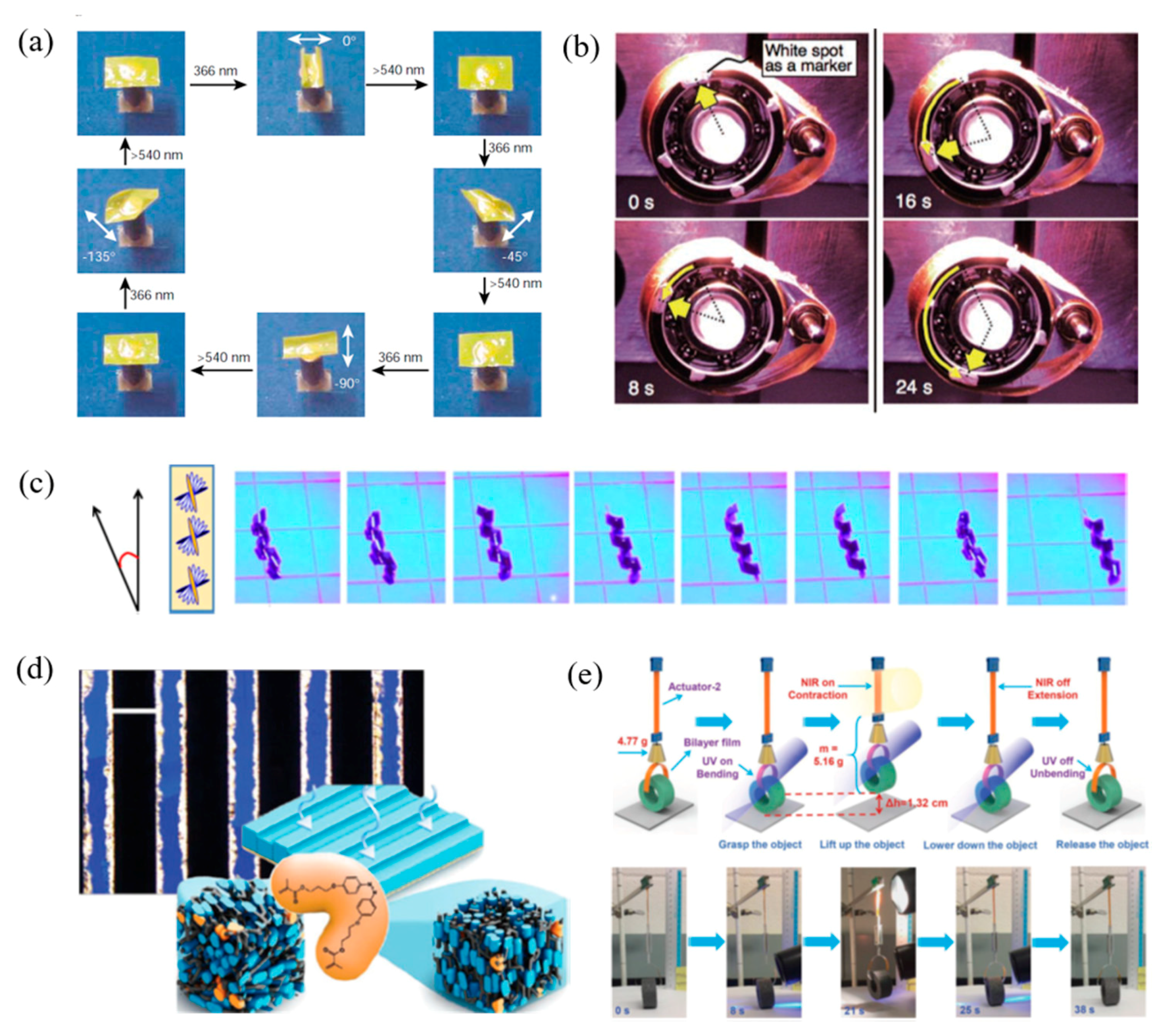
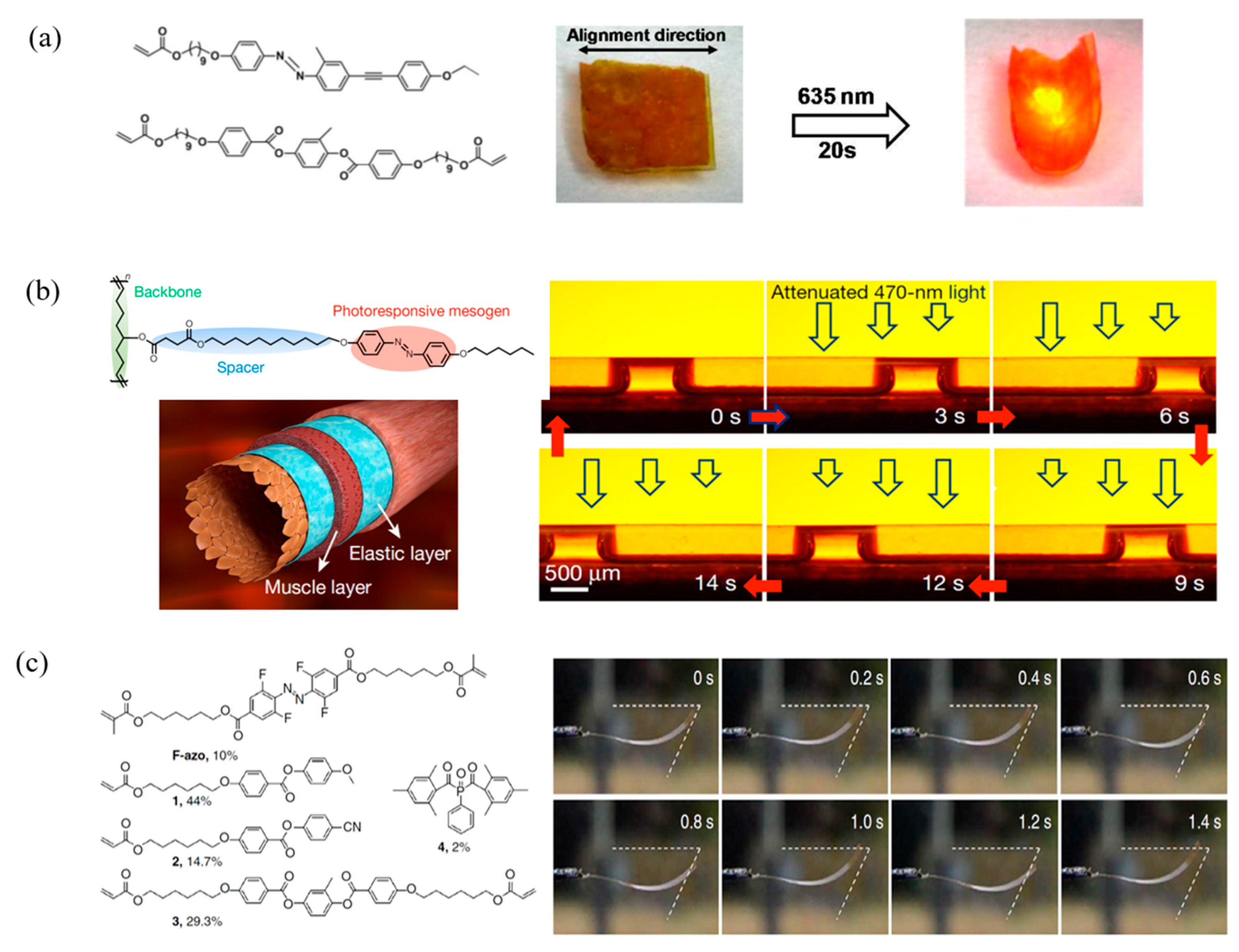
4.2. Photo Responsive LCPs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warner, M.; Terentjev, E.M. Liquid Crystal Elastomers; Oxford University Press; Oxford, UK, 5 April 2007; Volume 120, Available online: https://global.oup.com/academic/product/liquid-crystal-elastomers-9780199214860?cc=be&lang=en& (accessed on 5 April 2007).
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhao, Y. Microstructured Actuation of Liquid Crystal Polymer Networks. Adv. Funct. Mater. 2019, 1901890. [Google Scholar] [CrossRef]
- Warner, M.; Terentjev, E.M. Liquid Crystal Elastomers; OUP Oxford; Oxford, UK, 9 October 2003; Volume 120, Available online: https://global.oup.com/academic/product/liquid-crystal-elastomers9780198527671?cc=be&lang=en& (accessed on 9 October 2003).
- López-Valdeolivas, M.; Liu, D.; Broer, D.J.; Sánchez-Somolinos, C. 4D Printed Actuators with Soft-Robotic Functions. Macromol. Rapid Commun. 2018, 39, 1700710. [Google Scholar] [CrossRef]
- Li, M.H.; Keller, P.; Li, B.; Wang, X.; Brunet, M. Light-driven side-on nematic elastomer actuators. Adv. Mater. 2003, 15, 569–572. [Google Scholar] [CrossRef]
- Li, M.-H.; Keller, P. Artificial muscles based on liquid crystal elastomers. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 2763–2777. [Google Scholar] [CrossRef]
- Zhan, Y.; Schenning, A.P.; Broer, D.J.; Zhou, G.; Liu, D. Light-Driven Electrohydrodynamic Instabilities in Liquid Crystals. Adv. Funct. Mater. 2018, 28, 1707436. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Onck, P.R.; Broer, D.J. Reverse switching of surface roughness in a self-organized polydomain liquid crystal coating. Proc. Natl. Acad. Sci. USA 2015, 112, 3880–3885. [Google Scholar] [CrossRef]
- Liu, D.Q.; Bastiaansen, C.W.M.; den Toonder, J.M.J.; Broer, D.J. Photo-Switchable Surface Topologies in Chiral Nematic Coatings. Angew. Chem. Int. Edit. 2012, 51, 892–896. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, T.; Hui, Y.; Wang, W.; Yang, K.; Zhou, Q.; Wang, Y. Elaborate fabrication of well-defined side-chain liquid crystalline polyurethane networks with triple-shape memory capacity. J. Mater. Chem. A 2015, 3, 13435–13444. [Google Scholar] [CrossRef]
- Yu, Y.; Ikeda, T. Soft actuators based on liquid-crystalline elastomers. Angew. Chem. Int. Ed. 2006, 45, 5416–5418. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kondo, M.; Mamiya, J. i.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile polymer materials: Towards light-driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lv, J.-a.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable Azobenzene-Containing Liquid Crystal Polymers and Soft Actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef] [PubMed]
- Küpfer, J.; Finkelmann, H. Nematic liquid single crystal elastomers. Macromol. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Terentjev, E. Spontaneous thermal expansion of nematic elastomers. Eur. Phys. J. E 2001, 6, 181–188. [Google Scholar] [CrossRef]
- Yakacki, C.; Saed, M.; Nair, D.; Gong, T.; Reed, S.; Bowman, C. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol–acrylate reaction. Rsc Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- White, T.J.; Serak, S.V.; Tabiryan, N.V.; Vaia, R.A.; Bunning, T.J. Polarization-controlled, photodriven bending in monodomain liquid crystal elastomer cantilevers. J. Mater. Chem. 2009, 19, 1080–1085. [Google Scholar] [CrossRef]
- Urayama, K.; Arai, Y.O.; Takigawa, T. Volume phase transition of monodomain nematic polymer networks in isotropic solvents accompanied by anisotropic shape variation. Macromolecules 2005, 38, 3469–3474. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of liquid-crystal systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef]
- Zeng, H.; Wani, O.M.; Wasylczyk, P.; Kaczmarek, R.; Priimagi, A. Self-regulating iris based on light-actuated liquid crystal elastomer. Adv. Mater. 2017, 29, 1701814. [Google Scholar] [CrossRef]
- Gebhard, E.; Zentel, R. Ferroelectric liquid crystalline elastomers, 2. variation of mesogens and network density. Macromol. Chem. Phys. 2000, 201, 911–922. [Google Scholar] [CrossRef]
- Anwer, A.; Windle, A.H. Orientation kinetics of thermotropic main-chain liquid-crystalline polymers in a magnetic field. Polymer 1991, 32, 103–108. [Google Scholar] [CrossRef]
- Schuhladen, S.; Preller, F.; Rix, R.; Petsch, S.; Zentel, R.; Zappe, H. Iris-Like Tunable Aperture Employing Liquid-Crystal Elastomers. Adv. Mater. 2014, 26, 7247–7251. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nakano, M.; Yu, Y.; Tsutsumi, O.; Kanazawa, A. Anisotropic bending and unbending behavior of azobenzene liquid-crystalline gels by light exposure. Adv. Mater. 2003, 15, 201–205. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Ichimura, K.; Suzuki, Y.; Seki, T.; Hosoki, A.; Aoki, K. Reversible change in alignment mode of nematic liquid crystals regulated photochemically by command surfaces modified with an azobenzene monolayer. Langmuir 1988, 4, 1214–1216. [Google Scholar] [CrossRef]
- Fujimaki, M.; Kawahara, S.; Matsuzawa, Y.; Kurita, E.; Hayashi, Y.; Ichimura, K. Macrocyclic amphiphiles. 3. Monolayers of O-octacarboxymethoxylated calix [4] resorcinarenes with azobenzene residues exhibiting efficient photoisomerizability. Langmuir 1998, 14, 4495–4502. [Google Scholar] [CrossRef]
- Ahn, S. k.; Ware, T.H.; Lee, K.M.; Tondiglia, V.P.; White, T.J. Photoinduced topographical feature development in blueprinted azobenzene-functionalized liquid crystalline elastomers. Adv. Funct. Mater. 2016, 26, 5819–5826. [Google Scholar] [CrossRef]
- Gebhard, E.; Zentel, R. Ferroelectric liquid crystalline elastomers, 1. Variation of network topology and orientation. Macromol. Chem. Phys. 2000, 201, 902–910. [Google Scholar] [CrossRef]
- Brehmer, M.; Zentel, R. Ferroelectric liquid crystalline elastomers. Macromol. Chem. Phys. 1994, 195, 1891. [Google Scholar] [CrossRef]
- Skene, W.G.; Lehn, J.-M.P. Dynamers: Polyacylhydrazone reversible covalent polymers, component exchange, and constitutional diversity. Proc. Natl. Acad. Sci. USA 2004, 101, 8270–8275. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Dersch, R.; Wendorff, J.H.; Finkelmann, H. Photocrosslinkable Liquid Crystal Main-Chain Polymers: Thin Films and Electrospinning. Macromol. Rapid Commun. 2007, 28, 2062–2068. [Google Scholar] [CrossRef]
- Buguin, A.; Li, M.-H.; Silberzan, P.; Ladoux, B.; Keller, P. Micro-actuators: When artificial muscles made of nematic liquid crystal elastomers meet soft lithography. J. Am. Chem. Soc. 2006, 128, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Ohm, C.; Serra, C.; Zentel, R. A Continuous Flow Synthesis of Micrometer-Sized Actuators from Liquid Crystalline Elastomers. Adv. Mater. 2009, 21, 4859–4862. [Google Scholar] [CrossRef]
- Van Oosten, C.L.; Bastiaansen, C.W.; Broer, D.J. Printed artificial cilia from liquid-crystal network actuators modularly driven by light. Nat. Mater. 2009, 8, 677. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J. y.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364. [Google Scholar] [CrossRef]
- Kotikian, A.; Truby, R.L.; Boley, J.W.; White, T.J.; Lewis, J.A. 3D Printing of Liquid Crystal Elastomeric Actuators with Spatially Programed Nematic Order. Adv. Mater. 2018, 30, 1706164. [Google Scholar] [CrossRef]
- Yang, Z.; Herd, G.A.; Clarke, S.M.; Tajbakhsh, A.R.; Terentjev, E.M.; Huck, W.T. Thermal and UV shape shifting of surface topography. J. Am. Chem. Soc. 2006, 128, 1074–1075. [Google Scholar] [CrossRef]
- Yang, H.; Ye, G.; Wang, X.; Keller, P. Micron-sized liquid crystalline elastomer actuators. Soft Matter 2011, 7, 815–823. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.K.; Worrell, B.T.; Brown, T.; Cox, L.M.; Sowan, N.; Wang, C.; Podgorski, M.; Martinez, A.M.; Bowman, C.N. Enabling Applications of Covalent Adaptable Networks. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-Crystalline Dynamic Networks Doped with Gold Nanorods Showing Enhanced Photocontrol of Actuation. Adv. Mater. 2018, 30, 1706597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Yang, Y.; Xu, Y.-S.; Qian, X.; Wei, Y.; Ji, Y. Durable liquid-crystalline vitrimer actuators. Chem. Sci. 2019, 10, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; McBride, M.K.; Zhang, X.; Han, X.; Martinez, A.M.; Shao, R.; Zhu, C.; Visvanathan, R.; Clark, N.A.; Wang, Y.; et al. Reconfigurable LC Elastomers: Using a Thermally Programmable Monodomain To Access Two-Way Free-Standing Multiple Shape Memory Polymers. Macromolecules 2018, 51, 5812–5819. [Google Scholar] [CrossRef]
- Saed, M.O.; Gablier, A.; Terentejv, E.M. Liquid Crystalline Vitrimers with Full or Partial Boronic-Ester Bond Exchange. Adv. Funct. Mater. 2020, 30, 1906458. [Google Scholar] [CrossRef]
- Wang, Z.; He, Q.; Wang, Y.; Cai, S. Programmable actuation of liquid crystal elastomers via “living” exchange reaction. Soft matter 2019, 15, 2811–2816. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, H.; He, Q.; Cai, S. Reprogrammable, Reprocessible, and Self-Healable Liquid Crystal Elastomer with Exchangeable Disulfide Bonds. ACS Appl. Mater. Interfaces 2017, 9, 33119–33128. [Google Scholar] [CrossRef]
- McBride, M.K.; Hendrikx, M.; Liu, D.; Worrell, B.T.; Broer, D.J.; Bowman, C.N. Photoinduced Plasticity in Cross-Linked Liquid Crystalline Networks. Adv. Mater. 2017, 29, 1606509. [Google Scholar] [CrossRef]
- McBride, M.K.; Martinez, A.M.; Cox, L.; Alim, M.; Childress, K.; Beiswinger, M.; Podgorski, M.; Worrell, B.T.; Killgore, J.; Bowman, C.N. A readily programmable, fully reversible shape-switching material. Sci. Adv. 2018, 4, eaat4634. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.C.; Kotikian, A.; Li, S.; Aizenberg, J.; Lewis, J.A. 3D Printable and Reconfigurable Liquid Crystal Elastomers with Light-Induced Shape Memory via Dynamic Bond Exchange. Adv. Mater. 2020, 32, 1905682. [Google Scholar] [CrossRef] [PubMed]
- Ahir, S.V.; Terentjev, E.M. Photomechanical actuation in polymer–nanotube composites. Nat. Mater. 2005, 4, 491. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Zander, F.; Bergmann, G.; Brandt, H.; Wertmer, H.; Finkelmann, H. Nematic main-chain elastomers: Coupling and orientational behavior. Comptes Rendus Chimie 2009, 12, 85–104. [Google Scholar] [CrossRef]
- Yang, H.; Buguin, A.; Taulemesse, J.-M.; Kaneko, K.; Méry, S.; Bergeret, A.; Keller, P. Micron-sized main-chain liquid crystalline elastomer actuators with ultralarge amplitude contractions. J. Am. Chem. Soc. 2009, 131, 15000–15004. [Google Scholar] [CrossRef]
- Saed, M.O.; Ambulo, C.P.; Kim, H.; De, R.; Raval, V.; Searles, K.; Siddiqui, D.A.; Cue, J.M.O.; Stefan, M.C.; Shankar, M.R. Molecularly-Engineered, 4D-Printed Liquid Crystal Elastomer Actuators. Adv. Funct. Mater. 2019, 29, 1806412. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, Y. Non-uniform optical inscription of actuation domains in a liquid crystal polymer of uniaxial orientation: An approach to complex and programmable shape changes. Angew. Chem. Int. Ed. 2017, 56, 14202–14206. [Google Scholar] [CrossRef]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef]
- McBride, M.K.; Podgorski, M.; Chatani, S.; Worrell, B.T.; Bowman, C.N. Thermoreversible Folding as a Route to the Unique Shape-Memory Character in Ductile Polymer Networks. ACS Appl. Mater. Interfaces 2018, 10, 22739–22745. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, W.; Peng, R.; He, C.; Pang, X.; Shi, D.; Jiang, T.; Lin, Z. Graphene-enabled superior and tunable photomechanical actuation in liquid crystalline elastomer nanocomposites. Adv. Mater. 2015, 27, 6376–6381. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Lv, X.; Gao, L.; Fang, S.; Yu, H. Photoinduced triple shape memory polyurethane enabled by doping with azobenzene and GO. J. Mater. Chem. C 2016, 4, 9993–9997. [Google Scholar] [CrossRef]
- Kohlmeyer, R.R.; Chen, J. Wavelength-selective, IR light-driven hinges based on liquid crystalline elastomer composites. Angew. Chem. Int. Ed. 2013, 52, 9234–9237. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Huang, Y.Y.; Rungsawang, R.; Terentjev, E.M. Dispersion and alignment of carbon nanotubes in liquid crystalline polymers and elastomers. Adv. Mater. 2010, 22, 3436–3440. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, L.-X.; Lin, B.-P.; Zhang, X.-Q.; Sun, Y.; Yang, H. Near-infrared responsive liquid crystalline elastomers containing photothermal conjugated polymers. Macromolecules 2016, 49, 4023–4030. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.-H.; Deng, L.-L.; Lin, B.-P.; Yang, H. Near-Infrared Chromophore Functionalized Soft Actuator with Ultrafast Photoresponsive Speed and Superior Mechanical Property. J. Am. Chem. Soc. 2017, 139, 11333–11336. [Google Scholar] [CrossRef]
- Wang, M.; Lin, B.-P.; Yang, H. A plant tendril mimic soft actuator with phototunable bending and chiral twisting motion modes. Nat. Commun. 2016, 7, 13981. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.-J.; Wang, Z.-F.; Guo, L.-X.; Keller, P.; Lin, B.-P.; Sun, Y.; Zhang, X.-Q. Near-infrared-responsive gold nanorod/liquid crystalline elastomer composites prepared by sequential thiol-click chemistry. Chem. Commun. 2015, 51, 12126–12129. [Google Scholar] [CrossRef]
- Courty, S.; Mine, J.; Tajbakhsh, A.; Terentjev, E. Nematic elastomers with aligned carbon nanotubes: New electromechanical actuators. EPL (Europhysics Letters) 2003, 64, 654. [Google Scholar] [CrossRef]
- Marshall, J.E.; Ji, Y.; Torras, N.; Zinoviev, K.; Terentjev, E.M. Carbon-nanotube sensitized nematic elastomer composites for IR-visible photo-actuation. Soft Matter 2012, 8, 1570–1574. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Photomechanics: Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef]
- Lee, K.M.; Koerner, H.; Vaia, R.A.; Bunning, T.J.; White, T.J. Light-activated shape memory of glassy, azobenzene liquid crystalline polymer networks. Soft Matter 2011, 7, 4318–4324. [Google Scholar] [CrossRef]
- Wen, Z.-B.; Liu, D.; Li, X.-Y.; Zhu, C.-H.; Shao, R.-F.; Visvanathan, R.; Clark, N.A.; Yang, K.-K.; Wang, Y.-Z. Fabrication of Liquid Crystalline Polyurethane Networks with a Pendant Azobenzene Group to Access Thermal/Photoresponsive Shape-Memory Effects. ACS Appl. Mater. Interfaces 2017, 9, 24947–24954. [Google Scholar] [CrossRef] [PubMed]
- Wie, J.J.; Shankar, M.R.; White, T.J. Photomotility of polymers. Nat. Commun. 2016, 7, 13260. [Google Scholar] [CrossRef] [PubMed]
- Serak, S.; Tabiryan, N.; Vergara, R.; White, T.J.; Vaia, R.A.; Bunning, T.J. Liquid crystalline polymer cantilever oscillators fueled by light. Soft Matter 2010, 6, 779–783. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Vantomme, G.; Meijer, E.; Broer, D.J. Mastering the photothermal effect in liquid crystal networks: A general approach for self-sustained mechanical oscillators. Adv. Mater. 2017, 29, 1606712. [Google Scholar] [CrossRef]
- Kumar, K.; Knie, C.; Bléger, D.; Peletier, M.A.; Friedrich, H.; Hecht, S.; Broer, D.J.; Debije, M.G.; Schenning, A.P. A chaotic self-oscillating sunlight-driven polymer actuator. Nat. Commun. 2016, 7, 11975. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Sun, S.; Wei, J.; Wu, L.; Yu, Y. Humidity-and Photo-Induced Mechanical Actuation of Cross-Linked Liquid Crystal Polymers. Adv. Mater. 2017, 29, 1604792. [Google Scholar] [CrossRef]
- Liu, D.; Bastiaansen, C.W.M.; den Toonder, J.M.J.; Broer, D.J. Light-Induced Formation of Dynamic and Permanent Surface Topologies in Chiral–Nematic Polymer Networks. Macromolecules 2012, 45, 8005–8012. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Liu, D.; Mulder, D.J.; Leunissen, K.H.; van Gerven, J.; Schenning, A.P.; Broer, D.J. Photoresponsive Sponge-Like Coating for On-Demand Liquid Release. Adv. Funct. Mater. 2018, 28, 1705942. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, M.; Li, F.; Yu, Y. Red-light-controllable liquid-crystal soft actuators via low-power excited upconversion based on triplet–triplet annihilation. J. Am. Chem. Soc. 2013, 135, 16446–16453. [Google Scholar] [CrossRef]
- Lv, J.-a.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Tomberg, A.; Friščić, T.; Barrett, C.J. Shaping crystals with light: Crystal-to-crystal isomerization and photomechanical effect in fluorinated azobenzenes. J. Am. Chem. Soc. 2013, 135, 12556–12559. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Z.; Yang, K.; Raquez, J.-M. A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials. Molecules 2020, 25, 1241. https://doi.org/10.3390/molecules25051241
Wen Z, Yang K, Raquez J-M. A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials. Molecules. 2020; 25(5):1241. https://doi.org/10.3390/molecules25051241
Chicago/Turabian StyleWen, Zhibin, Keke Yang, and Jean-Marie Raquez. 2020. "A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials" Molecules 25, no. 5: 1241. https://doi.org/10.3390/molecules25051241
APA StyleWen, Z., Yang, K., & Raquez, J.-M. (2020). A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials. Molecules, 25(5), 1241. https://doi.org/10.3390/molecules25051241







