Variation Patterns of the Volatiles during Germination of the Foxtail Millet (Setaria Italic): The Relationship between the Volatiles and Fatty Acids in Model Experiments
Abstract
1. Introduction
2. Results and Discussion
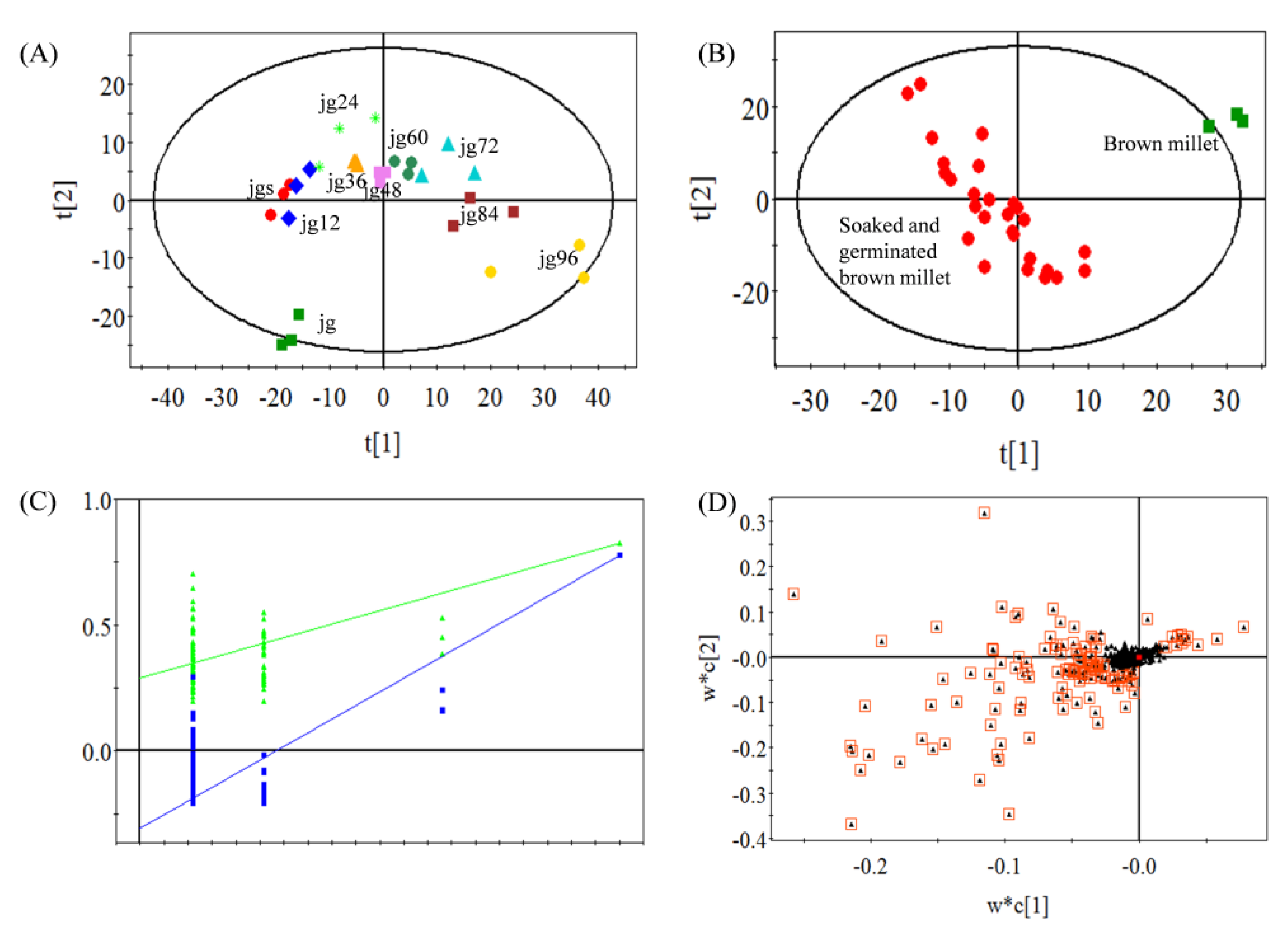
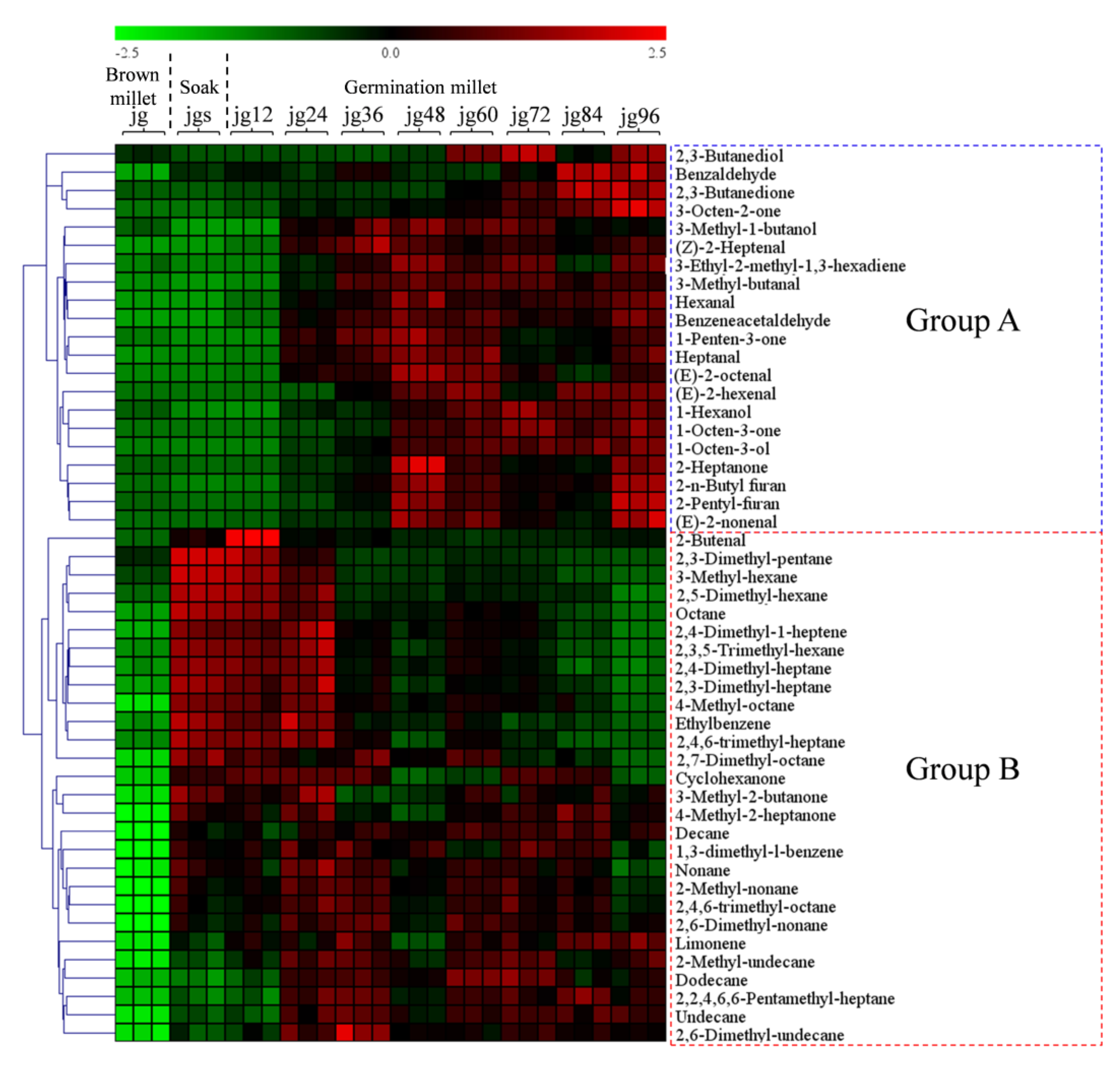
2.1. Variations of the Volatiles during the Germination of Foxtail Millet
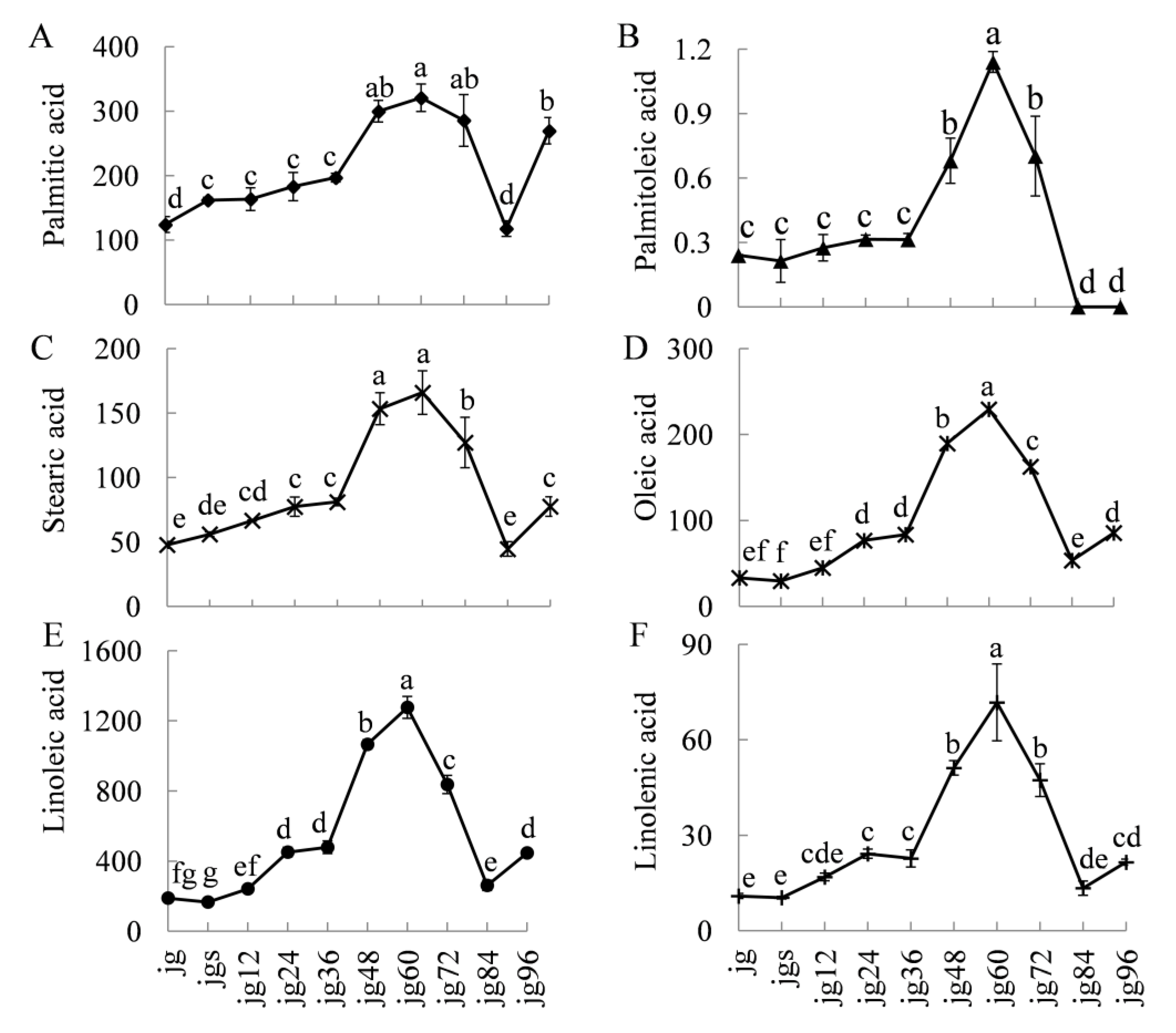
2.2. Variations of the Fatty Acid during Germination of the Foxtail Millet
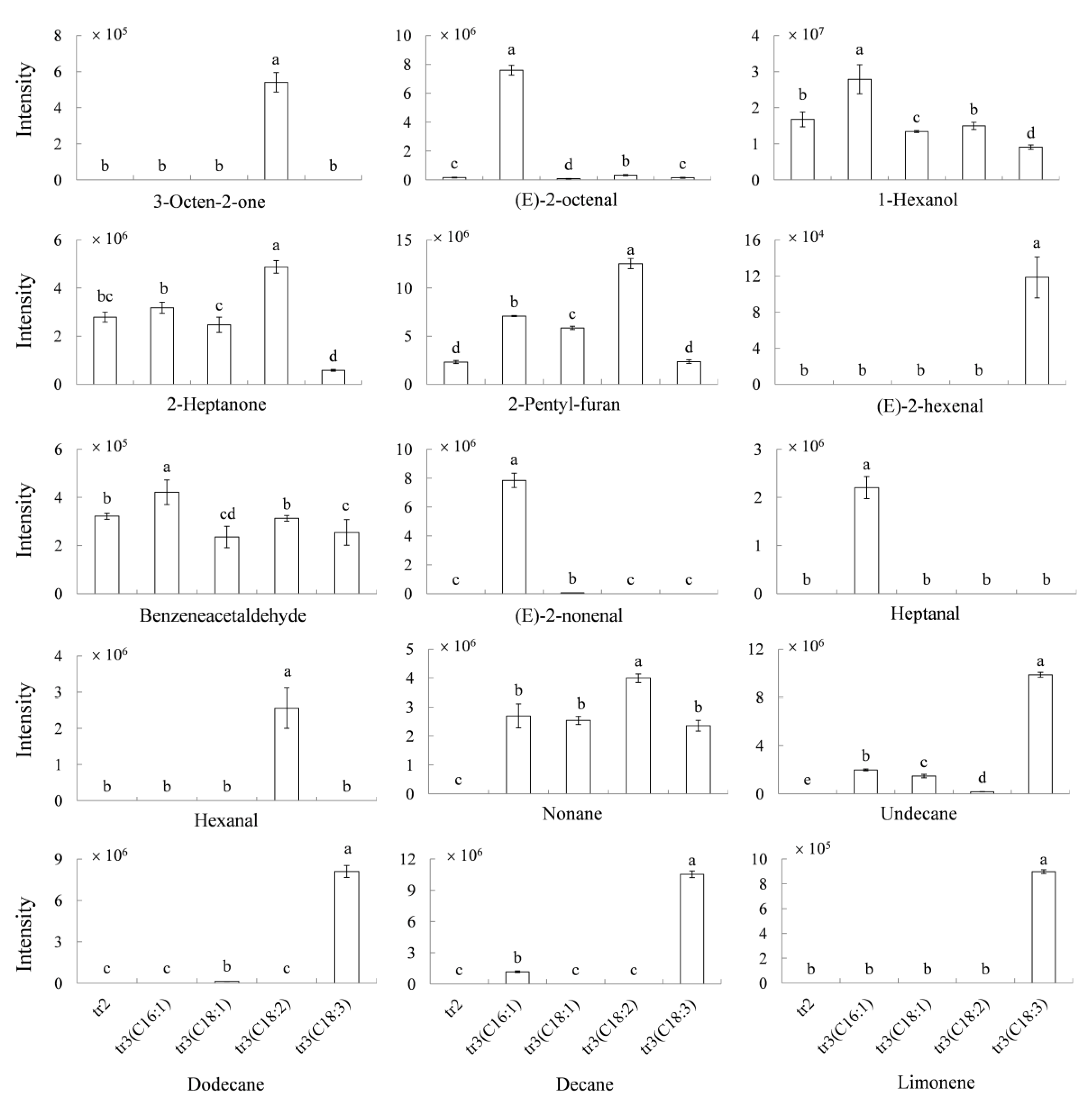
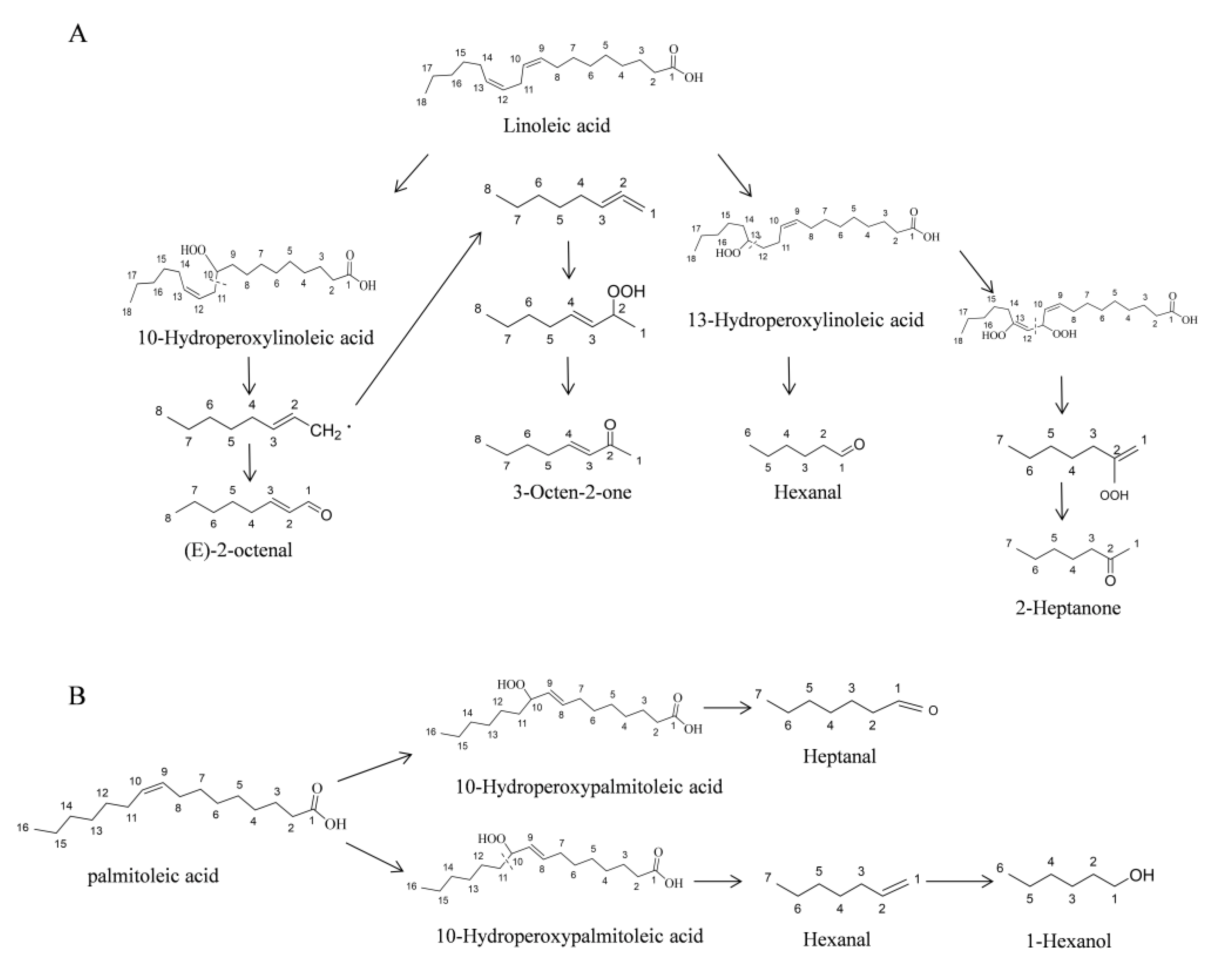
2.3. Relationships between the Volatiles and Fatty Acid during Millet Germination
3. Materials and Methods
3.1. Chemicals
3.2. Sample of Foxtail Millet
3.3. Determination of Volatiles Using HS-SPME-GC×GC-TOF/MS
3.4. Determination of Fatty Acid Using GC-FID
3.5. Determination of the Activity of Lipase
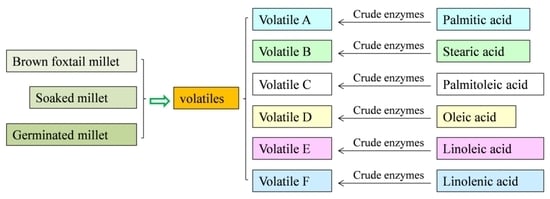
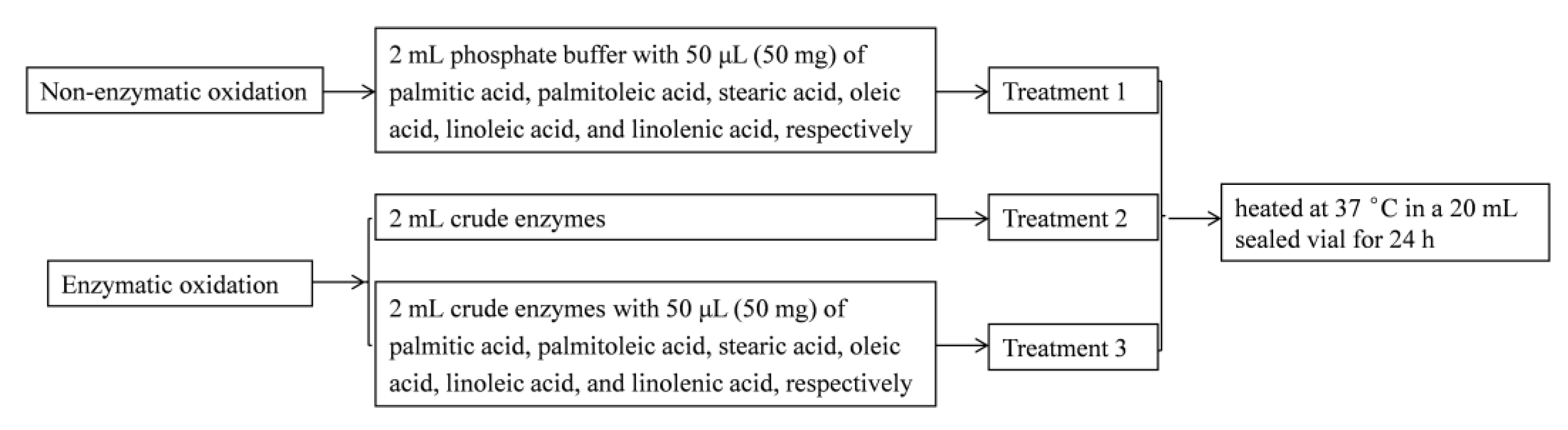
3.6. Model Experiments to Explain the Relationship between the Volatiles and the Fatty Acids with Crude Enzymes
3.7. Data Treatment and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.J.; Park, S.U. Influence of light on the free amino acid content and γ-aminobutyric acid synthesis in Brassica juncea seedlings. J. Agric. Food Chem. 2013, 61, 8624–8631. [Google Scholar] [CrossRef]
- Xia, Q.; Mei, J.; Yu, W.; Li, Y. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC–MS and chemometric methods. Eur. Food Res. Technol. 2017, 91, 103–114. [Google Scholar] [CrossRef]
- Coulibaly, A.; Chen, J. Evolution of energetic compounds, antioxidant capacity, some vitamins and minerals, phytase and amylase activity during the germination of foxtail millet. Am. J. Food Technol. 2011, 6, 40–51. [Google Scholar] [CrossRef]
- Chiou, R.Y.Y.; And, K.L.K.; Chen, W.L. Compositional characterization of peanut kernels after subjection to various germination times. J. Agric. Food Chem. 1994, 45, 3060–3064. [Google Scholar] [CrossRef]
- Guardado-Felix, D.; Serna-Saldivar, S.O.; Cuevas-Rodriguez, E.O.; Jacobo-Velazquez, D.A.; Gutierrez-Uribe, J.A. Effect of sodium selenite on isoflavonoid contents and antioxidant capacity of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2017, 226, 69–74. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Antioxidant activity, total phenolics, flavonoids and antinutritional characteristics of germinated foxtail millet (Setaria italica). Cogent Food Agric. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Hao, J.; Liu, X.; Liu, H.; Ning, Y.; Cheng, R.; Tan, B.; Jia, Y. Effect of the treatment by slightly acidic electrolyzed water on the accumulation of γ-aminobutyric acid in germinated brown millet. Food Chem. 2015, 186, 249–255. [Google Scholar] [CrossRef]
- Bai, Q.; Fan, G.; Gu, Z.; Cao, X.; Gu, F. Effects of culture conditions on γ-aminobutyric acid accumulation during germination of foxtail millet (Setaria italica L.). Eur. Food Res. Technol. 2008, 228, 169–175. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Wanasundara, U.N.; Shahidi, F. Changes in flax (Linum usitatissimum L.) seed lipids during germination. J. Am. Oil Chem. Soc. 1999, 76, 41–48. [Google Scholar] [CrossRef]
- Ghita Studsgaard, N.; Lone Melchior, L.; Leif, P. Formation of volatile compounds in model experiments with crude leek (Allium ampeloprasum Var. Lancelot) enzyme extract and linoleic acid or linolenic acid. J. Agric. Food Chem. 2004, 52, 2315–2321. [Google Scholar]
- Matsui, K.; Toyota, H.; Kajiwara, T.; Kakuno, T.; Hatanaka, A. Fatty acid hydroperoxide cleaving enzyme, hydroperoxide lyase, from tea leaves. Phytochemistry 1991, 30, 2109–2113. [Google Scholar] [CrossRef]
- Ohta, H.; Ida, S.; Mikami, B.; Morita, Y. Changes in lipoxygenase components of rice seedlings during germination. Plant. Cell Physiol. 1986, 27, 911–918. [Google Scholar] [CrossRef]
- Hisao, K.; Hidetoshi, K.; Hirotaka, K.; Masachika, T. Characterization of 9-fatty acid hydroperoxide lyase-like activity in germinating barley seeds that transforms 9(S)-hydroperoxy-10(E),12(Z)-octadecadienoic acid into (E)-nonenal. Biosci. Biotechnol. Biochem. 2005, 69, 1661–1668. [Google Scholar]
- Salas, J.J.; García-Gonzalez, D.L.; Ramón, A. Volatile compound biosynthesis by green leaves from an Arabidopsis thaliana hydroperoxide lyase knockout mutant. J. Agric. Food Chem. 2006, 54, 8199–8205. [Google Scholar] [CrossRef]
- Qin, G.; Tao, S.; Zhang, H.; Huang, W.; Wu, J.; Xu, Y.; Zhang, S. Evolution of the aroma volatiles of pear fruits supplemented with fatty acid metabolic precursors. Molecules 2014, 19, 20183–20196. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, S.; Li, Y.; Chen, F. Analysis of volatiles in brown rice, germinated brown rice, and selenized germinated brown rice during storage at different vacuum levels. J. Sci. Food Agric. 2018, 98, 2295–2301. [Google Scholar] [CrossRef]
- Zuo, Y.; Ju, S.; Liu, L.P.; Li, Y.; Xie, H. Analysis of volatile compounds in bean sprout by simultaneous distillation extraction and gas chromatography-mass spectrometry. Adv. Mater. Res. 2011, 396–398, 1570–1574. [Google Scholar] [CrossRef]
- Horiuchi, M.; Umano, K.; Shibamoto, T. Analysis of volatile compounds formed from fish oil heated with cysteine and trimethylamine oxide. J. Agric. Food Chem. 1998, 46, 5232–5237. [Google Scholar] [CrossRef]
- Antony, U.; Sripriya, G.; Chandra, T.S. The effect of fermentation on the primary nutrients in foxtail millet (Setaria italica). Food Chem. 1996, 56, 381–384. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, X.; Wang, G.; Wang, H.; Liu, J.; Zhao, W.; Zhang, Y. Crude fat content and fatty acid profile and their correlations in foxtail millet. Cereal Chem. 2015, 92, 455–459. [Google Scholar] [CrossRef]
- Sekiya, J.; Kajiwara, T.; Hatanaka, A. Volatile C6-aldehyde formation via hydroperoxides from C18-unsaturated fatty acids in etiolated alfalfa and cucumber seedlings. J. Agric. Chem. Soc. Jpn. 1979, 43, 969–980. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, N.; Fray, R.G.; Fisk, I.; Liu, C.; Li, H.; Han, Y. Characterization of volatile aroma compounds after in-vial cooking of foxtail millet porridge with gas chromatography-mass spectrometry. J. Cereal Sci. 2018, 82, 8–15. [Google Scholar] [CrossRef]
- Sekiya, J.; Kamiuchi, H.; Hatanaka, A. Lipoxygenase, hydroperoxide lyase and volatile C6-aldehyde formation from C18-fatty acids during development of Phaseolus vulgaris L. Plant. Cell Physiol. 1982, 23, 631–638. [Google Scholar]
- Monsoor, M.A.; Proctor, A. Volatile component analysis of commercially milled head and broken rice. J. Food Sci. 2010, 69, C632–C636. [Google Scholar] [CrossRef]
- Haslbeck, F.; Grosch, W. HPLC analysis of all positional isomers of the monohydroperoxides formed by soybean lipoxygenases during oxidation of linoleic acid. J. Food Biochem. 2010, 9, 1–14. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Combet, E.; Henderson, J.; Henderson, J.; Combet, E. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Model experiments about the formation of volatile carbonyl compounds. J. Am. Oil Chem. Soc. 1981, 58, 602–607. [Google Scholar] [CrossRef]
- Min, D.B.; Callison, A.L.; Lee, H.O. Singlet oxygen oxidation for 2-pentylfuran and 2-pentenyfuran formation in soybean oil. J. Food Sci. 2010, 68, 1175–1178. [Google Scholar] [CrossRef]
- Krishnamurthy, R.G.; Smouse, T.H.; Mookherjee, B.D.; Reddy, B.R.; Chang, S.S. Identification of 2-pentyl furan in fats and oils and its relationship to the reversion flavor of soybean oil. J. Food Sci. 2010, 32, 372–374. [Google Scholar] [CrossRef]
- An, A.; Capucine, B.; Fien, V.L.; Bruno, D.M.; Norbert, D.K. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agric. Food Chem. 2011, 59, 11058–11062. [Google Scholar]
- Giogios, I.; Grigorakis, K.; Nengas, I.; Papasolomontos, S.; Papaioannou, N.; Alexis, M.N. Fatty acid composition and volatile compounds of selected marine oils and meals. J. Sci. Food Agric. 2010, 89, 88–100. [Google Scholar] [CrossRef]
- Klensporf, D.; Jelen’, H.H. Analysis of volatile aldehydes in oat flakes by SPME-GC/MS. Pol. J. Food Nutr. Sci. 2005, 55, 389–395. [Google Scholar]
- Zhao, F.; Liu, J.; Wang, X.; Li, P.; Zhang, W.; Zhang, Q. Detection of adulteration of sesame and peanut oils via volatiles by GC × GC–TOF/MS coupled with principal components analysis and cluster analysis. Eur. J. Lipid Sci. Technol. 2013, 115, 337–347. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Eur. Food Res. Technol. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, X.; Fang, F.; Hong, X.; Wu, H.; Chen, D.; Huang, X. Immobilization of Enzymes on a Phospholipid Bionically Modified Polysulfone Gradient-Pore Membrane for the Enhanced Performance of Enzymatic Membrane Bioreactors. Molecules 2018, 23, 144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| ID | Volatile Compound | 1st Rt | 2nd Rt | RIcal a | RIref b | Probability | Odor Description c |
|---|---|---|---|---|---|---|---|
| Ketones | |||||||
| 1 | 2,3-Butanedione d | 3.40 | 1.79 | 566 | 595 | 1225 | fruity |
| 2 | 1-Penten-3-one d | 4.60 | 2.07 | 644 | 681 | 7650 | pungent |
| 3 | 3-Methyl-2-butanone d | 4.60 | 2.01 | 644 | 657 | 1443 | camphor |
| 4 | 2-Heptanone d | 8.73 | 2.18 | 880 | 891 | 8707 | fruity, floral |
| 5 | Cyclohexanone | 8.87 | 2.77 | 887 | 894 | 7841 | minty acetone |
| 6 | 4-Methyl-2-heptanone | 9.80 | 2.12 | 932 | 943 | 8771 | NF |
| 7 | 1-Octen-3-one d | 10.67 | 2.25 | 972 | 979 | 6443 | mushroom, earthy |
| 8 | 3-Octen-2-one d | 11.93 | 2.34 | 1035 | 1033 | 4690 | nutty, fruity |
| Aldehydes | |||||||
| 9 | 2-Butenal | 4.07 | 2.11 | 610 | 629 | 3657 | flower |
| 10 | 3-Methyl-butanal | 4.73 | 2.04 | 653 | 652 | 1162 | peach |
| 11 | Hexanal d | 6.73 | 2.19 | 776 | 800 | 8959 | grass |
| 12 | (E)-2-hexenal d | 8.00 | 2.33 | 844 | 854 | 5388 | green banana, cheesy |
| 13 | Heptanal d | 9.00 | 2.20 | 894 | 901 | 8838 | herbal |
| 14 | (Z)-2-Heptenal | 10.20 | 2.36 | 950 | 958 | 4277 | pungent, vegetable |
| 15 | Benzaldehyde d | 10.33 | 2.94 | 957 | 962 | 8697 | bitter almond |
| 16 | Benzeneacetaldehyde d | 12.07 | 3.00 | 1042 | 1045 | 8017 | green sweet, floral |
| 17 | (E)-2-octenal d | 12.33 | 2.35 | 1056 | 1060 | 7333 | fresh cucumber |
| 18 | (E)-2-nonenal d | 14.33 | 2.31 | 1161 | 1162 | 6268 | green cucumber |
| Alkanes | |||||||
| 19 | 2,3-Dimethyl-pentane | 4.73 | 1.60 | 652 | 672 | 2381 | NF |
| 20 | 3-Methyl-hexane | 5.33 | 1.61 | 691 | 676 | 859 | NF |
| 21 | 2,5-Dimethyl-hexane | 6.00 | 1.65 | 733 | 729 | 967 | NF |
| 22 | Octane d | 6.73 | 1.68 | 776 | 800 | 5265 | gasoline |
| 23 | 2,3,5-Trimethyl-hexane | 7.07 | 1.67 | 796 | 812 | 2323 | NF |
| 24 | 2,4-Dimethyl-heptane | 7.20 | 1.68 | 803 | 821 | 1831 | NF |
| 25 | 2,3-Dimethyl-heptane | 7.93 | 1.70 | 840 | 855 | 3634 | NF |
| 26 | 4-Methyl-octane | 8.13 | 1.70 | 850 | 863 | 5801 | NF |
| 27 | 2,4,6-trimethyl-heptane | 8.33 | 1.68 | 860 | 870 | 2161 | NF |
| 28 | Nonane d | 8.93 | 1.71 | 890 | 900 | 3280 | gasoline |
| 29 | 2,7-Dimethyl-octane | 9.53 | 1.69 | 919 | 928 | 5752 | NF |
| 30 | 2-Methyl-nonane | 10.33 | 1.71 | 9569 | 964 | 3699 | NF |
| 31 | 2,4,6-trimethyl-octane | 10.60 | 1.68 | 969 | 959 | 1045 | NF |
| 32 | 2,2,4,6,6-Pentamethyl-heptane | 10.93 | 1.70 | 9849 | 991 | 6719 | NF |
| 33 | Decane d | 11.07 | 1.76 | 991 | 1000 | 5144 | alkane |
| 34 | 2,6-Dimethyl-nonane | 11.53 | 1.72 | 1014 | 1018 | 1208 | NF |
| 35 | Undecane d | 13.13 | 1.74 | 1097 | 1100 | 1821 | alkane |
| 36 | 2-Methyl-undecane | 14.33 | 1.76 | 1161 | 1164 | 4077 | NF |
| 37 | Dodecane d | 15.00 | 1.79 | 1196 | 1200 | 3576 | alkane |
| 38 | 2,6-Dimethyl-undecane | 15.27 | 1.76 | 1212 | 1210 | 2783 | NF |
| Alcohols | |||||||
| 39 | 3-Methyl-1-butanol d | 6.07 | 2.05 | 736 | 736 | 1758 | fusel oil, whiskey |
| 40 | 2,3-Butanediol d | 6.53 | 2.38 | 765 | 788 | 9524 | fruity, creamy |
| 41 | 1-Hexanol d | 8.27 | 2.13 | 857 | 868 | 5363 | ethereal fusel oil |
| 42 | 1-Octen-3-ol d | 10.67 | 2.11 | 972 | 980 | 4162 | mushroom, earthy |
| Alkenes | |||||||
| 43 | 2,4-Dimethyl-1-heptene | 7.67 | 1.73 | 827 | 836 | 6350 | NF |
| 44 | Ethylbenzene | 8.13 | 2.23 | 850 | 855 | 4960 | NF |
| 45 | 1,3-dimethyl-l-benzene | 8.33 | 2.19 | 860 | 866 | 3038 | plastic |
| 46 | Limonene | 11.73 | 2.06 | 1024 | 1030 | 6216 | citrus |
| 47 | 3-Ethyl-2-methyl-1,3-hexadiene | 11.80 | 2.57 | 1028 | 1031 | 912 | NF |
| Furans | |||||||
| 48 | 2-n-Butyl furan d | 8.80 | 2.04 | 884 | 893 | 9053 | mild fruity wine |
| 49 | 2-Pentyl-furan d | 10.93 | 2.08 | 985 | 993 | 8130 | fruity, green, earthy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhu, Y.; Li, S.; Zhang, A.; Zhao, W.; Zhang, J.; Chen, Q.; Ren, S.; Liu, J.; Wang, H. Variation Patterns of the Volatiles during Germination of the Foxtail Millet (Setaria Italic): The Relationship between the Volatiles and Fatty Acids in Model Experiments. Molecules 2020, 25, 1238. https://doi.org/10.3390/molecules25051238
Li P, Zhu Y, Li S, Zhang A, Zhao W, Zhang J, Chen Q, Ren S, Liu J, Wang H. Variation Patterns of the Volatiles during Germination of the Foxtail Millet (Setaria Italic): The Relationship between the Volatiles and Fatty Acids in Model Experiments. Molecules. 2020; 25(5):1238. https://doi.org/10.3390/molecules25051238
Chicago/Turabian StyleLi, PengLiang, Yin Zhu, ShaoHui Li, AiXia Zhang, Wei Zhao, JiaLi Zhang, QinCao Chen, SuFen Ren, JingKe Liu, and HuiJun Wang. 2020. "Variation Patterns of the Volatiles during Germination of the Foxtail Millet (Setaria Italic): The Relationship between the Volatiles and Fatty Acids in Model Experiments" Molecules 25, no. 5: 1238. https://doi.org/10.3390/molecules25051238
APA StyleLi, P., Zhu, Y., Li, S., Zhang, A., Zhao, W., Zhang, J., Chen, Q., Ren, S., Liu, J., & Wang, H. (2020). Variation Patterns of the Volatiles during Germination of the Foxtail Millet (Setaria Italic): The Relationship between the Volatiles and Fatty Acids in Model Experiments. Molecules, 25(5), 1238. https://doi.org/10.3390/molecules25051238