Chemometric Analysis of Low-field 1H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. LF 1H NMR Analysis
2.2. Chemometric Analysis
3. Materials and Methods
3.1. Samples
3.2. Sample Preparation for LF 1H NMR Analysis
3.3. LF 1H NMR Analysis
3.4. Chemometrics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekar, T.; Kreft, S. Common risks of adulterated and mislabeled herbal preparations. Food Chem. Toxicol. 2019, 123, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Georgiev, M.I.; Orhan, I.E. Adulteration of herbal sexual enhancers and slimmers: The wish for better sexual well-being and perfect body can be risky. Food Chem. Toxicol. 2017, 108, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.S.; McDowell, A.; Strachan, C.J.; Gordon, K.C. Evaluation of vibrational spectroscopic methods to identify and quantify multiple adulterants in herbal medicines. Talanta 2015, 138, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Cai, W.; Shao, X. Rapid discrimination of slimming capsules based on illegal additives by electronic nose and flash gas chromatography. J. Sep. Sci. 2015, 38, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.; Assemat, G.; Martins, N.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J. Pharm. Biomed. Anal. 2016, 124, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Dunnick, J.K.; Hailey, J.R. Phenolphthalein exposure causes multiple carcinogenic effects in experimental model systems. Cancer Res. 1996, 56, 4922–4926. [Google Scholar]
- Rebiere, H.; Guinot, P.; Civade, C.; Bonnet, P.A.; Nicolas, A. Detection of hazardous weight-loss substances in adulterated slimming formulations using ultra-high-pressure liquid chromatography with diode-array detection. Food Addit. Contam. A 2012, 29, 161–171. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Lee, J.H.; Park, H.J.; Cho, S.H.; Cho, S.; Kim, W.S. Monitoring of 29 weight-loss compounds in foods and dietary supplements by LC-MS/MS. Food Addit. Contam. A 2014, 31, 777–783. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, Y.; Kee, C.L.; Low, M.Y.; Ge, X. Analysis of 40 weight loss compounds adulterated in health supplements by liquid chromatography quadrupole linear ion trap mass spectrometry. Drug Test. Anal. 2016, 8, 351–356. [Google Scholar] [CrossRef]
- Deconinck, E.; Cauwenbergh, T.; Bothy, J.L.; Custers, D.; Courselle, P.; De Beer, J.O. Detection of sibutramine in adulterated dietary supplements using attenuated total reflectance-infrared spectroscopy. J. Pharm. Biomed. Anal. 2014, 100, 279–283. [Google Scholar] [CrossRef]
- Dal Molin, T.R.; da Silveira, G.D.; Leal, G.C.; Muller, L.S.; Muratt, D.T.; de Carvalho, L.M.; Viana, C. A new approach to ion exchange chromatography with conductivity detection for adulterants investigation in dietary supplements. Biomed. Chromatogr. 2019, 33, e4669. [Google Scholar] [CrossRef] [PubMed]
- Blumich, B.; Singh, K. Desktop NMR and its applications from Materials Science to Organic Chemistry. Angew. Chem. Int. Ed. Engl. 2018, 57, 6996–7010. [Google Scholar] [CrossRef] [PubMed]
- Blumich, B. Low-field and benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A. Low-field benchtop NMR spectroscopy: Status and prospects in natural product analysis. Phytochem. Anal. 2020, 1–14. [Google Scholar] [CrossRef]
- Pages, G.; Gerdova, A.; Williamson, D.; Gilard, V.; Martino, R.; Malet-Martino, M. Evaluation of a benchtop cryogen-free low-field 1H NMR spectrometer for the analysis of sexual enhancement and weight loss dietary supplements adulterated with pharmaceutical substances. Anal. Chem. 2014, 86, 11897–11904. [Google Scholar] [CrossRef]
- Zivkovic, A.; Bandolik, J.J.; Skerhut, A.J.; Coesfeld, C.; Prascevic, M.; Zivkovic, L.; Stark, H. Quantitative analysis of multicomponent mixtures of over-the-counter pain killer drugs by Low-Field NMR spectroscopy. J. Chem. Educ. 2017, 94, 121–125. [Google Scholar] [CrossRef]
- Assemat, G.; Gouilleux, B.; Bouillaud, D.; Farjon, J.; Gilard, V.; Giraudeau, P.; Malet-Martino, M. Diffusion-ordered spectroscopy on a benchtop spectrometer for drug analysis. J. Pharm. Biomed. Anal. 2018, 160, 268–275. [Google Scholar] [CrossRef]
- Assemat, G.; Balayssac, S.; Gerdova, A.; Gilard, V.; Caillet, C.; Williamson, D.; Malet-Martino, M. Benchtop low-field 1H Nuclear Magnetic Resonance for detecting falsified medicines. Talanta 2019, 196, 163–173. [Google Scholar] [CrossRef]
- Keizers, P.H.J.; Bakker, F.; Ferreira, J.; Wackers, P.F.K.; van Kollenburg, D.; van der Aa, E.; van Beers, A. Benchtop NMR spectroscopy in the analysis of substandard and falsified medicines as well as illegal drugs. J. Pharm. Biomed. Anal. 2020, 178, 112939. [Google Scholar] [CrossRef]
- Sandasi, M.; Vermaak, I.; Chen, W.; Viljoen, A. The application of vibrational spectroscopy techniques in the qualitative assessment of material traded as Ginseng. Molecules 2016, 21, 472. [Google Scholar] [CrossRef]
- Parker, T.; Limer, E.; Watson, A.D.; Defernez, M.; Williamson, D.; Kemsley, E.K. 60 MHz 1H NMR spectroscopy for the analysis of edible oils. Trends Analyt. Chem. 2014, 57, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, X.; Chen, L. Rapid detection of peanut oil adulteration using low-field nuclear magnetic resonance and chemometrics. Food Chem. 2017, 216, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jakes, W.; Gerdova, A.; Defernez, M.; Watson, A.D.; McCallum, C.; Limer, E.; Colquhoun, I.J.; Williamson, D.C.; Kemsley, E.K. Authentication of beef versus horse meat using 60 MHz 1H NMR spectroscopy. Food Chem. 2015, 175, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. Alignment of 1D NMR data using the ICOSHIFT tool: A tutorial. In Magnetic Resonance In Food Science; Van Duynhoven, J., Belton, P.S., Webb, G.A., van As, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 14–24. [Google Scholar]
- Sousa, S.A.A.; Magalhaes, A.; Ferreira, M.M.C. Optimized bucketing for NMR spectra: Three case studies. Chemom. Intell. Lab. Syst. 2013, 122, 93–102. [Google Scholar] [CrossRef]
Sample Availability: Samples of dietary supplements are available from the authors. |
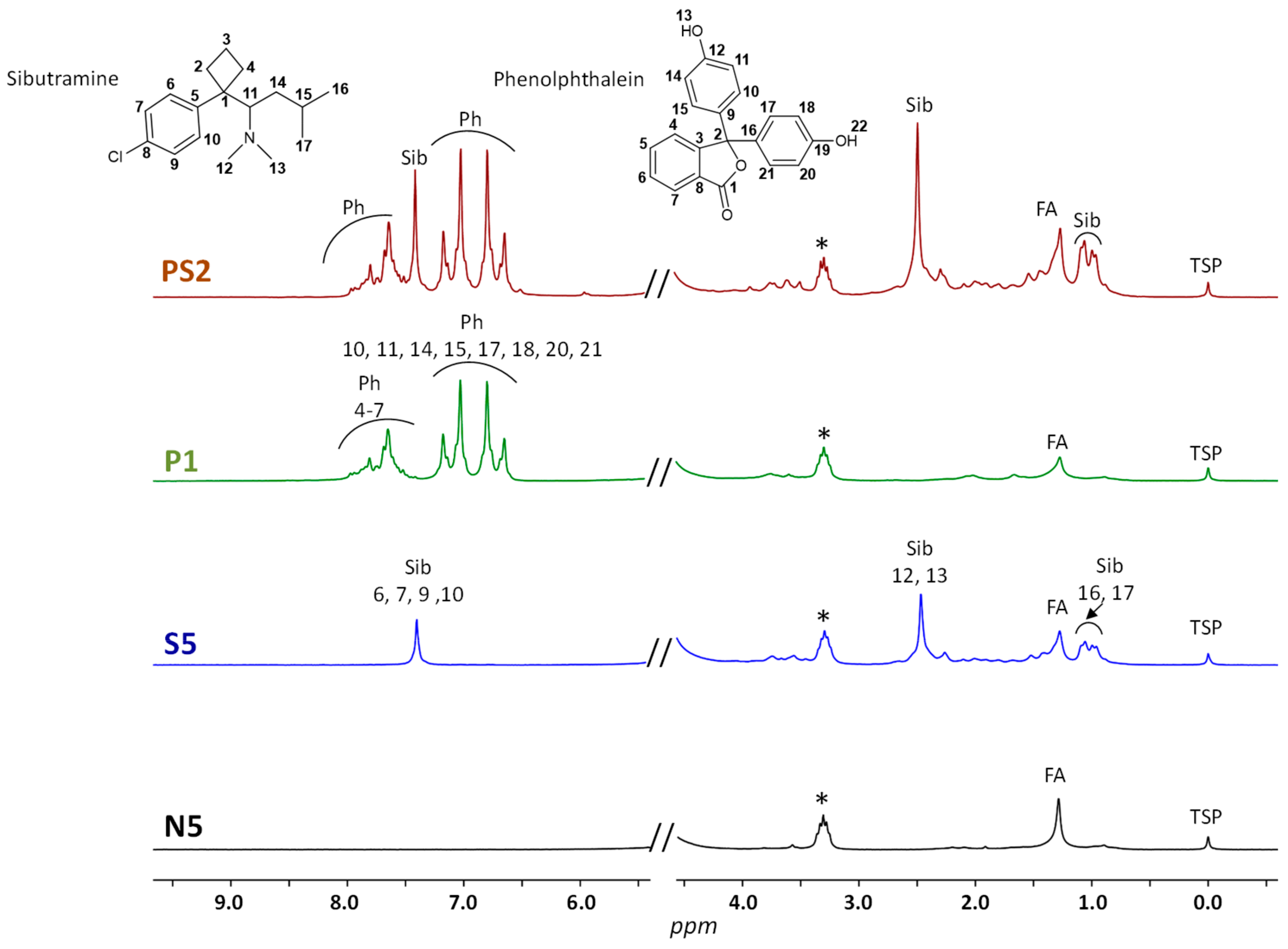
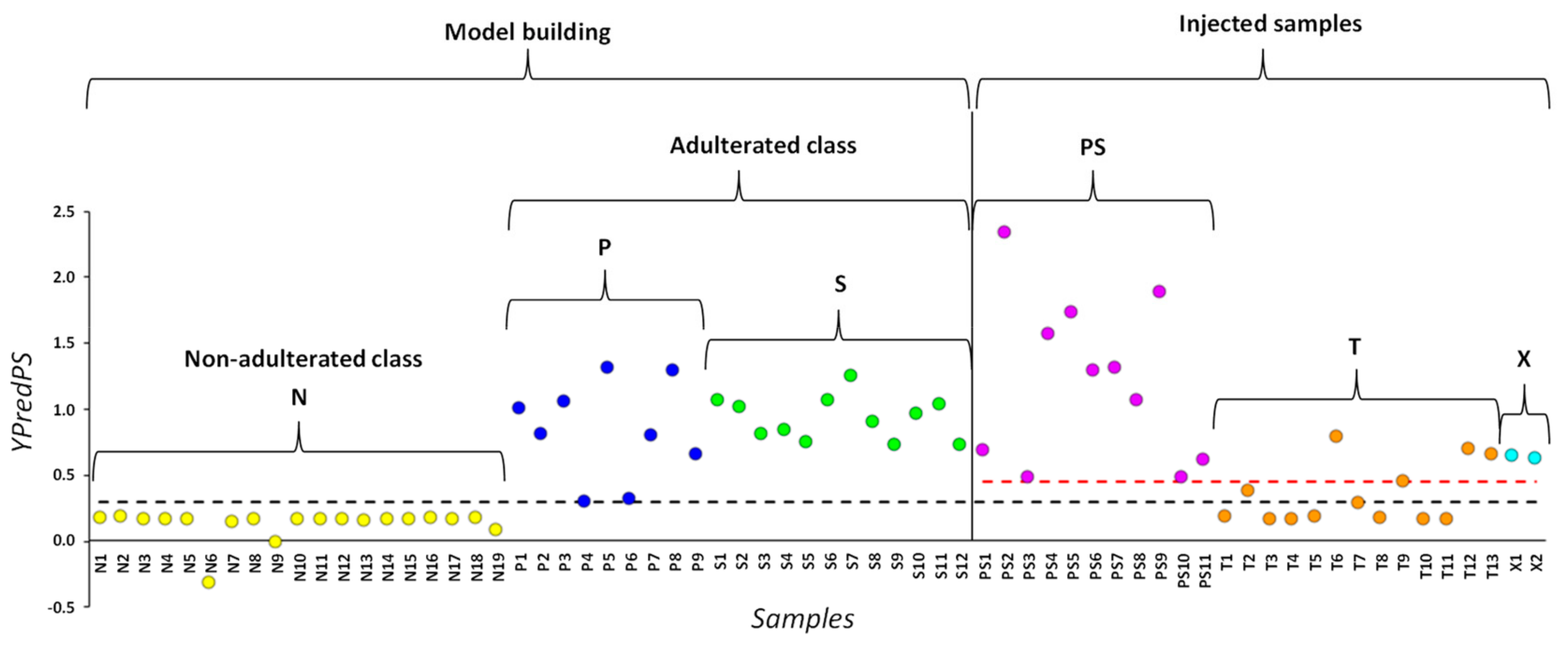
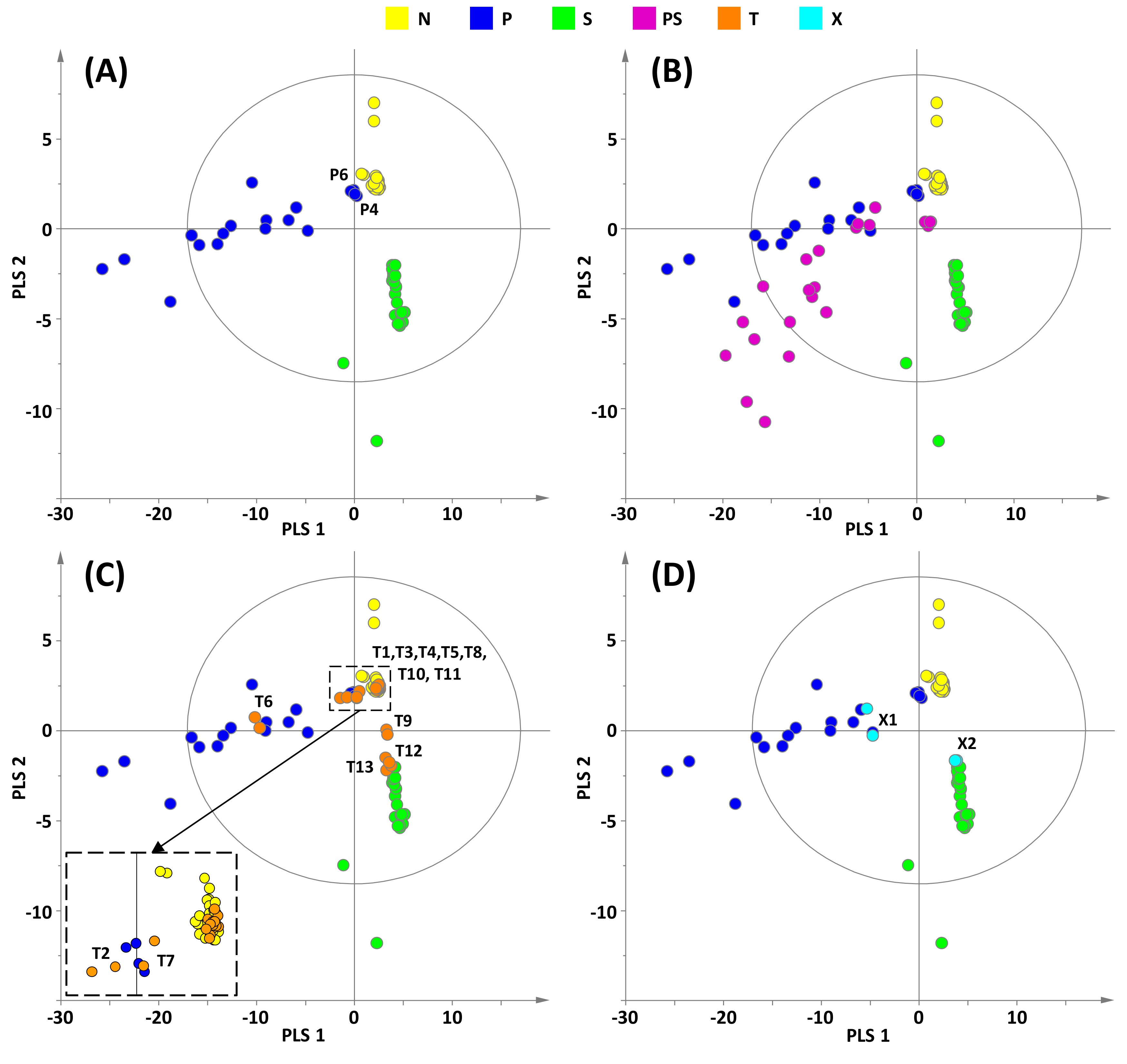
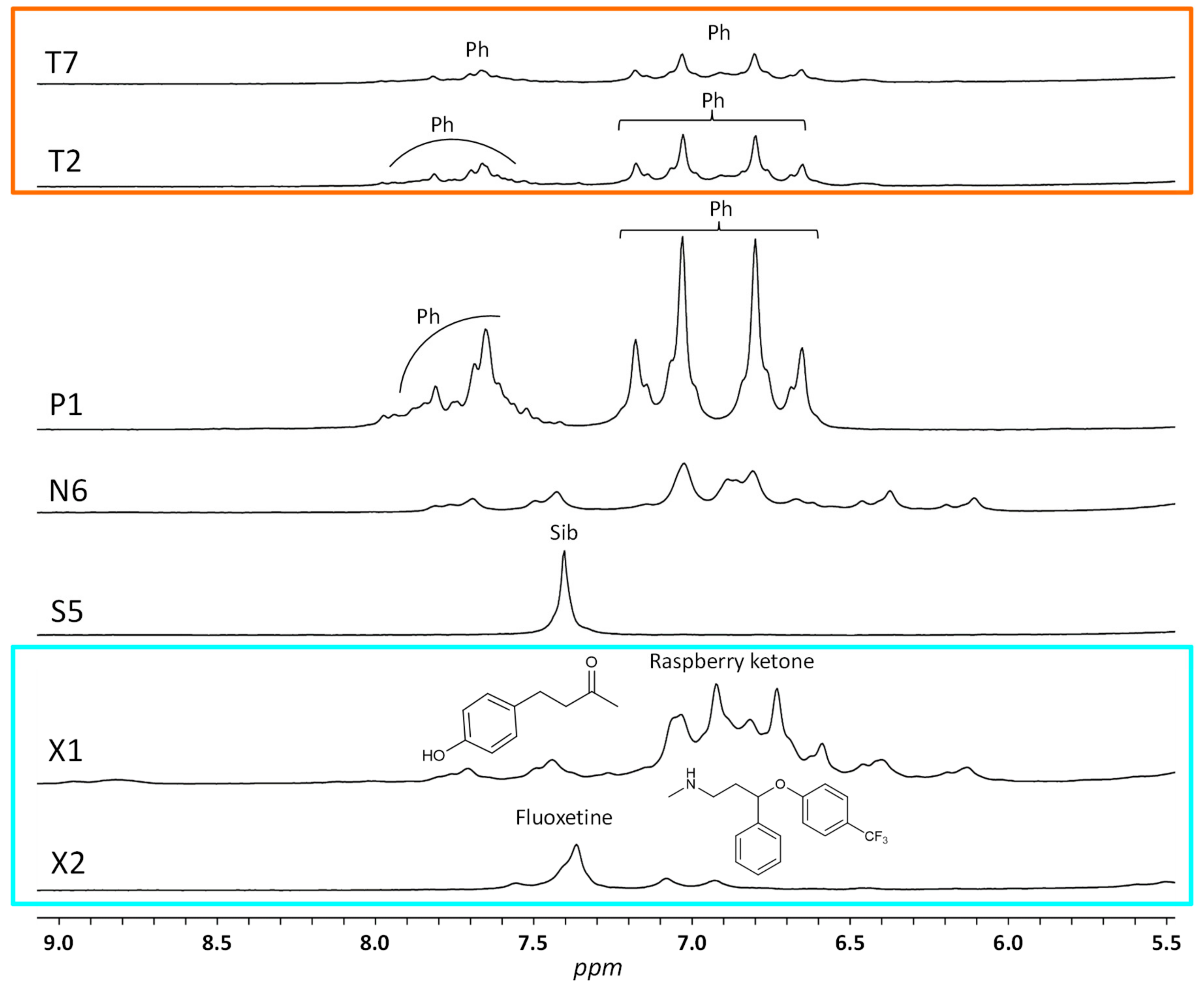
| Identification | Predictive Y-value Classification from the Two-Class PLS-DA | Projection on the Three-Class PLS-DA Model Shown in Figure 3A | ||
|---|---|---|---|---|
| YPredPS | Classification | Class membership | Adulterant | |
| T1 | 0.18 | natural | N | - |
| T2 | 0.37 | borderline | P | phenolphthalein |
| T3 | 0.16 | natural | N | - |
| T4 | 0.17 | natural | N | - |
| T5 | 0.18 | natural | N | - |
| T6 | 0.79 | adulterated | P | phenolphthalein |
| T7 | 0.30 | borderline | P | phenolphthalein |
| T8 | 0.17 | natural | N | - |
| T9 | 0.45 | adulterated | S | sibutramine |
| T10 | 0.17 | natural | N | - |
| T11 | 0.17 | natural | N | - |
| T12 | 0.69 | adulterated | S | sibutramine |
| T13 | 0.65 | adulterated | S | sibutramine |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Balayssac, S.; Danoun, S.; Malet-Martino, M.; Gilard, V. Chemometric Analysis of Low-field 1H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds. Molecules 2020, 25, 1193. https://doi.org/10.3390/molecules25051193
Wu N, Balayssac S, Danoun S, Malet-Martino M, Gilard V. Chemometric Analysis of Low-field 1H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds. Molecules. 2020; 25(5):1193. https://doi.org/10.3390/molecules25051193
Chicago/Turabian StyleWu, Nao, Stéphane Balayssac, Saïda Danoun, Myriam Malet-Martino, and Véronique Gilard. 2020. "Chemometric Analysis of Low-field 1H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds" Molecules 25, no. 5: 1193. https://doi.org/10.3390/molecules25051193
APA StyleWu, N., Balayssac, S., Danoun, S., Malet-Martino, M., & Gilard, V. (2020). Chemometric Analysis of Low-field 1H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds. Molecules, 25(5), 1193. https://doi.org/10.3390/molecules25051193







