Antigenotoxic, Anti-photogenotoxic, and Antioxidant Properties of Polyscias filicifolia Shoots Cultivated In Vitro
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Phytochemical Investigations
4.3.1. Preparation of Extracts and HPLC-UV-Vis Determination of Phenolic Compounds
4.3.2. UHPLC-DAD-MS/MS Analysis
Preparation of Samples to Analysis
Ultra-high-resolution Mass Spectrometry Parameters
4.3.3. Determination of Total Phenolic Compounds Content
4.3.4. Determination of Total Flavonoid Content
4.3.5. Determination of Antioxidant Activity
4.4. Antigenotoxicity Assessment
4.4.1. Metabolic Activation
4.4.2. Bacterial Strain
4.4.3. Umu-Test
4.4.4. Determination of Antigenotoxicity and Anti-photogenotoxicity by the umu-test
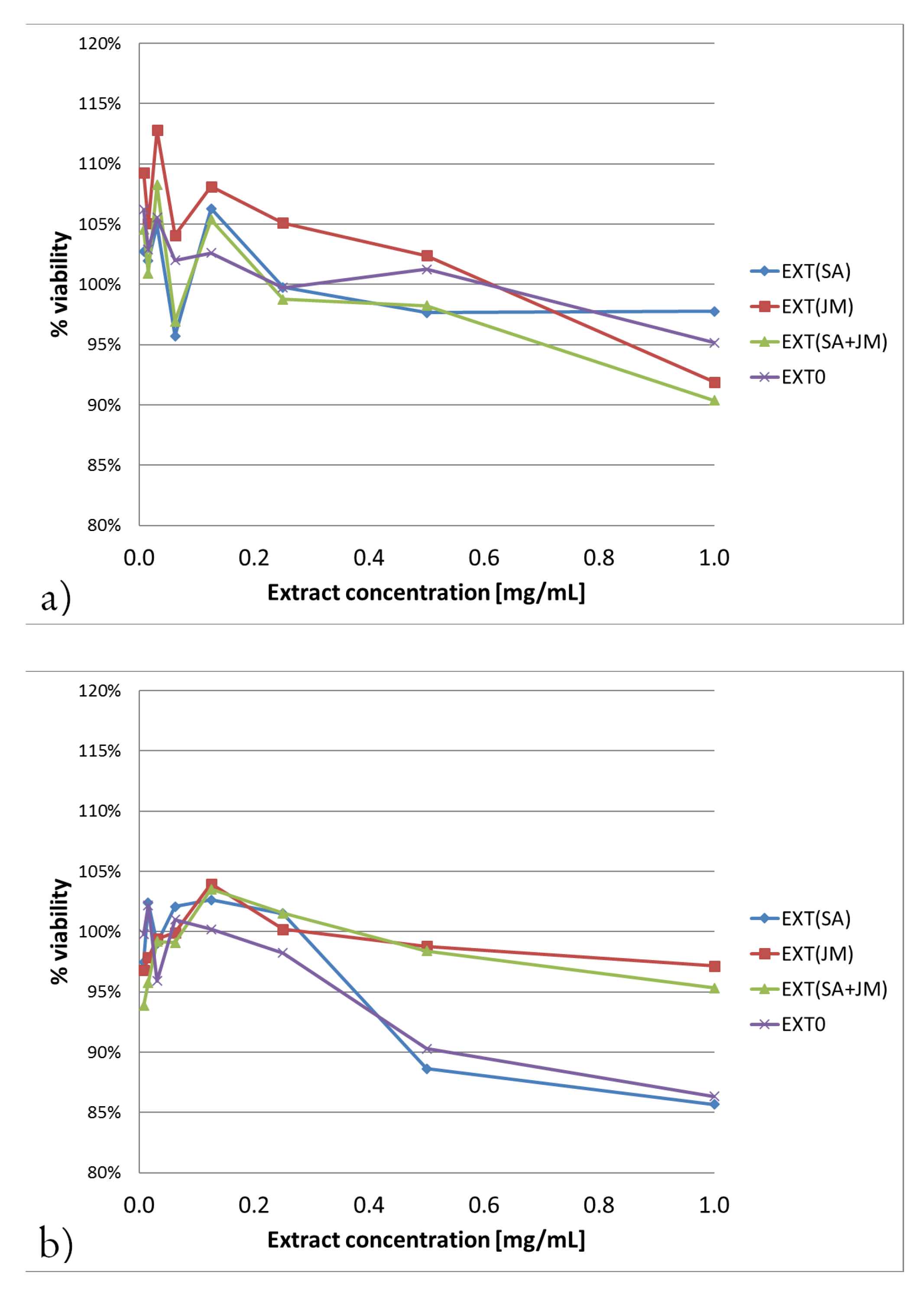
4.5. Determination of Cytotoxicity
4.5.1. Cell line and Culture Conditions
4.5.2. Neutral Red Uptake (NRU) Assay
4.5.3. MTT Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mut. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. F. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, A.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Rocha, L.; Monteiro, M.; Teodoro, A. Anticancer Properties of Hydroxycinnamic Acids—A Review. Cancer Clin. Oncol. 2012, 1. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Basgedik, B.; Ugur, A.; Sarac, N. Antimicrobial, antioxidant, antimutagenic activities, and phenolic compounds of Iris germanica. Ind. Crop. Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Bhatia, A.; Arora, S.; Singh, B.; Kaur, G.; Nagpal, A. Anticancer potential of Himalayan plants. Phytochem. Rev. 2011, 10, 309–323. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Berhow, M.A.; Wagner, E.D.; Vaughn, S.F.; Plewa, M.J. Characterization and antimutagenic activity of soybean saponins. Mut. Res. 2000, 448, 11–22. [Google Scholar] [CrossRef]
- Horn, R.C.; Vargas, V.M.F. Antimutagenic activity of extracts of natural substances in the Salmonella/microsome assay. Mutagenesis 2003, 18, 113–118. [Google Scholar] [CrossRef]
- Kruawan, K.; Kangsadalampai, K. Antioxidant activity, phenolic compound contents and antimutagenic activity of some water extract of herbs. Thai J. Pharm. Sci. 2005, 30, 28–35. [Google Scholar]
- Sroka, Z. Antioxidative and Antiradical Properties of Plant Phenolics. Z. Naturforsch. C. 2005, 60, 833–843. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.; Corrêa, M.; Salgado, H. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203. [Google Scholar] [CrossRef]
- Gaurav, V.; Kolewe, M.; Roberts, S. Pharmaceutically Active Natural Product Synthesis and Supply via Plant Cell Culture Technology. Mol. Pharmaceut. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Wilson, S.; Roberts, S. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2011, 10, 249–268. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Future Sci. 2017, 3, FSO226. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.; Jin, Y.W.; Lee, E.K.; Loake, G. Plant cell culture strategies for the production of natural products. BMB Rep. 2015, 49. [Google Scholar] [CrossRef]
- Ramírez Estrada, K.; Hidalgo Martinez, D.; Moyano, E.; Goleniowski, M.; Cusido, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, A.; Sykłowska-Baranek, K.; Kosmider, A.; Granica, S.; Miszczak, K.; Nowicka, G.; Kasztelan, A.; Podsadni, P.; Turlo, J.; Pietrosiuk, A. Stimulation of phenolic compounds production in the in vitro cultivated Polyscias filicifolia Bailey shoots and evaluation of the antioxidant and cytotoxic potential of plant extracts. Acta Soc. Bot. Pol. 2018, 87, 1–16. [Google Scholar] [CrossRef]
- Marczewska, J.; Drozd, E.; Drozd, J.; Anuszewskal, E.; Sliwińska, A.; Nosov, A.; Olszowska, O. Assessment of cytotoxic and genotoxic activity of alcohol extract of Polyscias filicifolia shoot, leaf, cell biomass of suspension culture and saponin fraction. Acta Pol. Pharm. 2011, 68, 703–710. [Google Scholar] [PubMed]
- Stich, H.F.; Rosin, M.P.; Wu, C.H.; Powrie, W.D. A comparative genotoxicity study of chlorogenic acid (3-O-caffeoylquinic acid). Mutat. Res.-Genet. Tox. 1981, 90, 201–212. [Google Scholar] [CrossRef]
- Alarcón-Herrera, N.; Flores-Maya, S.; Bellido, B.; García-Bores, A.M.; Mendoza, E.; Ávila Acevedo, G.; Hernández-Echeagaray, E. Protective effects of chlorogenic acid in 3-nitropropionic acid induced toxicity and genotoxicity. Food Chem. Toxicol. 2017, 109, 1018–1025. [Google Scholar] [CrossRef]
- Maistro, E.L.; Angeli, J.P.F.; Andrade, S.F.; Mantovani, M.S. In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 2011, 10, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, S.; Hur, J.; Sung, J.; Huh, J.; Moon, C.; Hui, J. In vitro Antigenotoxic Activity of Novel Ginseng Saponin Metabolites Formed by Intestinal Bacteria. Planta Med. 1998, 64, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Saini, A.; Kaur, I.P. Ginseng extract exhibits antimutagenic activity against induced mutagenesis in various strains of Salmonella typhimurium. Indian J. Exp. Biol. 2006, 44, 838–841. [Google Scholar] [PubMed]
- Bailleul, B.; Daubersies, P.; Galiègue-Zouitina, S.; Loucheux-Lefebvre, M.H. Molecular Basis of 4-Nitroquinoline 1-Oxide Carcinogenesis. Jpn. J. Cancer Res. 1989, 80, 691–697. [Google Scholar] [CrossRef]
- Nunoshiba, T.; Demple, B. Potent Intracellular Oxidative Stress Exerted by the Carcinogen 4-Nitroquinoline-N-oxide. Cancer Res. 1993, 53, 3250–3252. [Google Scholar]
- Skrzypczak, A.; Przystupa, N.; Zgadzaj, A.; Parzonko, A.; Sykłowska-Baranek, K.; Paradowska, K.; Nałęcz-Jawecki, G. Antigenotoxic, anti-photogenotoxic and antioxidant activities of natural naphthoquinone shikonin and acetylshikonin and Arnebia euchroma callus extracts evaluated by the umu-test and EPR method. Toxicol. In Vitro 2015, 30, 364–372. [Google Scholar] [CrossRef]
- Dueñas-García, I.; Heres-Pulido, M.; Arellano-Llamas, M.; la Cruz-Núñez, J.D.; Cisneros-Carrillo, V.; Palacios-López, C.; Acosta-Anaya, L.; Santos-Cruz, L.; Castañeda-Partida, L.; Durán-Díaz, A. Lycopene, resveratrol, vitamin C and FeSO4 increase damage produced by pro-oxidant carcinogen 4-nitroquinoline-1-oxide in Drosophila melanogaster: Xenobiotic metabolism implications. Food Chem. Toxicol. 2017, 103, 233–245. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Rad. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Maurya, D.; Devasagayam, T. Antioxidant and prooxidant nature of hydroxycinnamic acid derivates ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Sergediene, E.; Jönsson, K.; Szymusiak, H.; Tyrakowska, B.; Rietjens, I.; Cenas, N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: Description of quantitative structure-activity relationships. FEBS Lett. 2000, 462, 392–396. [Google Scholar] [CrossRef]
- Bhat, S.; Azmi, A.; Hadi, S. Prooxidant DNA breakage induced by caffeic acid in human peripheral lymphocytes: Involvement of endogenous copper and a putative mechanism for anticancer properties. Toxicol. Appl. Pharm. 2007, 218, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Cohen, M.; Grace, S.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Heim, K.; Tagliaferro, A.; Bobilya, D. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Yamada, J.; Tomita, Y. Antimutagenic Activity of Caffeic Acid and Related Compounds. Biosci. Biotech. Bio. 1996, 60, 328–329. [Google Scholar] [CrossRef]
- Abraham, S.K.; Schupp, N.; Schmid, U.; Stopper, H. Antigenotoxic effects of the phytoestrogen pelargonidin chloride and the polyphenol chlorogenic acid. Mol. Nutr. Food Res. 2007, 51, 880–887. [Google Scholar] [CrossRef]
- Abraham, S.K.; Eckhardt, A.; Oli, R.G.; Stopper, H. Analysis of in vitro chemoprevention of genotoxic damage by phytochemicals, as single agents or as combinations. Mut. Res. 2012, 744, 117–124. [Google Scholar] [CrossRef]
- Tomasz, M. Mitomycin C: Small, fast and deadly (but very selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar] [CrossRef]
- Pan, S.S.; Iracki, T.; Bachur, N.R. DNA alkylation by enzyme-activated mitomycin C. Mol. Pharmacol. 1986, 29, 622–628. [Google Scholar]
- Dusre, L.; Covey, J.M.; Collins, C.; Sinha, B.K. DNA damage, cytotoxicity and free radical formation by mitomycin C in human cells. Chem.-Biol. Interact. 1989, 71, 63–78. [Google Scholar] [CrossRef]
- Veres, Z.; Török, G.; Tóth, É; Vereczkey, L.; Jemnitz, K. The spectrum of enzymes involved in activation of 2-aminoanthracene varies with the metabolic system applied. Mutat. Res. 2005, 586, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Linsmaier, E.; Skoog, F. Organic Growth Factor Requirements of Tobacco Tissue Cultures. Physiol. Plantarum. 1965, 18. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthova, J.; Malachova, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2016, 73. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Oda, Y.; Nakamura, S.I.; Oki, I.; Kato, T.; Shinagawa, H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res. 1985, 147, 219–229. [Google Scholar] [CrossRef]
- Reifferscheid, G.; Heil, J.; Oda, Y.; Zahn, R. A microplate version of the SOS/umu-test for rapid detection of genotoxins and genotoxic potentials of environmental samples. Mutat. Res. 1991, 253, 215–222. [Google Scholar] [CrossRef]
- Water Quality — Determination of the Genotoxicity of Water and Waste Water Using the umu-Test; Standard; ISO: Geneva, Switzerland, 2000.
- Oppenländer, T. A Comprehensive Photochemical and Photophysical Assay Exploring the Photoreactivity of Drugs. Chimia 1988, 42, 331–342. [Google Scholar] [CrossRef]
- Skrzypczak, A.; Bonisławska, A.; Nałęcz-Jawecki, G.; Kobylińska, J.; Koszyk, I.; Demkowicz, K.; Sawicki, J. Short-term bacterial tests as a convenient method for screening assessment of photogenotoxic properties of pharmaceuticals. Fresen. Environ. Bull. 2014, 19, 147–153. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

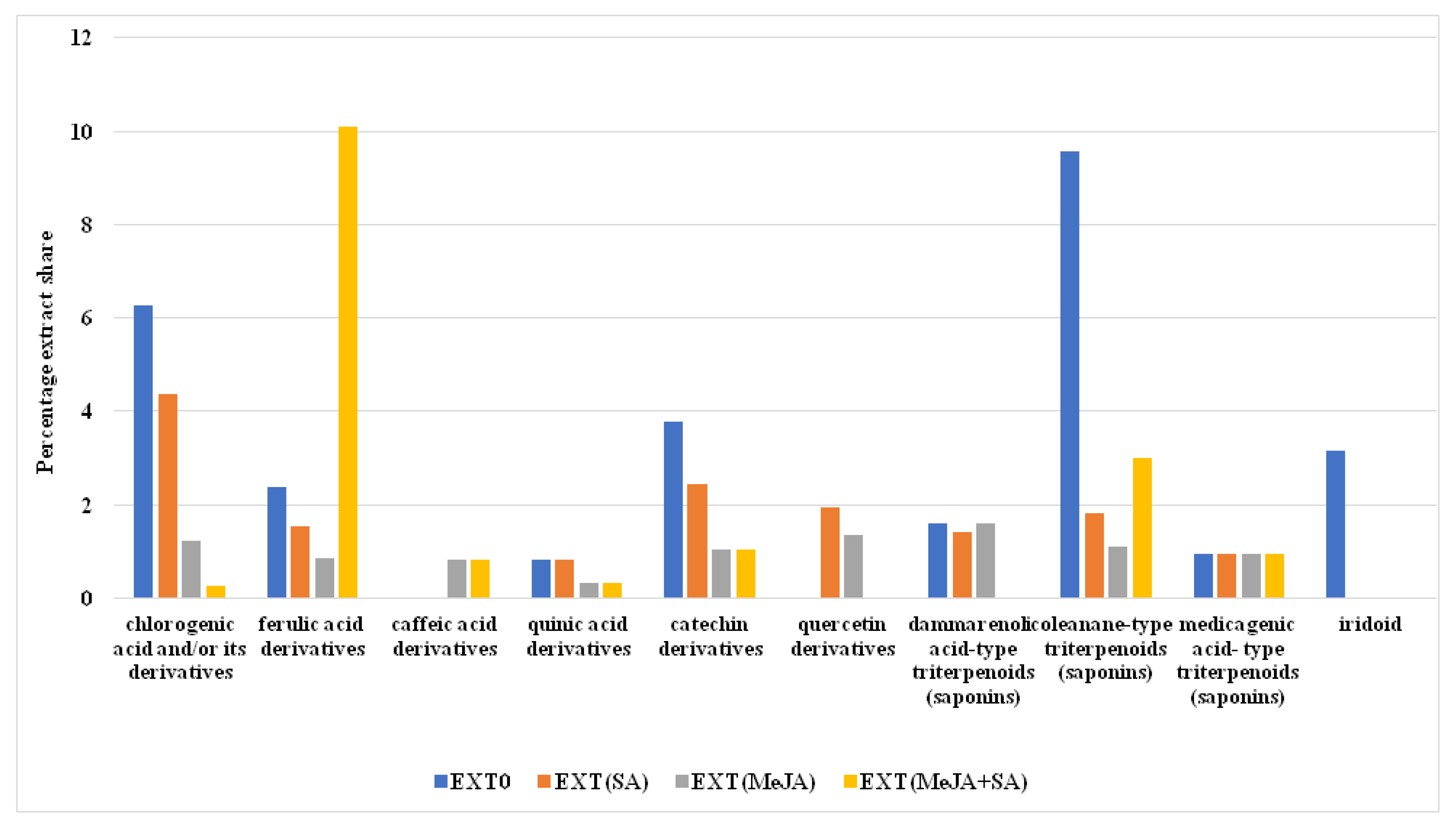
| Extract Type | CGA mg/g DW | CA mg/g DW | FA mg/g DW | Total Content Phenolics mg GAE/g DW | Total Content Flavonoids mg QE/g DW |
|---|---|---|---|---|---|
| EXT0 | 1.18 ± 0.07 * | 0.6 ± 0.13 | 0.02± 0.001 | 1.5 ± 0.35 * | 1.0 ± 0.17 * |
| EXT(SA) | 2.8 ± 0.13 | 0.8 ± 0.22 | 0.07 ± 0.08 | 3.9 ± 0.53 * | 2.5 ± 0.35 * |
| EXT(MeJA) | 3.1 ± 0.24 | 0. 8 ± 0.29 | 0.09 ± 0.01 | 5.3 ± 0.49 * | 3.4 ± 0.35 * |
| EXT(MeJA+SA) | 4.2± 0.17 * | 0.9 ± 0.23 | 0.1 ± 0.23 | 7.8 ± 0.21 * | 4.3 ± 0.27 * |
| IR (mean ± SD) | Tested Material Concentration [mg/mL] | ||||
|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1 | 2 | |
| EXT0 (−S9) EXT0 (+S9) EXT(SA) (−S9) EXT(SA) (+S9) EXT(MeJA) (−S9) EXT(MeJA) (+S9) EXT(SA+MeJA) (−S9) EXT(SA+MeJA) (+S9) | 0.9 ± 0.10 | 1.0 ± 0.25 1.1 ± 0.20 0.96 ± 0.08 0.99 ± 0.07 0.7 ± 0.89 0.97 ± 0.08 0.7 ± 0.77 1.0 ± 0.13 | 1.1 ± 0.43 1.05 ± 0.03 1.17 ± 0.07 1.04 ± 0.05 1.4 ± 0.28 0.99 ± 0.06 1.1 ± 0.16 1.05 ± 0.05 | 1.0 ± 0.16 1.05 ± 0.06 1.16 ± 0.05 1.1 ± 0.15 1.3 ± 0.19 1.01 ± 0.08 1.2 ± 0.20 1.00 ± 0.02 | 0.97 ± 0.08 1.18 ± 0.09 1.2 ± 0.31 1.16 ± 0.09 1.0 ± 0.16 1.20 ± 0.06 1.2 ± 0.25 |
| IR (mean±SD) | |
|---|---|
| CGA (−S9) CGA (+S9) FA (−S9) FA (+S9) CA (−S9) CA (+S9) | 1.3 ± 0.19 1.0 ± 0.21 1.2 ± 0.10 1.1 ± 0.14 1.1 ± 0.16 1.0 ± 0.11 |
| Concentration of EXTs [mg/mL], PhAs [g/mL] | 4NQO 0.25 [g/mL] | 4NQO 0.05 [g/mL] | MMC 0.02 [g/mL] | MMC 0.004 [g/mL] | ||||
|---|---|---|---|---|---|---|---|---|
| IR (mean ± SD) | Antigenotox. | IR (mean ± SD) | Antigenotox. | IR (mean ± SD) | Antigenotox. | IR (mean ± SD) | Antigenotox. | |
| 0 (control) EXT0 2 1 0.5 0.25 EXT(SA) 2 1 0.5 0.25 0.125 EXT(MeJA) 2 1 0.5 0.25 EXT(MeJA+SA) 2 1 0.5 0.25 CGA 10 5 2.5 1.25 0.625 0.3125 FA 10 5 2.5 1.25 0.625 0.3125 CA 10 5 2.5 1.25 0.625 0.3125 | 9 ± 2.6 8 ± 1.2 10 ± 2.7 8 ± 2.7 7 ± 1.4* not tested 11 ± 3.0* 10 ± 2.5 7 ± 1.7* 8 ± 1.6 13 ± 2.4* 11 ± 2.6* 12 ± 2.2* 14 ± 4.1* 13 ± 1.2* 11 ± 2.9* 11 ± 5.1 11 ± 5.1 11 ± 2.6 11 ± 3.0 10 ± 1.4 9 ± 1.8 10 ± 3.0 8 ± 2.8 12 ± 2.2 11 ± 2.5 9 ± 1.5 9 ± 1.7 8 ± 2.4 8 ± 2.5 11 ± 1.8 10 ± 1.5 9 ± 1.2 8 ± 1.6 8 ± 2.3 7 ± 2.1* | - 20% −25% 20% −47% −26% −32% −53% −45% −24% 25% | 3.7 ± 0.99 3.7 ± 0.52 4.3 ± 0.96* 3.5 ± 0.83 3.0 ± 0.36 not tested 5.2 ± 0.56* 4.3 ± 0.66 3.1 ± 0.34 2.9 ± 0.51 4.9 ± 0.42* 4 ± 1.0 4 ± 1.2 4 ± 1.4 4.8 ± 0.46* 4.9 ± 0.31* 5.1 ± 0.33* 5.4 ± 0.37* 4.1 ± 0.96 3.9 ± 0.83 4.0 ± 0.85 3.7 ± 0.78 3.4 ± 0.79 3.8 ± 0.96 4.0 ± 0.70 3.6 ± 0.67 3.6 ± 0.75 3.8 ± 0.75 3.5 ± 0.77 3.9 ± 0.97 3.9 ± 0.99 3.7 ± 0.69 3.6 ± 0.47 3.6 ± 0.78 3.3 ± 0.64 4 ± 1.0 | - −17% −43% −33% −30% −35% −39% −48% | 5.3 ± 0.70 6 ± 1.0 6 ± 1.5 6 ± 1.3 6 ± 1.3 5 ± 1.2 4.9 ± 0.94* 5 ± 1.1* 6 ± 1.2 not tested 5 ± 1.6* 5 ± 1.4 6 ± 1.1 7 ± 1.5* 5 ± 1.7 5 ± 1.0 6 ± 1.5 6 ± 1.0 4 ± 1.1 4 ± 1.5 5 ± 1.1 4.5 ± 0.86 5 ± 1.1 5 ± 1.1 5 ± 1.2 5 ± 1.2 5 ± 1.2 4.8 ± 0.71 5 ± 1.1* 5.2 ± 0.55 5 ± 1.2 5 ± 1.1 5 ± 1.2 5 ± 1.1 5.1 ± 0.91 5.0 ± 0.44 | - 16% 16% 14% −16% −14% | 2.4 ± 0.42 2.1 ± 0.39 2.4 ± 0.47 2.3 ± 0.32 2.0 ± 0.13* 2.3 ± 0.27 2.4 ± 0.39 2.2 ± 0.40 2.4 ± 0.45 not tested 2.2 ± 0.40 2.3 ± 0.37 2.5 ± 0.43 2.6 ± 0.41 2.5 ± 0.53 2.6 ± 0.34 2.6 ± 0.38 2.6 ± 0.28 2.2 ± 0.40 2.4 ± 0.55 2.5 ± 0.71 2.7 ± 0.85 2.6 ± 0.81 2.2 ± 0.57 2.3 ± 0.51 2.5 ± 0.62 2.6 ± 0.93 2.4 ± 0.71 2.5 ± 0.98 2.2 ± 0.70 2.3 ± 0.67 2.7 ± 0.81 2.5 ± 0.75 2.4 ± 0.88 2.6 ± 0.80 2.6 ± 0.74 | - 17% |
| Tested Extracts Concentration (mg/mL) | 2AA 10 (g/mL) | 2AA 1 (g/mL) | ||
|---|---|---|---|---|
| IR (mean ± SD) | Antigenotox. | IR (mean ± SD) | Antigenotox. | |
| 0 (control) EXT0 2 1 0.5 0.25 EXT(SA) 2 1 0.5 0.25 EXT(MeJA) 2 1 0.5 0.25 EXT(MeJA+SA) 2 1 0.5 0.25 | 6 ± 1.5 3.6 ± 0.67* 5.8 ± 0.86 6 ± 1.4 6 ± 1.6 3 ± 1.1* 4 ± 1.2* 5 ± 1.5* 5 ± 1.1 2.8 ± 0.42* 3.7 ± 0.70* 4.9 ± 0.87* 5 ± 1.2 2.0 ± 0.24* 3.2 ± 0.34* 4.3 ± 0.45* 4.7 ± 0.95* | - 38% 43% 29% 16% 51% 36% 16% 66% 45% 26% 19% | 4.5 ± 0.99 2.9 ± 0.77* 4.4 ± 0.57 4 ± 1.0 5 ± 1.4 2.6 ± 0.89* 3.4 ± 0.88* 4.1 ± 0.93 4.4 ± 0.56 2.4 ± 0.21* 3.2 ± 0.56* 3.8 ± 0.75* 4 ± 1.2 1.7 ± 0.27* 2.7 ± 0.34* 3.4 ± 0.46* 4.1 ± 0.57 | - 35% 41% 24% 45% 28% 16% 63% 39% 24% |
| Tested Extracts Concentration (mg/mL) | 2AA 10 (g/mL) | 2AA 1 (g/mL) | ||
|---|---|---|---|---|
| IR (mean ± SD) | Antigenotox. | IR (mean ± SD) | Antigenotox. | |
| 0 (control) CGA 10 5 2.5 1.25 0.625 0.3125 FA 10 5 2.5 1.25 0.625 0.3125 CA 10 5 2.5 1.25 0.625 0.3125 | 4 ± 1.3 4.6 ± 0.66* 5 ± 1.5* 3.3 ± 0.80 3.2 ± 0.77 3.2 ± 0.88 2.9 ± 0.43 5 ± 1.4 4 ± 1.3 3.2 ± 0.45 3.3 ± 0.63 3.1 ± 0.75 2.9 ± 0.43 3.7 ± 0.80 3.6 ± 0.89 3.2 ± 0.51 2.7 ± 0.34* 3.4 ± 0.90 2.8 ± 0.60 | - −29% −36% 24% | 3.37 ± 0.72 4 ± 1.2* 5 ± 1.4* 3.2 ± 0.47 3.6 ± 0.95 3.1 ± 0.60 3 ± 1.0 4 ± 1.0 3.4 ± 0.96 2.8 ± 0.58 2.9 ± 0.66 2.7 ± 0.37* 3.1 ± 0.73 3.8 ± 0.85 3.7 ± 0.77 3.1 ± 0.53 3.1 ± 0.29 3.1 ± 0.55 3 ± 1.0 | - −27% −34% 19% |
| Tested Concentration EXT (mg/mL) PhAs (g/mL) | CPZ 10 (g/mL) | CPZ 5 (g/mL) | ||
|---|---|---|---|---|
| IR (mean ± SD) | Anti-Photogenotox. | IR (mean ± SD) | Anti-Photogenotox. | |
| 0 (control) EXT(MeJA + SA) 1 0.5 0.25 0.125 CGA 10 5 2.5 1.25 FA 10 5 2.5 1.25 CA 10 5 2.5 1.25 | 5.1 ± 0.78 3.3 ± 0.65* 4.6 ± 0.75 5 ± 1.0 4 ± 1.3* 4.8 ± 0.43 5.0 ± 0.70 4.5 ± 0.58* 4.6 ± 0.83 4.3 ± 0.46* 4.5 ± 0.73 4 ± 1.1* 4.1 ± 0.97* 4.9 ± 0.90 5 ± 1.2 4.5 ± 0.49* 4.9 ± 0.62 | - 34% 14% 11% 15% 14% 18% 10% | 3.6 ± 0.40 2.3 ± 0.38* 3.1 ± 0.58* 3.0 ± 0.80* 3.2 ± 0.82 3.4 ± 0.26 3.3 ± 0.34 3.1 ± 0.53* 3.3 ± 0.50 3.2 ± 0.20* 3.0 ± 0.37* 3.0 ± 0.41* 3.0 ± 0.43* 3.4 ± 0.25 3.3 ± 0.33 3.2 ± 0.40* 3.4 ± 0.54 | - 37% 13% 16% 13% 11% 15% 20% 19% 11% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figat, R.; Śliwińska, A.; Stochmal, A.; Soluch, A.; Sobczak, M.; Zgadzaj, A.; Sykłowska-Baranek, K.; Pietrosiuk, A. Antigenotoxic, Anti-photogenotoxic, and Antioxidant Properties of Polyscias filicifolia Shoots Cultivated In Vitro. Molecules 2020, 25, 1090. https://doi.org/10.3390/molecules25051090
Figat R, Śliwińska A, Stochmal A, Soluch A, Sobczak M, Zgadzaj A, Sykłowska-Baranek K, Pietrosiuk A. Antigenotoxic, Anti-photogenotoxic, and Antioxidant Properties of Polyscias filicifolia Shoots Cultivated In Vitro. Molecules. 2020; 25(5):1090. https://doi.org/10.3390/molecules25051090
Chicago/Turabian StyleFigat, Ramona, Anita Śliwińska, Anna Stochmal, Agata Soluch, Magdalena Sobczak, Anna Zgadzaj, Katarzyna Sykłowska-Baranek, and Agnieszka Pietrosiuk. 2020. "Antigenotoxic, Anti-photogenotoxic, and Antioxidant Properties of Polyscias filicifolia Shoots Cultivated In Vitro" Molecules 25, no. 5: 1090. https://doi.org/10.3390/molecules25051090
APA StyleFigat, R., Śliwińska, A., Stochmal, A., Soluch, A., Sobczak, M., Zgadzaj, A., Sykłowska-Baranek, K., & Pietrosiuk, A. (2020). Antigenotoxic, Anti-photogenotoxic, and Antioxidant Properties of Polyscias filicifolia Shoots Cultivated In Vitro. Molecules, 25(5), 1090. https://doi.org/10.3390/molecules25051090







