Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail
Abstract
1. Introduction
2. Results and Discussion
2.1. Olfactometry Analysis of Mango and Vodka Cocktail
2.2. Quantitative Analysis and OAV of Odor-active Compounds
2.3. Olfactory Properties of Compounds
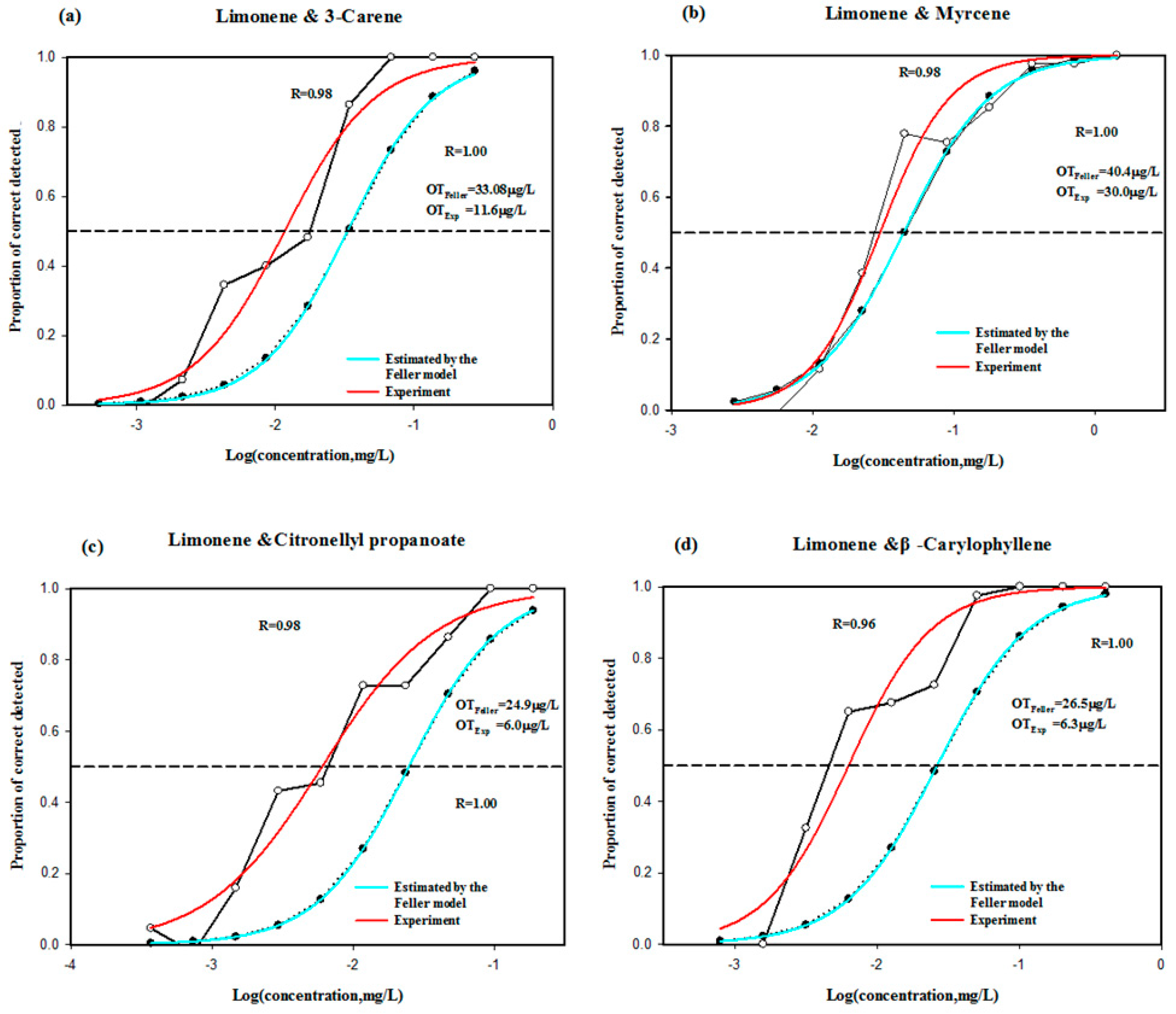
2.4. Odor Interaction Effects Evaluated by γ
3. Materials and Methods
3.1. Materials
3.2. Chemicals
3.3. Solid Phase Micro Extraction (SPME) Absorption of Aroma Compounds
3.4. Sensory Analyses
3.5. SPME-GC-FID-O Analysis of Mango and Vodka Cocktail
3.6. SPME-GC-MS Analysis of Mango and Vodka Cocktail
3.7. Calibration of Standard Curves
3.8. Odor Threshold and OAV Analysis
3.9. The Measurement of γ
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Czerny, M.; Brueckner, R.; Kirchhoff, E.; Schmitt, R.; Buettner, A. The influence of molecular structure on odor qualities and odor detection thresholds of volatile alkylated phenols. Chem. Senses. 2011, 36, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; She, Y.; Xiao, Z.; Xu, L.; Niu, Y.; Zhu, J. Investigations on the Aroma Active Compounds in Fresh and Aged Longjing Tea by SPME/GC-MS/GC-O/OAV. Food Ind. 2016, 37, 279–285. [Google Scholar]
- Wu, C.; Liu, J.; Yan, L.; Chen, H.; Shao, H.; Meng, T. Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Atanasova, B.; Thomas-Danguin, T.; Langlois, D.; Nicklaus, S.; Etievant, P. Perceptual interactions between fruity and woody notes of wine. Flavour Frag J. 2004, 19, 476–482. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: a flavour chemistry view. Part 1: intensity and detectability. A review. Flavour Frag J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; de Revel, G.; Teissedre, P. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef]
- Eri, S.; Costa, N.; Trinnaman, L. The Flavor of the Classic Margarita Cocktail. ACS Natl. Meet. Book Abstr. 2006, 179–191. [Google Scholar]
- Xin, S.; Zhu, N.; Wang, X.; Zhou, L. Comparative Study of Chinese Liquor and Cocktail Base Wine Based on Electronic Tongue Assessment and Sensory Evaluation. Liquor Making Sci. Tech. 2012, 7, 35–38. [Google Scholar]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Commercial Rums by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef]
- Johnson, A.; Hopfer, H.; Heymann, H.; Ebeler, S. Aroma Perception and Chemistry of Bitters in Whiskey Matrices: Modeling the Old-Fashioned. Chemosens Percep. 2017, 1–14. [Google Scholar] [CrossRef]
- Pino, J.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Sumby, K.; Jiranek, V.; Grbin, P. Ester synthesis and hydrolysis in an aqueous environment, and strain specific changes during malolactic fermentation in wine with Oenococcus oeni. Food Chem. 2013, 141, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Quijano, C.; Salamanca, G.; Pino, J. Aroma volatile constituents of Colombian varieties of mango (Mangifera indica L). Flavour Frag J. 2007, 22, 401–406. [Google Scholar] [CrossRef]
- Reddy, L.; Kumar, Y.; Reddy, O. Analysis of volatile aroma constituents of wine produced from Indian mango (Mangifera indica L.) by GC-MS. Indian J. Microbiol. 2010, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yao, Z.; Xiao, Q.; Xiao, Z.; Ma, N.; Zhu, J. Characterization of the key aroma compounds in different light aroma type Chinese liquors by GC-olfactometry, GC-FPD, quantitative measurements, and aroma recombination. Food Chem. 2017, 233, 204–215. [Google Scholar] [CrossRef]
- Cho, S.; Kim, J.; Kim, S.; Park, S.; Kim, J.; Choi, I. A comparative study on the fuel properties of biodiesel from woody essential oil depending on terpene composition. Fuel 2018, 218, 375–384. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, H.; Yao, Q.; Wang, S.; Wei, C.; Ma, X. Changes of Aroma Components in‘Keitt’Mango during the Postharvest Period. Agric. Biotechnol. 2014, 3, 14–17. [Google Scholar]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the differences in the aroma of cherry wines from different price segments using gas chromatography–mass spectrometry, odor activity values, sensory analysis, and aroma reconstitution. Food Sci. Biotechno. 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, Z. Characterization of the Major Odor-Active Compounds in Dry Jujube Cultivars by Application of Gas Chromatography-Olfactometry and Odor Activity Value. J. Agric. Food Chem. 2018, 7722–7734. [Google Scholar] [CrossRef] [PubMed]
- Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media; Boelens Aroma Chemical Information Service: Huizen, The Netherlands, 2003. [Google Scholar]
- Niu, Y.; Yao, Z.; Xiao, Z.; Zhu, G.; Zhu, J.; Chen, J. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 2018, 113, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Klotzbücher, B.; Wurzbache, M.; Hanke, S.; Kattein, U.; Back, W.; Becker, T.; Krottenthaler, M. A new validation of relevant substances for the evaluation of beer aging depending on the employed boiling system. J. Inst. Brew. 2010, 116, 41–48. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Xiao, Z. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef]
- Atanasova, B.; Thomas-Danguin, T.; Chabanet, C.; Langlois, D.; Nicklaus, S.; Etievant, P. Perceptual interactions in odour mixtures: odour quality in binary mixtures of woody and fruity wine odorants. Chem Senses 2005, 30, 209–217. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.; Abraham, M. Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pharmacol. Biochem. Behav. 2008, 89, 279–291. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| NO. | RI | Compound | Odor Descriptor | Aroma Intensity | |
|---|---|---|---|---|---|
| HP-Wax | DB-5 | ||||
| 1 | 1028 | 776 | Methyl 2-methylbutanoate | sweet, fruity, fatty, green | 3.7 |
| 2 | 1036 | 942 | (1R)-(+)-a-pinene | woody | 0.2 |
| 3 | 1034 | 809 | Ethyl butanoate | fruity | 3.1 |
| 4 | 1077 | 948 | Camphene | woody, herbal | 1.0 |
| 5 | 1111 | 986 | β-Pinene | dry woody, green | 1.3 |
| 6 | 1123 | 870 | Isoamyl acetate | sweet, fruity | 0.8 |
| 7 | 1156 | 1012 | 3-carene | sweet, fruity | 4.5 |
| 8 | 1153 | 987 | Myrcene | woody, green | 4.2 |
| 9 | 1196 | 1007 | Methyl hexanoate | fruity | 2.0 |
| 10 | 1207 | 1023 | Limonene | citrus, sweet | 4.7 |
| 11 | 1259 | 1064 | γ-terpinene | woody, citrus | 2.0 |
| 12 | 1266 | 1029 | (Z)-β-ocimene | warm floral, herb, sweet | 3.6 |
| 13 | 1281 | 1050 | Isoamyl butanoate | fruity, green | 3.8 |
| 14 | 1288 | 1018 | Hexyl acetate | fruity, green, sweet | 3.0 |
| 15 | 1295 | 1100 | Terpinolene | woody, sweet, citrus | 3.4 |
| 16 | 1336 | 1005 | (Z)-3-Hexenyl Acetate | green, sweet, fruity | 1.0 |
| 17 | 1368 | 872 | 1-Hexanol | sweet, green | 1.3 |
| 18 | 1368 | Allyl hexanoate | sweet, fruity | 3.1 | |
| 19 | 1398 | 865 | Leaf alcohol | green | 0.6 |
| 20 | 1453 | 1203 | Ethyl octoate | fruity, wine | 3.8 |
| 21 | 1490 | 964 | Benzaldehyde | sweet, bitter almond | 0.2 |
| 22 | 1542 | 1106 | Linalool | green, floral, sweet | 2.0 |
| 23 | 1593 | 1302 | Menthyl acetate | minty, fruity | 0.2 |
| 24 | 1588 | 1290 | Isobornyl acetate | herb, woody, sweet, minty | 0.1 |
| 25 | 1603 | 1415 | β-caryophyllene | sweet, woody, spice | 4.0 |
| 26 | 1621 | 1392 | Hexyl hexanoate | herbal, green, fruity | 0.4 |
| 27 | 1660 | 1391 | Ethyl caprate | sweet, fruity | 0.3 |
| 28 | 1658 | 1393 | (Z)-3-Hexenyl hexanoate | fruity, green | 2.8 |
| 29 | 1744 | 1449 | Citronellyl propanoate | floral, green, fruity | 4.1 |
| 30 | 1825 | 1248 | Nerol | sweet, citrus | 0.8 |
| 31 | 1834 | Allyl cyclohexyl propionate | sweet, fruity | 3.4 | |
| 32 | 1853 | 1271 | Geraniol | sweet, floral, fruity | 1.1 |
| 33 | 1851 | 1338 | Benzyl butanoate | fruity | 0.3 |
| 34 | 1944 | 1276 | γ-Octanoic lactone | fruity, fatty, sweet | 3.2 |
| 35 | 2187 | 1509 | γ-Decalactone | sweet, fruity, fatty | 3.9 |
| 36 | 1376 | Neryl acetate | floral | 2.3 | |
| Compound | Standard Curve | R2 | Range (L–H) (μg/L) | Wine | ||
|---|---|---|---|---|---|---|
| Slope | Intercept | Av (μg/L) | RSDa (%) | |||
| Methyl 2-methylbutanoate | 0.588 | −0.0277 | 0.998 | 5.62–2249 | 195 | 3 |
| (1R)-(+)-a-pinene | 0.380 | −0.00710 | 0.998 | 33.1–13,248 | 1364 | 7 |
| Ethyl butanoate | 0.312 | 0.00840 | 0.981 | 4.70–1880 | 205 | 2 |
| Camphene | 0.123 | −0.000100 | 0.982 | 0.445–178 | 56.7 | 8 |
| β-Pinene | 0.0560 | 0.00370 | 0.988 | 2.97–1188 | 752 | 5 |
| Isoamyl acetate | 7.36 | 0.00260 | 0.998 | 1.39–554 | 2.55 | 3 |
| 3-carene | 0.0830 | −0.00180 | 0.993 | 29.4–11,772 | 5460 | 3 |
| Myrcene | 0.0852 | 0.00280 | 0.990 | 60.4–24,150 | 10,900 | 7 |
| Methyl hexanoate | 1.67 | 0.00440 | 0.998 | 3.42–1369 | 29 | 2 |
| Limonene | 0.0951 | 0.108 | 0.999 | 81.6–32,627 | 12,100 | 6 |
| γ-terpinene | 0.188 | 0.00160 | 0.995 | 4.71–1885 | 379 | 2 |
| (Z)-β-ocimene | 0.184 | −0.000700 | 0.989 | 6.57–2629 | 556 | 8 |
| Isoamyl butanoate | 2.07 | 0.0558 | 0.996 | 12.1–4821 | 62.8 | 3 |
| Hexyl acetate | 2.63 | −0.0214 | 0.997 | 9.20–3688 | 62 | 6 |
| Terpinolene | 0.278 | 0.00280 | 0.982 | 5.38–2151 | 288 | 7 |
| (Z)-3-Hexenyl Acetate | 1.52 | 0.108 | 0.999 | 29.1–11,640 | 225 | 5 |
| 1-Hexanol | 0.259 | −0.000500 | 0.999 | 3.92–1570 | 236 | 3 |
| Allyl hexanoate | 2.56 | −0.0317 | 0.996 | 33.9–13,566 | 334 | 8 |
| Leaf alcohol | 0.134 | 0.00570 | 0.981 | 4.59–1837 | 487 | 6 |
| Ethyl octoate | 1.86 | 0.0455 | 0.996 | 10.7–4294 | 64.6 | 6 |
| Benzaldehyde | 1.62 | −0.00210 | 0.997 | 0.266–106 | 3.83 | 2 |
| Linalool | 2.95 | 0.137 | 0.997 | 11.6–4640 | 14.1 | 3 |
| Menthyl acetate | 2.88 | 0.0427 | 0.996 | 3–1199 | 1.23 | 5 |
| Isobornyl acetate | 11.4 | −0.125 | 0.994 | 1.44–576 | 12.9 | 6 |
| β-caryophyllene | 0.707 | −0.111 | 0.998 | 32.5–13,011 | 867 | 2 |
| Hexyl hexanoate | 0.826 | 0.00110 | 0.999 | 24.8–9905 | 461 | 2 |
| Ethyl caprate | 1.26 | 0.000400 | 0.997 | 0.371–148 | 4.22 | 4 |
| (Z)-3-Hexenyl hexanoate | 2.02 | 0.0128 | 0.997 | 3.64–1457 | 21.5 | 8 |
| Citronellyl propanoate | 0.435 | −0.00170 | 0.988 | 4.62–1846 | 11.9 | 7 |
| Nerol | 2.08 | −0.0208 | 0.993 | 8.57–3426 | 73.5 | 5 |
| Allyl cyclohexylpropionate | 2.57 | −0.0289 | 0.992 | 26.2–10,475 | 168 | 7 |
| Geraniol | 2.95 | −0.0554 | 0.987 | 3.83–1533 | 38.8 | 4 |
| Benzyl butanoate | 3.38 | 0.0859 | 0.995 | 21.8–8703 | 73.9 | 2 |
| γ-Octanoic lactone | 0.653 | −0.0102 | 0.981 | 0.401–160 | 143 | 7 |
| γ-Decalactone | 1.53 | −0.195 | 0.986 | 5.39–2155 | 131 | 9 |
| Neryl acetate | 2.55 | 0.336 | 0.993 | 29.1–11,640 | 44.2 | 6 |
| NO | Compound | Odor Thresholda (μg/L) | OAV |
|---|---|---|---|
| 1 | Methyl 2-methylbutanoate | 0.4 | 487 |
| 2 | (1R)-(+)-a-pinene | 26000 | <1 |
| 3 | Ethyl butanoate | 44 | 10 |
| 4 | Camphene | 1860 | <1 |
| 5 | β-Pinene | 1500 | <1 |
| 6 | Isoamyl acetate | 30 | <1 |
| 7 | 3-carene | 44 | 124 |
| 8 | Myrcene | 100 | 109 |
| 9 | Methyl hexanoate | 87 | <1 |
| 10 | Limonene | 10 | 1219 |
| 11 | γ-terpinene | 1000 | <1 |
| 12 | (Z)-β-ocimene | 34 | 16 |
| 13 | Isoamyl butanoate | 0.13 | 483 |
| 14 | Hexyl acetate | 10 | 6 |
| 15 | Terpinolene | 41 | 7 |
| 16 | (Z)-3-Hexenyl Acetate | 1500 | <1 |
| 17 | 1-Hexanol | 5200 | <1 |
| 18 | Allyl hexanoate | 40 | 8 |
| 19 | Leaf alcohol | 1000 | <1 |
| 20 | Ethyl octoate | 5 | 32 |
| 21 | Benzaldehyde | 5000 | <1 |
| 22 | Linalool | 15 | <1 |
| 23 | Menthyl acetate | 2000 | <1 |
| 24 | Isobornyl acetate | 1800 | <1 |
| 25 | β-caryophyllene | 64 | 14 |
| 26 | Hexyl hexanoate | 6400 | <1 |
| 27 | Ethyl caprate | 200 | <1 |
| 28 | (Z)-3-Hexenyl hexanoate | 1.4 | 15 |
| 29 | Citronellyl propanoate | 1.8 | 7 |
| 30 | Nerol | 500 | <1 |
| 31 | Allyl cyclohexyl propionate | 2 | 84 |
| 32 | Geraniol | 100 | <1 |
| 33 | Benzyl butanoate | 16 | 5 |
| 34 | γ-Octanoic lactone | 14 | 10 |
| 35 | γ-Decalactone | 10 | 13 |
| 36 | Neryl acetate | 1.6 | 28 |
| OIbmixture | γa Values | |||
|---|---|---|---|---|
| 3-carene | Myrcene | β-caryophyllene | Citronellyl Propanoate | |
| 2.00 | 3.60 | 1.59 | 1.67 | 1.52 |
| 3.00 | 2.43 | 0.92 | 1.87 | 3.84 |
| 4.00 | 1.85 | 1.95 | 2.31 | 5.29 |
| 5.00 | 1.06 | 1.10 | 3.25 | 8.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules 2020, 25, 1083. https://doi.org/10.3390/molecules25051083
Niu Y, Wang P, Xiao Q, Xiao Z, Mao H, Zhang J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules. 2020; 25(5):1083. https://doi.org/10.3390/molecules25051083
Chicago/Turabian StyleNiu, Yunwei, Pinpin Wang, Qing Xiao, Zuobing Xiao, Haifang Mao, and Jun Zhang. 2020. "Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail" Molecules 25, no. 5: 1083. https://doi.org/10.3390/molecules25051083
APA StyleNiu, Y., Wang, P., Xiao, Q., Xiao, Z., Mao, H., & Zhang, J. (2020). Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules, 25(5), 1083. https://doi.org/10.3390/molecules25051083




