An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review
Abstract
1. Introduction
2. Saponins Elimination Methods
2.1. Wet Methods
2.2. Dry Methods
2.3. Genetic Methods
3. Extraction Methods of Quinoa Saponins
4. Chemical Characterization of Quinoa Saponins
5. Biological Activities of Quinoa Saponins
6. Conclusions
Funding
Conflicts of Interest
References
- Repo-Carrasco-Valencia, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crop. Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Jancurová, M.; Minarovicova, L.; Dandár, A. Quinoa—A review. J. Food. Sci. 2009, 27, 71–79. [Google Scholar]
- Abugoch James, L.E. Chapter 1 Quinoa (Chenopodium quinoa Willd.). Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [PubMed]
- Bhargava, A.; Srivastava, S. Quinoa: Botany, Production and Uses; CABI: Wallingford, UK; Boston, MA, USA, 2013; p. 247. [Google Scholar]
- Bazile, D.; Baudron, F. The dynamics of the global expansion of quinoa growing in view of its high biodiversity. In State of the Art Report of Quinoa in the World in 2013; FAO & CIRAD: Rome, Italy, 2015; pp. 42–55. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 January 2020).
- Coulter, L.; Lorenz, K. Quinoa-composition, nutritional value, food applications. Leb. Wiss. Technol. 1990, 23, 203–207. [Google Scholar]
- Kozioł, M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Quispe, I.; Vega-Galvez, A.; Miranda, M.; Lemus-Mondaca, R.; Lozano, M.; Ah-Hen, K. A Kinetic approach to saponin extraction during washing of quinoa (Chenopodium quinoa Willd.) seeds. J. Food Process. Eng. 2012, 36, 202–210. [Google Scholar] [CrossRef]
- Przybylski, R.; Chauhan, G.; Eskin, N. Characterization of quinoa (Chenopodium quinoa) lipids. Food Chem. 1994, 51, 187–192. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124–982. [Google Scholar] [CrossRef]
- Repo de Carrasco, R.; Zelada, R. Determination of antioxidant capacity and phenolic compounds in Andean cereals: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Rev. Soc. Quim. Peru 2008, 74, 85–99. [Google Scholar]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, A.; Ortega, A.; Trujillo, D.; Benítez, R. Saponins of quinoa (Chenopodium quinoa Willd.): A by-product with high biological potential. Rev. Colomb. Cienc. Quím. Farm. 2016, 45, 438–469. [Google Scholar] [CrossRef]
- Improta, F.; Kellems, R.O. Comparison of raw, washed and polished quinoa (Chenopodium quinoa Willd.) to wheat, sorghum or maize based diets on growth and survival of broiler chicks. Livest. Res. Rural Dev. 2001, 13, 1. [Google Scholar]
- Satheesh, N.; Fanta, S. Review on structural, nutritional and anti-nutritional composition of Teff (Eragrostis tef) in comparison with quinoa (Chenopodium quinoa Willd.). Cogent Food Agric. 2018, 4, 1–27. [Google Scholar] [CrossRef]
- Ward, S.M. Response to selection for reduced grain saponin content in quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 2000, 68, 157–163. [Google Scholar] [CrossRef]
- Telleria Rios, M.; Sgarbieri, V. Evaluacion quimica y biologica de la quinoa (Chenopodium quinoa Willd). Influencia de la extraccion de las saponinas por tratamiento termico. Arch. Latinoam. Nutr. 1978, 28, 254–263. [Google Scholar]
- Meyer, B.N.; Heinstein, P.F.; Burnouf-Radosevich, M.; Delfel, N.E.; McLaughlin, J.L. Bioactivity-directed isolation and characterization of quinoside A: One of the toxic/bitter principles of quinoa seeds (Chenopodium quinoa Willd.). J. Agric. Food Chem. 1990, 38, 205–208. [Google Scholar] [CrossRef]
- Gee, J.M.; Ridout, C.L.; Wortley, G.M.; Hurrell, R.F.; Johnson, I.T. Saponins of quinoa (Chenopodium quinoa): Effects of processing on their abundance -in quinoa products and their biological effects on intestinal mucosal tissue. J. Sci. Food Agric. 1993, 63, 201–209. [Google Scholar] [CrossRef]
- Pappier, U.; Fernandez Pinto, V.; Larumbe, G.; Vaamonde, G. Effect of processing for saponin removal on fungal contamination of quinoa seeds (Chenopodium quinoa Willd.). Int. J. Food Microbiol. 2008, 125, 153–157. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; San Martin, R.; Sanders, M.; Miranda, M.; Lara, E. Characteristics and mathematical modeling of convective drying of quinoa (Chenopodium quinoa Willd.): Influence of temperature on the kinetic parameters. J. Food Process. Preserv. 2010, 34, 945–963. [Google Scholar] [CrossRef]
- Irigoyen, R.M.T.; Giner, S.A. Extraction Kinetics of Saponins from quinoa Seed (Chenopodium quinoa Willd). Int. J. Food Stud. 2018, 7, 76–88. [Google Scholar]
- Ruales, J.; Nair, B. Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa Willd) seeds. Food Chem. 1993, 48, 137–143. [Google Scholar] [CrossRef]
- Nickel, J.; Spanier, L.P.; Botelho, F.T.; Gularte, M.A.; Helbig, E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem. 2016, 209, 139–143. [Google Scholar] [CrossRef]
- Ridout, C.L.; Price, K.R.; DuPont, M.S.; Parker, M.L. Quinoa saponins -analysis and preliminary investigations into the effects of reduction by processing. J. Sci. Food Agric. 1991, 54, 165–176. [Google Scholar] [CrossRef]
- Ruales, J.; Valencia-Chamorro, S.; Nair, B. Effect of processing on the physico chemical characteristics of quinoa flour (Chenopodium quinoa Willd). Starch Starke 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Brady, K.; Ho, C.-T.; Rosen, R.T.; Sang, S.; Karwe, M. Effects of processing on the nutraceutical profile of quinoa. Food Chem. 2007, 100, 1209–1216. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Encina-Zelada, C.; Binaghi, J.; Greco, C.B.; Ronayne de Ferrer, P. Effects of roasting and boiling of quinoa, kiwicha and kañiwa on composition and availability of minerals in vitro. J. Sci. Food Agric. 2010, 90, 2068–2073. [Google Scholar] [CrossRef]
- Armada, M.; Chavarría, J.A.; Trejo, A.V. Diseño y construcción de un prototipo escarificador de quinua. Rev. Argent. Ing. 2013, 1, 53–58. [Google Scholar]
- Dick Mastebroek, H.; Limburg, H.; Gilles, T.; Marvin, H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 2000, 80, 152–156. [Google Scholar] [CrossRef]
- Risi, J.; Galwey, N. The pattern of genetic diversity in the Andean grain crop quinoa (Chenopodium quinoa Willd). I. Associations between characteristics. Euphytica 1989, 41, 147–162. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Hill, J.; Stølen, O. Stability of quantitative traits in quinoa (Chenopodium quinoa). Theor. Appl. Genet. 1996, 93, 110–116. [Google Scholar] [CrossRef]
- Palomino, G.; Hernández, L.T.; de la Cruz Torres, E. Nuclear genome size and chromosome analysis in Chenopodium quinoa and C. berlandieri subsp. nuttalliae. Euphytica 2008, 164, 221. [Google Scholar] [CrossRef]
- Maughan, P.J.; Bonifacio, A.; Jellen, E.N.; Stevens, M.R.; Coleman, C.E.; Ricks, M.; Mason, S.L.; Jarvis, D.E.; Gardunia, B.W.; Fairbanks, D.J. A genetic linkage map of quinoa (Chenopodium quinoa) based on AFLP, RAPD, and SSR markers. Theor. Appl. Genet. 2004, 109, 1188–1195. [Google Scholar] [CrossRef]
- Reynolds, D.J. Genetic Dissection of Triterpenoid Saponin Production in Chenopodium Quinoa Using Microarray Analysis. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2009. [Google Scholar]
- Jarvis, D.E.; Kopp, O.R.; Jellen, E.N.; Mallory, M.A.; Pattee, J.; Bonifacio, A.; Coleman, C.E.; Stevens, M.R.; Fairbanks, D.J.; Maughan, P.J. Simple sequence repeat marker development and genetic mapping in quinoa (Chenopodium quinoa Willd.). J. Genet. 2008, 87, 39–51. [Google Scholar] [CrossRef]
- Maughan, P.J.; Smith, S.M.; Rojas-Beltrán, J.A.; Elzinga, D.; Raney, J.A.; Jellen, E.N.; Bonifacio, A.; Udall, J.A.; Fairbanks, D.J. Single Nucleotide Polymorphism Identification, Characterization, and Linkage Mapping in Quinoa. Plant Genome 2012, 5, 114–125. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Dini, I.; Schettino, O.; Simioli, T.; Dini, A. Studies on the Constituents of Chenopodium quinoa Seeds: Isolation and Characterization of New Triterpene Saponins. J. Agric. Food Chem. 2001, 49, 741–746. [Google Scholar] [CrossRef]
- Güçlü Üstündağ, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Gianna, V.; Manuel Montes, J.; Calandri, E.; Alberto Guzmán, C. Impact of several variables on the microwave extraction of Chenopodium quinoa willd saponins. Int. J. Food Sci. Technol. 2012, 47, 1593–1597. [Google Scholar] [CrossRef]
- Verza, S.G.; Silveira, F.; Cibulski, S.; Kaiser, S.; Ferreira, F.; Gosmann, G.; Roehe, P.M.; Ortega, G.G. Immunoadjuvant activity, toxicity assays, and determination by UPLC/Q-TOF-MS of triterpenic saponins from Chenopodium quinoa seeds. J. Agric. Food Chem. 2012, 60, 3113–3118. [Google Scholar] [CrossRef]
- Lozano, M.; Gonzales, E.; Flores, Y.; Almanza, G.R. Effect in acute inflammation of sapogenin extract and isolated sapogenins from quinoa waste (Chenopodium quinoa Willd). Boliv. J. Chem. 2013, 30, 115–121. [Google Scholar]
- Dini, I.; Tenore, G.C.; Schettino, O.; Dini, A. New Oleanane Saponins in Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 3976–3981. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar] [CrossRef]
- Ma, W.-W.; Heinstein, P.F.; McLaughlin, J.L. Additional toxic, bitter saponins from the seeds of Chenopodium quinoa. J. Nat. Prod. 1989, 52, 1132–1135. [Google Scholar] [CrossRef]
- Zhu, N.; Sheng, S.; Sang, S.; Jhoo, J.W.; Bai, N.; Karwe, M.V.; Rosen, R.T.; Ho, C.T. Triterpene saponins from debittered quinoa (Chenopodium quinoa) Seeds. J. Agric. Food Chem. 2002, 50, 865–867. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography–diode array detection–electrospray ionization–time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Herrera, T.; García-Risco, M.; Fornari, T.; Reglero, G.; Martin, D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Acid hydrolysis of saponin-rich extracts of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich extracts and other bioactive compounds. J. Sci. Food Agric. 2019, 99, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins—Polar isoprenoids with important and diverse biological activities. R. Soc. Chem. 2011, 28, 1261. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Mizui, F.; Kasai, R.; Ohtani, K.; Tanaka, O. Saponins from Bran of quinoa, Chenopodium quinoa Willd. II. Chem. Pharm. Bull. 1990, 38, 375–377. [Google Scholar] [CrossRef]
- Madl, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass Spectrom. 2006, 17, 795–806. [Google Scholar]
- Kuljanabhagavad, T.; Wink, M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem. Rev. 2009, 8, 473–490. [Google Scholar] [CrossRef]
- Holstein, S.; Hohl, R. Isoprenoids: Remarkable diversity of form and function. Lipids 2004, 39, 293–309. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2001, 75, 31–49. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Oleanane Saponins in “Kancolla”, a Sweet Variety of Chenopodium quinoa. J. Nat. Prod. 2002, 65, 1023–1026. [Google Scholar] [CrossRef]
- Joshi, R.; San Martin, R.; Sáez-Navarrete, C.; Alarcon, J.; Sainz, J.; Antolin, M.; Martin, A.R.; Sebastian, L. Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions. Crop. Prot. 2008, 27, 553–557. [Google Scholar] [CrossRef]
- San Martin, R.; Ndjoko, K.; Hostettmann, K. Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponins. Crop. Prot. 2008, 27, 310–319. [Google Scholar] [CrossRef]
- Zouaoui, S.A.; Megherbi-Benali, A.; Toumi Benali, F.; Ouaar, D. Contribution to the study of the antifungal potency of the seeds of Chenopodium quinoa wild against two phytopathogenic fungi of the barley: Pyrenophora tritici-repentis and Rhynchosporium secalis. Bull. Société R. Sci. Liège 2018, 87, 100–111. [Google Scholar]
- Stuardo, M.; San Martin, R. Antifungal properties of quinoa (Chenopodium quinoa Willd) alkali treated saponins against Botrytis cinerea. Ind. Crop. Prod. 2008, 27, 296–302. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crop. Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa secondary metabolites and their biological activities or functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef]
- Escalante, A.; Santecchia, C.; Lopez, S.; Gattuso, M.; Ravelo, A.; Monache, F.; Gonzales-Sierra, M.; Zacchino, S. Isolation of antifungal saponins from Phytolacca tetramera, an Argentinean species in critic risk. J. Ethnopharmacol. 2002, 82, 29–34. [Google Scholar] [CrossRef]
- Sotheeswaran, S.; Kenchington, W. Hemolysis test for saponins: A caution. J. Chem. Educ. 1989, 66, 1058. [Google Scholar] [CrossRef]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Plé, K.; Voutquenne, L. Structure-activity relationships of some hederagenin diglycosides: Haemolysis, cytotoxicity and apoptosis induction. Biochim. Biophys. Acta 2006, 1760, 1418–1427. [Google Scholar] [CrossRef]
- Gauthier, C.; Legault, J.; Girard-Lalancette, K.; Mshvildadze, V.; Pichette, A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins. Bioorg. Med. Chem. 2009, 17, 2002–2008. [Google Scholar] [CrossRef]
- Amini, E.; Nabiuni, M.; Baharara, J.; Parivar, K.; Asili, J. Hemolytic and cytotoxic effects of saponin like compounds isolated from Persian Gulf brittle star (Ophiocoma erinaceus). J. Coast. Life Med. 2014, 2, 614–620. [Google Scholar]
- Soltani, M.; Parivar, K.; Baharara, J.; Kerachian, M.A.; Asili, J. Hemolytic and cytotoxic properties of saponin purified from Holothuria leucospilota sea cucumber. Rep. Biochem. Mol. Biol. 2014, 3, 43–50. [Google Scholar]
- Vo, N.; Fukushima, E.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2016, 71, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Voutquenne, L.; Lavaud, C.; Massiot, G.; Men-Olivier, L. Structure-activity relationships of haemolytic saponins. Pharm. Biol. 2008, 40, 253–262. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.; Li, B.; Laarveld, B. Adjuvant action of Chenopodium quinoa saponins on the induction of antibody responses to intragastric and intranasal administered antigens in mice. Comp. Immunol. Microbiol. Infect. Dis. 1998, 21, 225–236. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Gao, Y.; Shi, Z.; Yibo, H.; Ren, G. Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef]
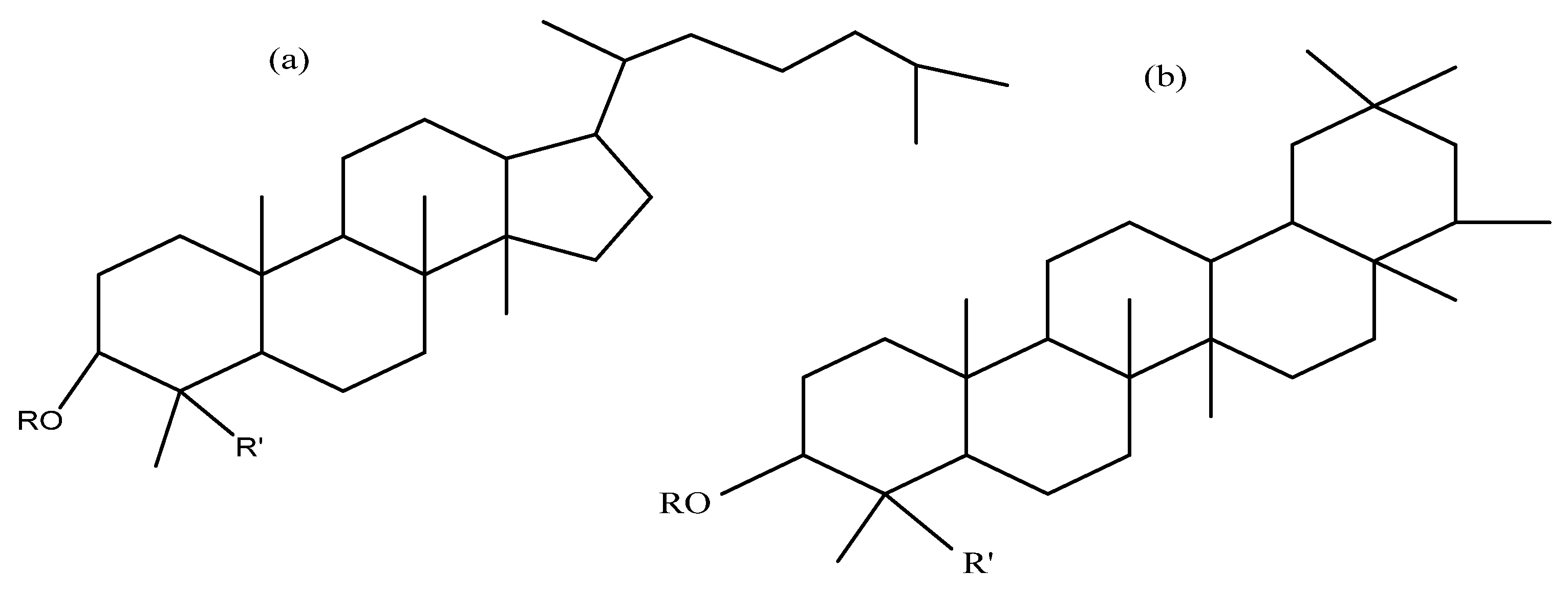
| Washing | Drying | % Removal of Saponins | Yield % | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Wash | Treatment Times (min) | Agitation | Temperature °C | Quinoa: Water | Duration (h) | Temperature °C | |||
| 3 | 30 | Yes | - | 1:10 | 16 | 40 | 13.44%–84.87% | 1.03–0.18 | [27] |
| 5 | - | No | - | 1:10 | - | Ambient | 72% | 0.39 | [21] |
| 1 | - | Yes | - | 1:5 | 4–5 | - | 97.7% | 0.06 | [22] |
| 1 | 10 | Yes | 50 | 1:5 | 4–5 | - | 80% | 0.16 | [22] |
| 1 | 15–120 | Yes | 20–60 | 1:10 | 7 | 70 | 64.35%–80.75% | 1.13–0.61 | [10] |
| 1 | 5–70 | Yes | 20–70 | 1:5 | - | 50 | 80% | 0.311–0.062 | [24] |
| - | 15 | - | - | - | - | 17.42% | 2.75 | [26] | |
| 1 | 0-180 | - | - | - | - | 40–80 | 96% | 0.25 | [23] |
| 1 | 20 | - | - | - | 12 | 50 | - | - | [28] |
| Extraction Technique | Objective | State of Input Material | Solid/Solvent | Solvent Used | Extraction Duration (h) | Extraction Temperature (° C) | Mechanical Aid | Ref. |
|---|---|---|---|---|---|---|---|---|
| Maceration | Study of immunoadjuvant activity, toxicity assays, and determination of triterpenic saponins quinoa seeds | Seed powder | 1:10 | 40% EtOH | 1 | 50 | Magnetic stirring | [46] |
| Study of the anti-inflammatory activity of the saponin extract | Seed powder | 1:9 | 50% EtOH | 3 | Constant stirring at 200 rpm | [47] | ||
| Evaluation of preconditioning, extrusion, and roasting effects on compounds of quinoa | Seed powder | 1:3 1:2 | Acetate ethyl MeOH | 24 24 | - | Magnetic rod | [29] | |
| Isolation and identification of quinoa saponins | Seed powder | - | 90% MeOH | - | - | - | [48] | |
| Study of saponin extraction kinetics by applying diffusion equations | Seed powder | 1:10 | 50% EtOH | 0.5 | - | - | [24] | |
| Isolation and characterization of quinoa saponins | Seed powder | 3:25 | Petroleum ether MeOH EtOH Bu-OH | - | Room temperature | - | [49] | |
| Powdered seed coats | 3:25 | |||||||
| Powdered flowers | 2:25 | |||||||
| Isolation and identification of saponins from C. quinoa | Seed powder | H2O2 H2O2/BuOH | - | 60 | - | [50] | ||
| Study of the effects of five treatment types on the saponin content, total phenolic compound content, and the antioxidant capacity of quinoa seeds grown in Brazil | Seed powder | 1:10 | 50% EtOH | 72 | - | - | [26] | |
| Description of the leaching kinetics of saponins in C. quinoa | Seed powder | 1:13 | 50% EtOH | 2 | - | Constant stirring (200 rpm) | [10] | |
| Identification of triterpenoid saponins | Seed powder | 4kg/- | 95% EtOH | 3*72 | Room temperature | - | [51] | |
| Study of the influence of drying temperature on kinetic parameters through the application of empirical models | Seed powder | 1:10 | MeOH | 0.5 | Magnetic stirrer | [23] | ||
| Soxhlet | Isolation and characterization of saponins in quinoa seeds by analytical and chemical methods | Seed powder | 900g/- | Petroleum ether MeOH | 72 | Room temperature | Soxhlet extractor | [52] |
| Description of the method for the separation and analysis of saponins in quinoa | Seed powder | 1:40 | Chloroform MeOH | 16 30 | - | Soxhlet extractor | [27] | |
| Sonication | Identification of phenolic compounds and saponins of C. quinoa Willd | Seed powder | 1:15 | (4:1) MeOH/Water | 1/3 | Room temperature | Ultrasonic bath | [53] |
| The production of rich saponin extracts from edible seeds (quinoa, soya beans, red lentils, fenugreek, and lupine) | Seed powder | 1:10 | EtOH EtOH/Water Water | 0.25 | Room temperature | Ultrasonic probe | [54] | |
| Optimization of processes of obtaining saponin-rich extracts from different plants | Seed powder | 1:10 | MeOH | 0.25 | Room temperature | Ultrasonic probe | [55] | |
| Microwave | Evaluation of the efficiency of saponin extraction | Seed powder | 1:20 | 20% EtOH 20% Isopropanol | 1/3 | 90° C | Microwave | [45] |
| Compound | R1 | R2 | Formula | MW | Quinoa Part | Origin | Ref. |
|---|---|---|---|---|---|---|---|
Oleanolic Acid | |||||||
| Monodesmosidic saponins | |||||||
| Oleanolic acid 3-O-β -d-glucopyranoside | -glc | H | C36H58O8 | 618 | Seeds | m | [50] |
| Oleanolic acid 3-O-β-d-glucuropyranoside | -glcUA | H | C36H56O9 | 632 | Seeds | b | [51,52] |
| Oleanolic acid 3-O-β -d-xylopyranosyl-(1,3)-β-d-glucuronopyranoside | –xyl (1–3) glcUA | H | C41H64O13 | Seeds | m | [50] | |
| Oleanolic acid 3-O-β -d-xylopyranosyl(l,3)-β -d-glucuronopyranosyl-methyl-ester | –xyl (1–3)-6-O-Me glcUA | H | C42H66O13 | 778 | Seeds | m | [50] |
| Bidesmosidic saponins | |||||||
| Oleanolic acid 3-O- [β -d- glucopyranosyl-(1,3)- α-l- arabinopyranosyl]-28-O-β-d-glucopyranoside | –glc (1–3) ara | -glc | C47H76O17 | 912 | Seeds, bran flowers, and fruits | a, b, c, e, g | [43,49,59,63] |
| Oleanolic acid 3-O-α-l-arabinopyranosyl-(1,3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | –ara (1–3) glcUA | -glc | C47H74O18 | 926 | Seeds | a, e, p | [43,59,63] |
| Oleanolic acid 3-O-[β-d-glucuronopyranosyl]-28-O-β-d-glucopyranoside | –glcUA | -glc | C42H66O14 | 794 | Flowers, fruits, seeds, and bran | a, c, p, g | [49,52,53,58,59,63] |
| Oleanolic acid 3-O-β-d-xylopyranosyl-(1,3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | –xyl (1–3) glcUA | -glc | C47H74O18 | 926 | Flowers, fruits, seeds, and bran | a, c, g, sa, p | [49,50,53,58,59] |
| Oleanolic acid 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl 28-O-β-d-glucopyranoside | –glc (1–2) glc (1–3) ara | -glc | C53H86O22 | 1074 | Flowers, fruits, seeds, and bran | a, b, c, g, sa | [49,51,58,59] |
Hederagenin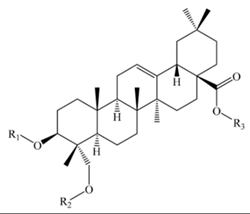 | |||||||
| Monodesmosidic saponins R3= -H | |||||||
| Hederagenin 3-O-β-d-glucopyranosyl-(1,3)- α -l-arabinopyranoside | –glc (1–3) ara | -H | C41H66O13 | 766 | Seeds and bran | b, c | [51,52] |
| Hederagenin 3-O-α-l-Arabinopyranoside | -ara | -H | - | - | Seeds | c | [52] |
| Bidesmosidic saponins R3= -H | |||||||
| Hederagenin 3-O-[β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl]-28-O-β-d-glucopyranoside | –glc (1–3) ara | -glc | C47H76O18 | 928 | Flowers, fruits, seeds and bran | a, b, c, e, g, sa, p | [25,48,49,51,52,53,58,59,63] |
| Hederagenin 3-O- α- l-arabinopyranosyl-28-O-β-d-glucopyranoside | -ara | -glc | C41H66O13 | 766 | Flowers, fruits, seeds, and bran | a, c, g, sa | [49,52,58,59] |
| Hederagenin 3-O-β-d-Glucopyranosyl-(1,3)-α-l-galactopyranosyl 28-O- β-d-glucopyranoside | –glc (1–3) gal | -glc | C48H78O19 | 958 | Flowers, fruits, seeds, and bran | a, c, b, g, sa | [49,51,58,59] |
| Hederagenin 3-O- β- d-Glucuronopyranosyl 28-O- β-d-glucopyranoside | –glcUA | -glc | C42H66O15 | 810 | Flowers, fruits, seeds, and bran | a, c, g, sa | [49,58,59] |
| Hederagenin 3-O-β-d-Xylopyranosyl-(1,3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | –xyl (1–3) glcUA | -glc | C47H74O19 | 942 | Seeds and bran | a, sa | [53,58,59] |
| Hederagenin 3-O-β-d-Glucopyranosyl-(1,4)-β-d-glucopyranosyl-(1,4)-β-d-glucopyranosyl-28-O-β-d-glucopyranoside | –glc (1–4) glc (1–4) glc | -glc | C54H88O24 | 1120 | Seeds | a, e | [48,59] |
| Tridesmosidic saponins R3= -glc | |||||||
| 3,23-bis(O-β-d-Glucopyranosyloxy) olean-12-en28-oicacid28-O-α-l-arabinopyanosyl-(1,3)-β-d-glucopyranoside | -glc | -glc-glc | C53H86O23 | 1090 | Seeds | e | [20] |
Phytolaccagenic acid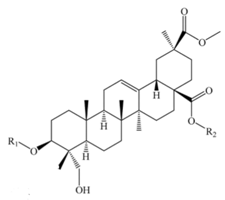 | |||||||
| Monodesmosidic saponins | |||||||
| Phytolaccagenic acid 3-O-β-d-glucopyranosyl (1,3)-α-l-arabinopyranoside | –glc (1–3) ara | -H | C42H66O15 | 810 | Seeds | b, c | [51,52] |
| Bidesmosidic saponins | |||||||
| Phytolaccagenic acid 3-O- [α -l-arabinopyranosyl-(1,3)-β-d-glucuronopyranosyl]-28-O-β-d-glucopyranoside | –ara (1–3) glcUA | -glc | C48H74O21 | 986 | Seeds | e | [43,48] |
| Phytolaccagenic acid 3-O-[β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl]-28-O-β-d-glucopyranoside | –glc (1–3) ara | -glc | C48H76O20 | 972 | Flowers, fruits, seeds, and bran | a, b, c, e, g, m, p, sa | [43,49,51,52,53,58,59,63] |
| Phytolaccagenic acid 3-O-[β-d-glucopyranosyl-(1,3)-β-d-xylopyranosyl-(1,2)- β-d-glucopyranosyl]-28-O-β-d-glucopyranoside | –glc (1–2) xyl (1–3) glc | -glc | C54H86O25 | 1134 | Seeds | a, e, sa | [43,59] |
| Phytolaccagenic acid 3-O-α -l-arabinopyranosyl 28-O-β-d-glucopyranoside | -ara | -glc | C42H66O15 | 810 | Flowers, fruits, seeds, and bran | a, b, c, g, p, sa | [49,51,52,53,58,59] |
| Phytolaccagenic acid 3-O-β-d-galactopyranosyl-(1,3)-β-d-glucopyranosyl 28-O-β-d-glucopyranoside | –gal (1–3) glc | -glc | C49H78O21 | 1002 | Seeds | b | [51] |
| Phytolaccagenic acid 3-O-β-d-Glucopyranosyl-(1,3)-β-d-galactopyranosyl 28-O-β-d-glucopyranoside | –glc (1–3) gal | -glc | C49H78O21 | 1002 | Flowers, fruits, seeds, and bran | a, c, g, sa | [49,53,58,59] |
| Phytolaccagenic acid 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl 28-O-β-d-glucopyranoside | –glc (1–2) glc (1–3) ara | -glc | C54H86O25 | 1134 | Flowers, fruits, seeds, and bran | a, c, e, g, sa | [43,49,51,53,58,59] |
| Phytolaccagenic acid 3-O-β-d-Glucopyranosyl-(1,4)-β-d-glucopyranosyl-(1,4)-β-d-glucopyranosyl 28-O-β-d-glucopyranoside | –glc (1–4) glc (1–4) glc | -glc | C55H88O26 | 1164 | Flowers, fruits, seeds, and bran | a, c, e, g | [48,49,59] |
Serjanic acid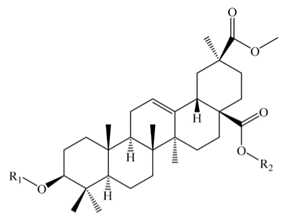 | |||||||
| Bidesmosidic saponins | |||||||
| Serjanic acid 3-O-[β-d-glucopyranosyl-(1,3)- α -l-arabinopyranosyl]-28-O-β-d-glucopyranoside = 30-O-methyl spergulagenate 3-O-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl-28-O-β-d-glucopyranoside | –glc (1–3) ara | -glc | C48H76O19 | 956 | Flowers, fruits, seeds, and bran | a, b, c, g, p | [49,51,53,59,63] |
| Serjanic acid 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl-28-O-β-d-glucopyranoside= 30-O-methyl spergulagenate 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-L-arabinopyranosyl-28-O-β-d -glucopyranoside | –glc (1–2) glc (1–3) ara | -glc | C54H86O24 | 1118 | Flowers, fruits, seeds, and bran | b, c, e, g, sa | [49,51,58] |
| Serjanic acid 3-O-α-l-arabinopyranosyl-28-O-β-d-glucopyranoside | -ara | -glc | C42H66O14 | 794 | Flowers, fruits, seeds, and bran | c, g | [49] |
| Serjanic acid 3-O-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | –glcUA | -glc | C43H66O16 | 838 | Flowers, fruits, seeds, and bran | c, g | [49] |
| Serjanic acid 3-O-α-l-arabinopyranosyl-(1,3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranosyl ester | -ara (1-3) glc | -glc | C48H74O20 | 970 | Seeds | p | [53] |
Spergulagenic acid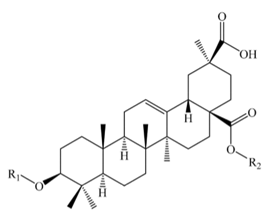 | |||||||
| Bidesmosidic saponins | |||||||
| Spergulagenic acid 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranoside | -glc (1-2) glc (1-3) ara | -H | - | - | Bran | sa | [58] |
| Spergulagenic acid 3-O-β-d-glucopyranosyl-(1,2)-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl-28-O-β-d-glucopyranoside | Glc (1-2) glc (1-3) ara | -glc | - | - | Seeds | e | [48] |
| Spergulagenic acid 3-O-α-l-arabinopyranosyl-(1,3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | –ara (1–3) glcUA | -glc | C48H74O20 | 970 | Seeds | e | [48] |
| 28-oic acid | |||||||
3β-Hydroxy-23-oxo-olean-12-en -28-oic acid= Gypsogenin | |||||||
| 3β-O-β-d-glucopyranosyl-(1,3)- α -l-arabinopyranosyl-oxy-23-oxo-olean-12-en-28-oic acid β-d-glucopyranoside | –glc (1–3) ara | -glc | C47H74O18 | 926 | Flowers, fruits, seeds, and bran | c, g | [49] |
3β-Hydroxy-27-oxo-olean-12-en -28-oic acid | |||||||
| 3β-O-β-d-glucopyranosyl-(1,3)-α- l -arabinopyranosyl-oxy-27-oxo-olean-12-en-28-oic acid β-d-glucopyranoside | –glc (1–3) ara | -glc | C47H74O18 | 926 | Flowers, fruits, seeds, and bran | c, g | [49] |
3β, 23 α, 30 β-Trihydroxy-olean-12-en-28-oic acid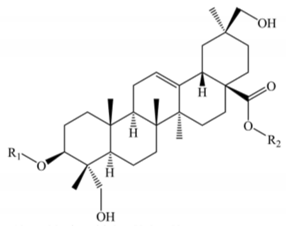 | |||||||
| 3,23,30-trihydroxyolean-12-en-28-oic acid 3-O-β-d-Glucopyranosyl-(1,3)-α-l-arabinopyranosyl-28-O-β-d-glucopyranoside | –glc (1–3) ara | -glc | C47H76O19 | 944 | Flowers, fruits, seeds, and bran | a, c, g | [49,53,59] |
| Aglycone | Structure Name | Quinoa Part | Biological Activity | Species | Ref. |
|---|---|---|---|---|---|
| - | - | Seeds | Intestinal permeability | Rat | [21] |
| - | - | Seeds | Molluscicidal activity | Pomacea canaliculata | [68,69] |
| - | - | Seeds | Antifungal activity | Pyrenophora triticirepentis and Rhynchosporium secalis | [79] |
| - | - | Seeds | Botrytis cinerea | [66] | |
| Phytolaccagenic acid | 3-O-β-d-glucopyranosyl-(1,3) -α-l-arabinopyranosyl phytolaccagenic acid | Seeds | Candida albicans | [52] | |
| - | Bran | Anti-inflammatory activity | - | [47] | |
| Oleanolic acid | Methyl oleanate | Bran | |||
| 3-O-β-d-glucuronopyranosyl oleanolic acid 28-O-β-d- glucopyranosyl ester | Hemolytic activity | - | [49,52,58,63] | ||
| Serjanic acid | 3-O-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester3-O-β-d-glucuronopyranosyl serjanic acid 28-O-β-d- glucopyranosyl ester | Seeds, bran, flowers, and fruits | Cytotoxic activity in HeLa cell lines | - | [49,58] |
| Gypsogenin (3β-Hydroxy-23-oxo-olean-12-en-28-oic acid) | 3-O-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl 23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester 3β-[(O-β-d-glucopyranosyl-(1,3)-α-l-arabinopyranosyl) oxy]-23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester | [49] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hazzam, K.; Hafsa, J.; Sobeh, M.; Mhada, M.; Taourirte, M.; EL Kacimi, K.; Yasri, A. An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules 2020, 25, 1059. https://doi.org/10.3390/molecules25051059
El Hazzam K, Hafsa J, Sobeh M, Mhada M, Taourirte M, EL Kacimi K, Yasri A. An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules. 2020; 25(5):1059. https://doi.org/10.3390/molecules25051059
Chicago/Turabian StyleEl Hazzam, Khadija, Jawhar Hafsa, Mansour Sobeh, Manal Mhada, Moha Taourirte, Kamal EL Kacimi, and Abdelaziz Yasri. 2020. "An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review" Molecules 25, no. 5: 1059. https://doi.org/10.3390/molecules25051059
APA StyleEl Hazzam, K., Hafsa, J., Sobeh, M., Mhada, M., Taourirte, M., EL Kacimi, K., & Yasri, A. (2020). An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules, 25(5), 1059. https://doi.org/10.3390/molecules25051059





