The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
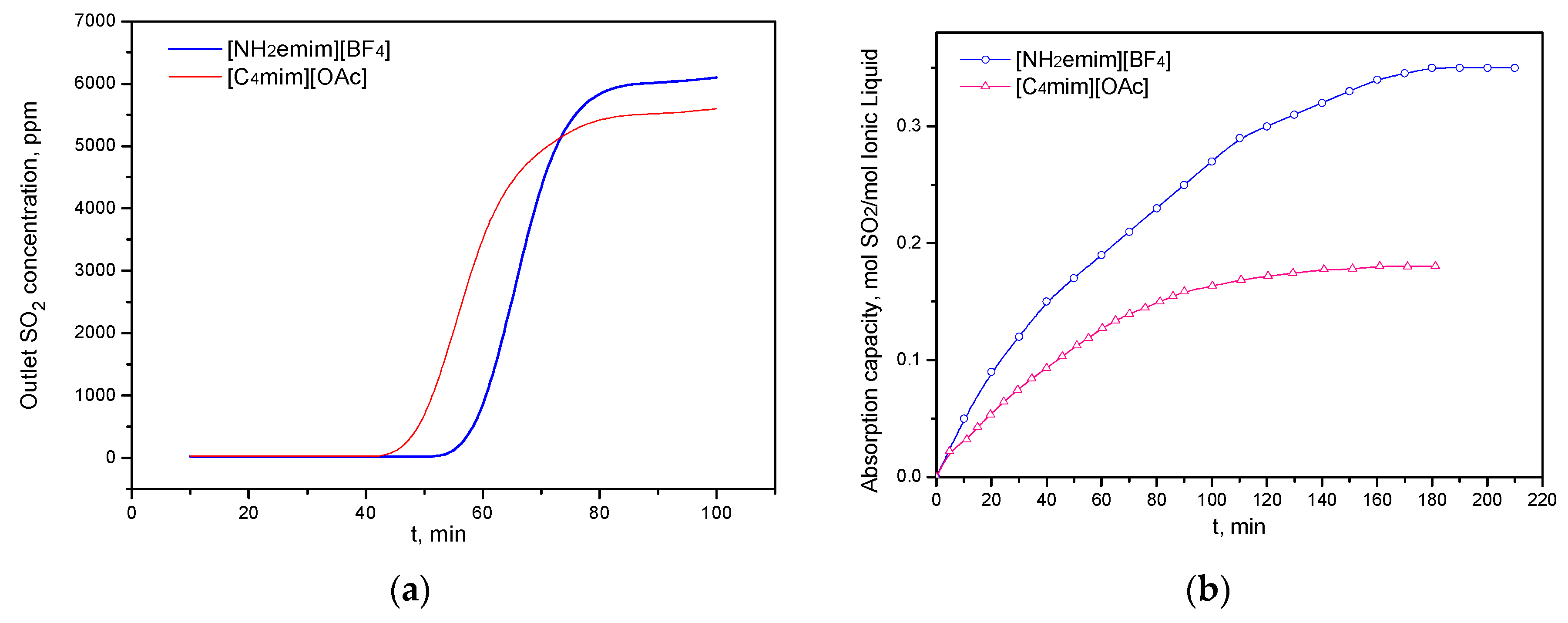
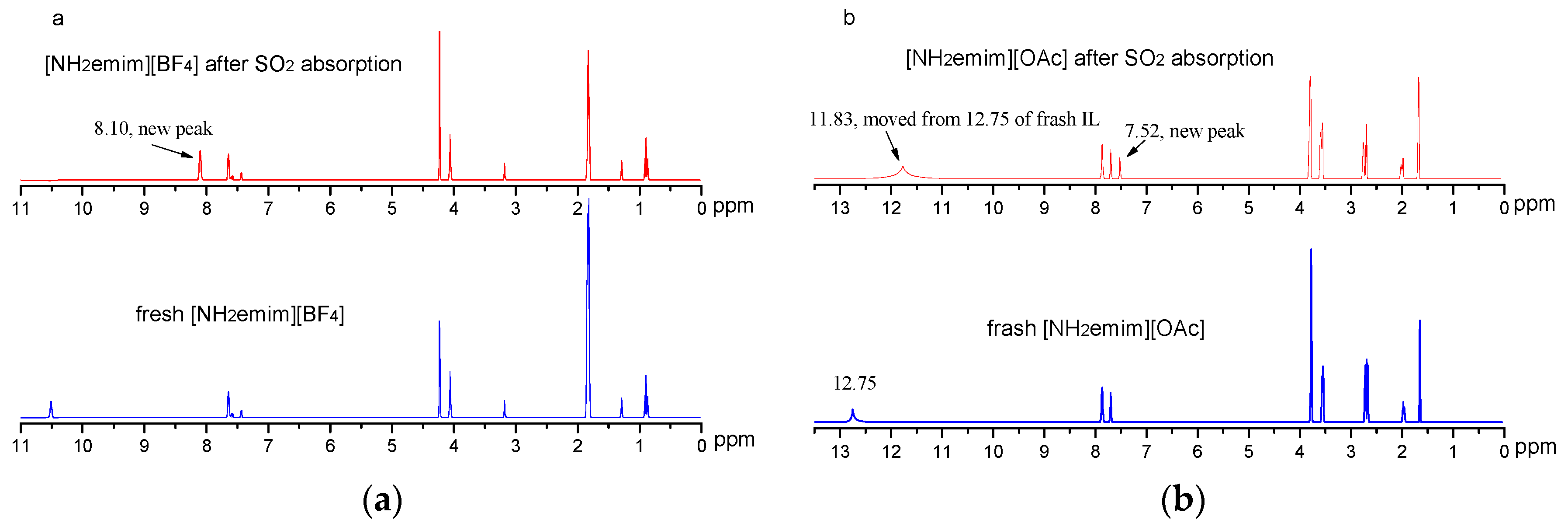
2.1. CO2 and SO2 Absorption Performance of the Single ILs
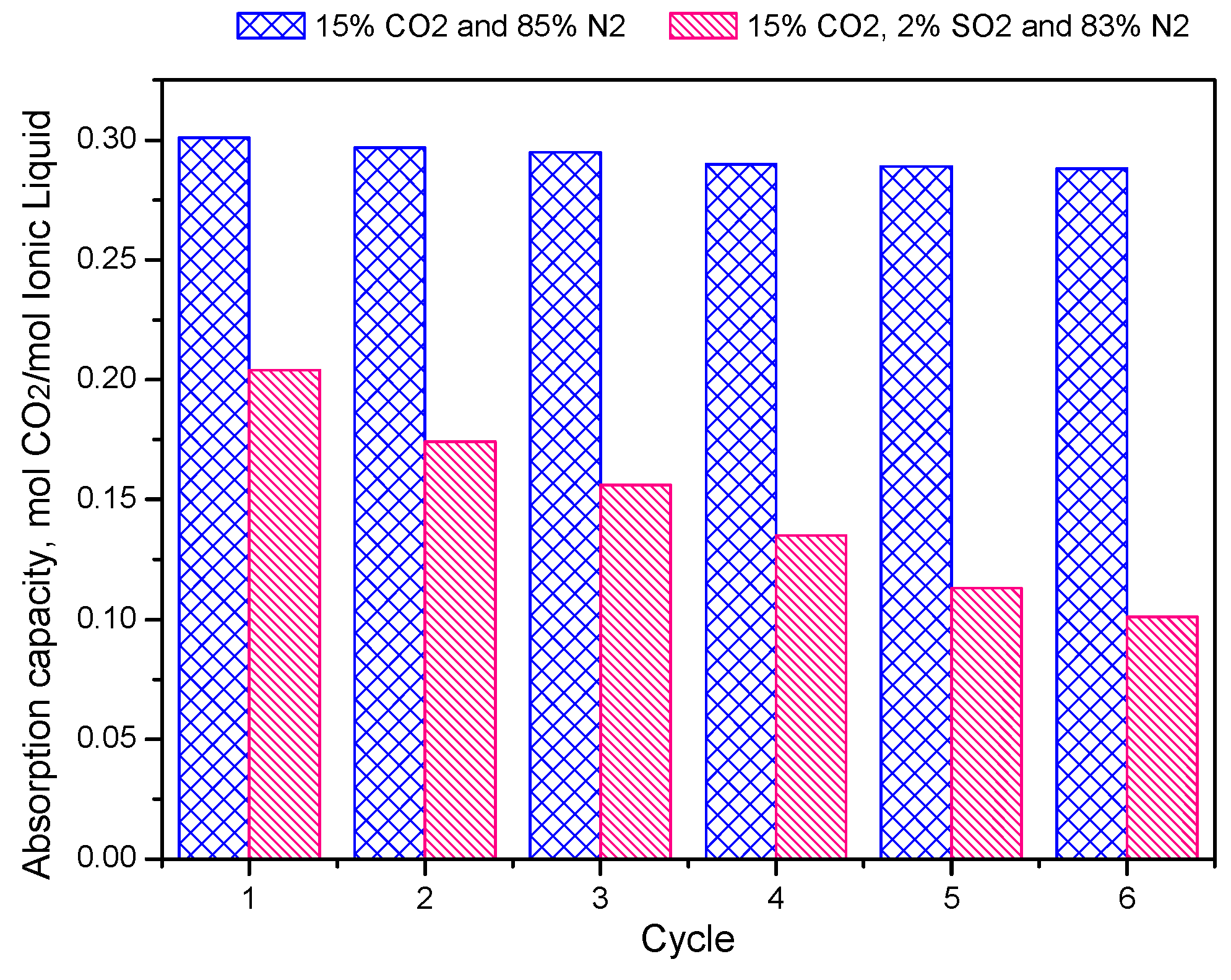
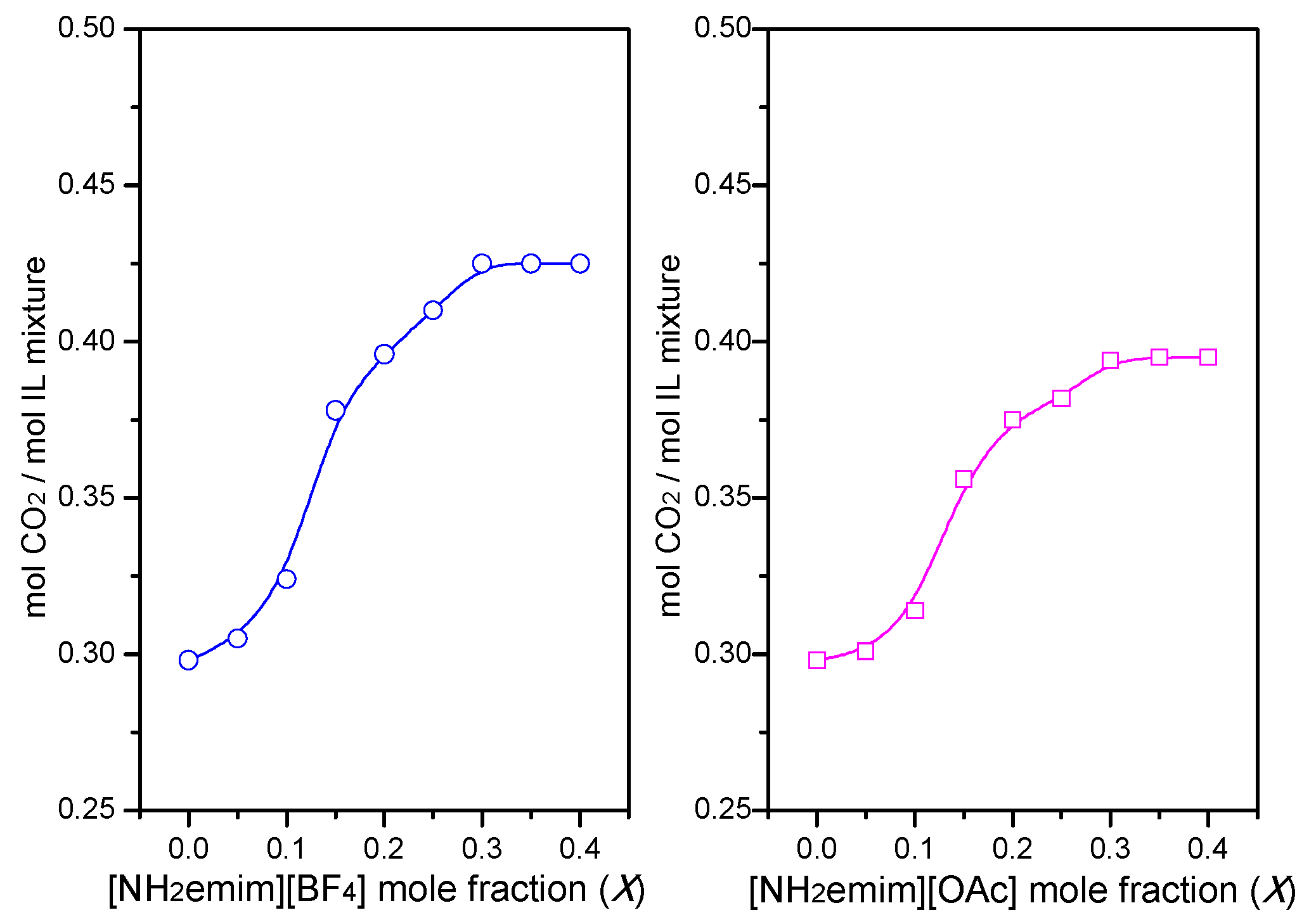
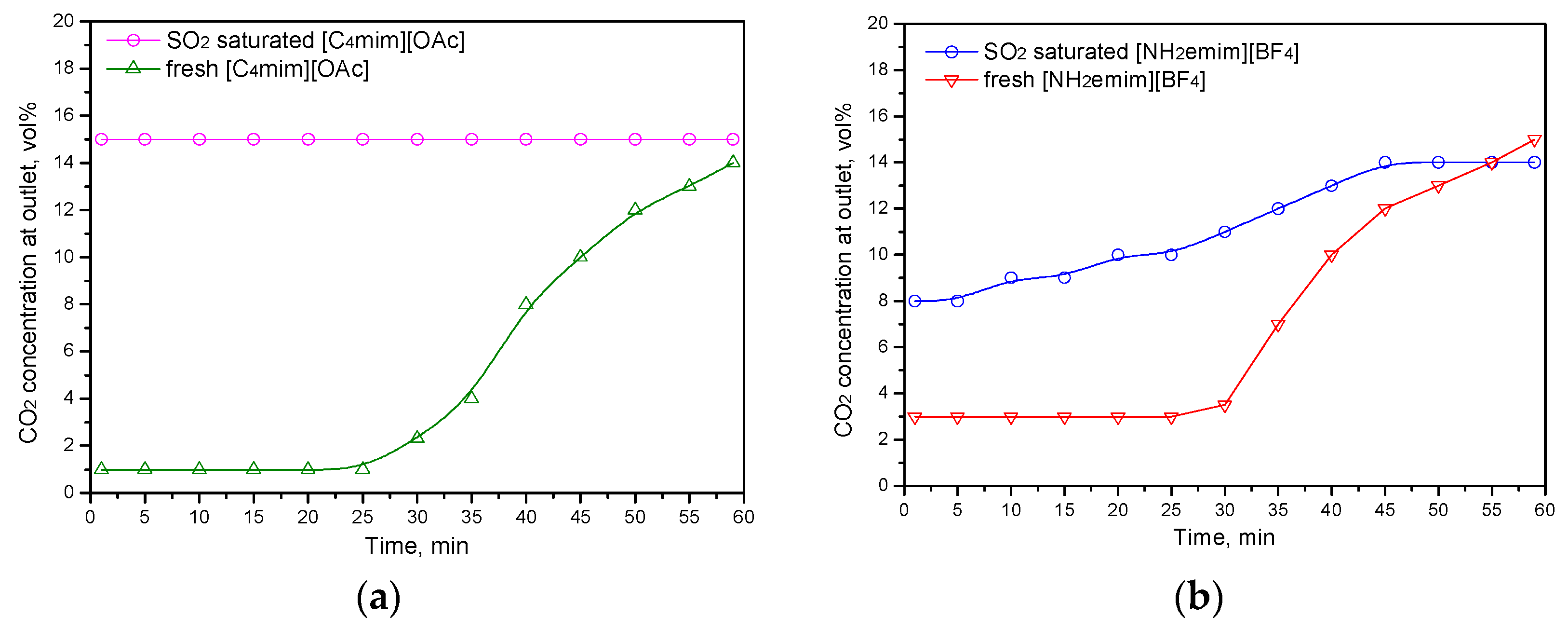
2.2. CO2/SO2 Absorption Properties in IL Mixtures
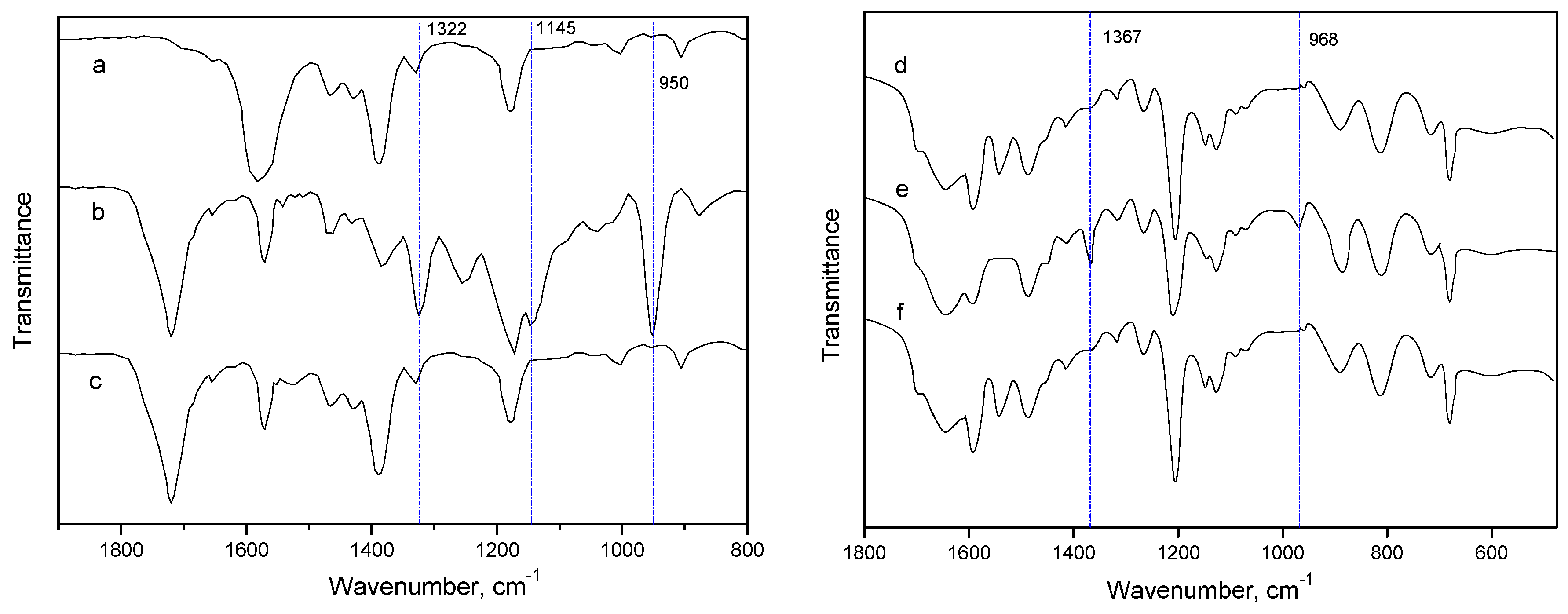
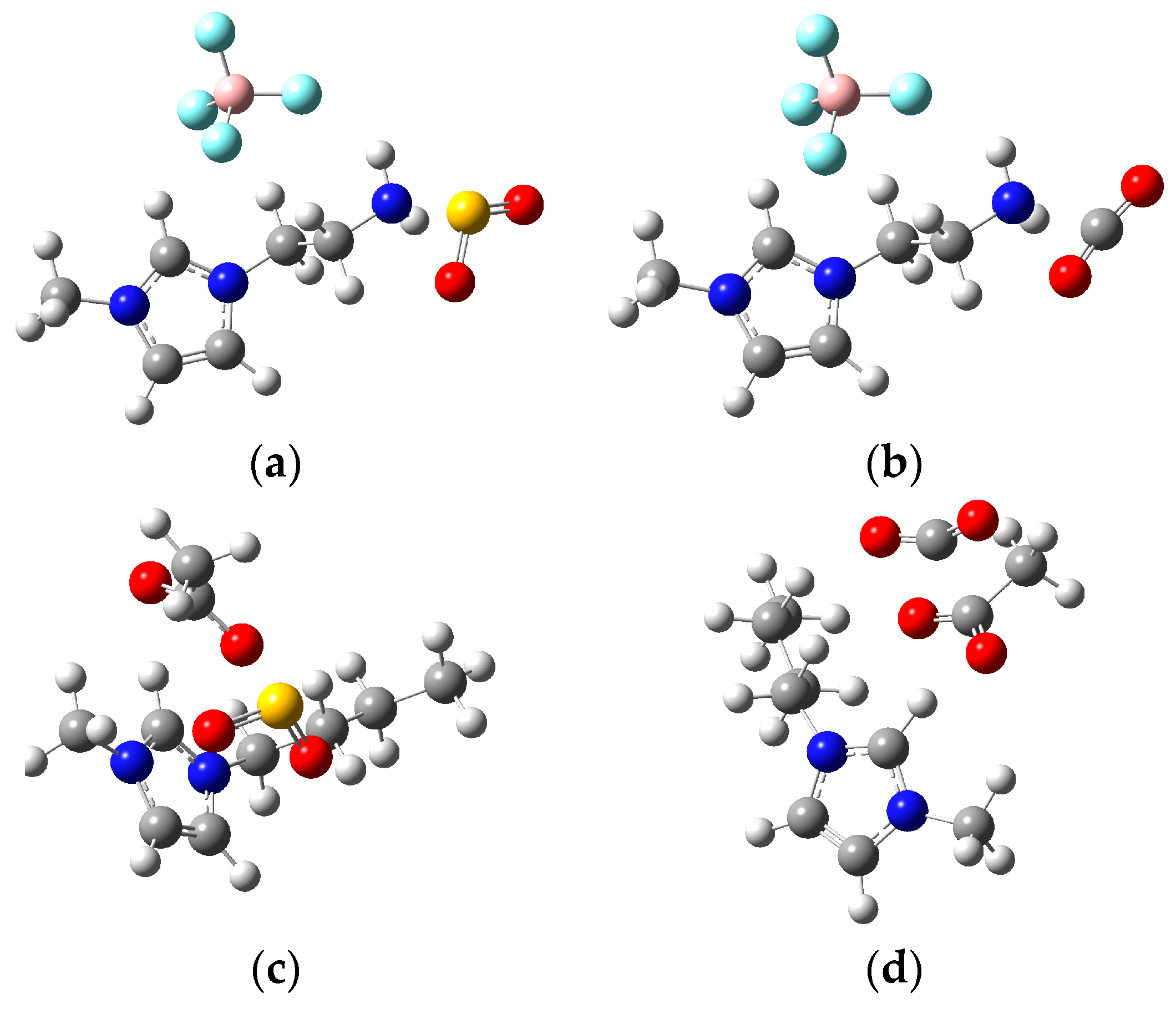
2.3. Quantum Chemical Calculation on the Interaction of IL Mixture with CO2/SO2
3. Materials and Methods
3.1. Materials
3.2. Ionic Liquid Preparation
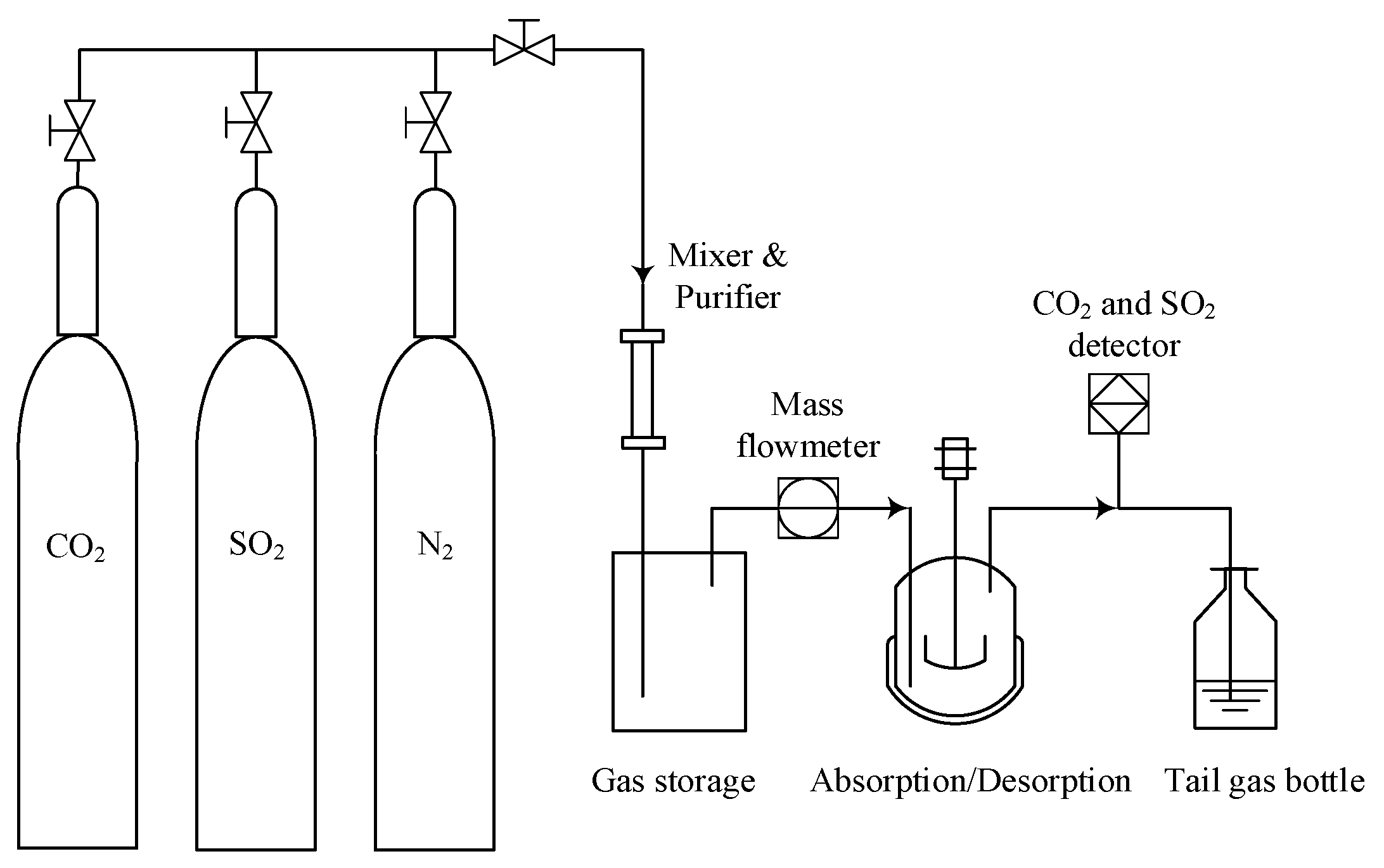
3.3. CO2 and SO2 Absorption
3.4. Theoretical Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Wang, C.M.; Cui, G.K.; Luo, X.Y.; Xu, Y.J.; Li, H.R.; Dai, S. Highly efficient and reversible SO2 capture by tunable azole-based ionic liquids through multiple-site chemical absorption. J. Am. Chem. Soc. 2011, 133, 11916–11919. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Separation of carbon dioxide and sulfur dioxide using room-temperature ionic liquid [bmim][MeSO4]. Energy Fuels 2010, 24, 1001–1008. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Solubilities and diffusivities of carbon dioxide in ionic liquids: Bmim PF6 and bmim BF4. Ind. Eng. Chem. Res. 2005, 44, 4453–4464. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, K.; Liu, Z.; Yao, X.; Liu, X.; Zeng, S.; Dong, K.; Zhang, S. Insight into the performance of acid gas in ionic liquids by molecular simulation. Ind. Eng. Chem. Res. 2019, 58, 1443–1453. [Google Scholar] [CrossRef]
- Shang, Y.; Li, H.P.; Zhang, S.J.; Xu, H.; Wang, Z.X.; Zhang, L.; Zhang, J.M. Guanidinium-based ionic liquids for sulfur dioxide sorption. Chem. Eng. J. 2011, 175, 324–329. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; Hodgson, J.L.; Weis, D.C.; Pas, S.J.; MacFarlane, D.R. Physical absorption of CO2 in protic and aprotic ionic liquids: An interaction perspective. J. Phys. Chem. B 2015, 119, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Karousos, D.S.; Kouvelos, E.; Sapalidis, A.; Pohako-Esko, K.; Bahlmann, M.; Schulz, P.S.; Wasserscheid, P.; Siranidi, E.; Vangeli, O.; Falaras, P.; et al. Novel inverse supported ionic liquid absorbents for acidic gas removal from flue gas. Ind. Eng. Chem. Res. 2016, 55, 5748–5762. [Google Scholar] [CrossRef]
- Li, X.S.; Zhang, L.Q.; Zheng, Y.; Zheng, C.G. Effect of SO2 on CO2 absorption in flue gas by ionic liquid 1-ethyl-3-methylimidazolium acetate. Ind. Eng. Chem. Res. 2015, 54, 8569–8578. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.E.; Li, H.; Dai, S. Tuning the basicity of ionic liquids for equimolar CO2 capture. Angew. Chem. Int. Ed. 2011, 50, 4918–4922. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.G.; Dai, C.N.; Chen, B.H. Gas solubility in ionic liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, B.F.; de la Fuente, J.C.; Gurkan, B.E.; Zadigian, D.J.; Price, E.A.; Huang, Y.; Brennecke, J.F. Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide. Ind. Eng. Chem. Res. 2011, 50, 111–118. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.Q.; Gao, L.X.; Pi, K.W.; Zhang, J.Y.; Zheng, C.G. Improvement of the CO2 absorption performance using ionic liquid [NH2emim][BF4] and [Emim][BF4]/[Bmim][BF4] mixtures. Energy Fuels 2013, 27, 461–466. [Google Scholar] [CrossRef]
- Damas, G.B.; Dias, A.B.A.; Costa, L.T. A quantum chemistry study for ionic liquids applied to gas capture and separation. J. Phys. Chem. B 2014, 118, 9046–9064. [Google Scholar] [CrossRef]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Rezaei, F.; Jones, C.W. Stability of supported amine adsorbents to SO2 and NOx in postcombustion CO2 capture. 1. Single-component adsorption. Ind. Eng. Chem. Res. 2013, 52, 12192–12201. [Google Scholar] [CrossRef]
- Hallenbeck, A.P.; Kitchin, J.R. Effects of O2 and SO2 on the capture capacity of a primary-amine based polymeric CO2 sorbent. Ind. Eng. Chem. Res. 2013, 52, 10788–10794. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Chemical absorption of sulfur dioxide in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2010, 49, 1370–1377. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, L.; Gao, G. Using ionic liquid mixtures to improve the so2 absorption performance in flue gas. Energy Fuels 2017, 31, 1771–1777. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, H.S.; Kim, C.S.; Jung, K.D. Behaviors of SO2 absorption in BMIm OAc as an absorbent to recover SO2 in thermochemical processes to produce hydrogen. Int. J. Hydrog. Energy 2010, 35, 10173–10178. [Google Scholar] [CrossRef]
- Gurau, G.; Rodríguez, H.; Kelley, S.P.; Janiczek, P.; Kalb, R.S.; Rogers, R.D. Demonstration of chemisorption of carbon dioxide in 1,3-dialkylimidazolium acetate ionic liquids. Angew. Chem. Int. Ed. 2011, 50, 12024–12026. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.Z.; Zhong, Q.; He, C.; Wang, J. Hydroxyl ammonium ionic liquids synthesized by water-bath microwave: Synthesis and desulfurization. J. Hazard. Mater. 2010, 177, 807–813. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Mutalib, M.I.A.; Ariwahjoedi, B. Estimation of physicochemical properties of ionic liquids [H2N-C2mim][BF4] and [H2N-C3mim][BF4]. J. Chem. Eng. Data 2011, 56, 2557–2562. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16 (Revision A.01); Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Li, H.P.; Chang, Y.H.; Zhu, W.S.; Jiang, W.; Zhang, M.; Xia, J.X.; Yin, S.; Li, H.M. A DFT Study of the Extractive Desulfurization Mechanism by BMIM (+) AlCl4 (-) Ionic Liquid. J. Phys. Chem. B 2015, 119, 5995–6009. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101–194118. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hujo, W.; Kirchner, B. Performance of dispersion-corrected density functional theory for the interactions in ionic liquids. Phys. Chem. Chem. Phys. 2012, 14, 4875–4883. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The Calculations of Small Molecular Interaction by the Difference of Separate Total Energies. Some Procedures with Reduced Error. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bernales, V.S.; Marenich, A.V.; Contreras, R.; Cramer, C.J.; Truhlar, D.G. Quantum Mechanical Continuum Solvation Models for Ionic Liquids. J. Phys. Chem. B 2012, 116, 9122–9129. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
Sample Availability: Samples of the ionic liquids are available from the authors. |

| Ionic Liquids | T, K | Gas | Absorption Capacity, mol CO2/mol IL |
|---|---|---|---|
| [C4mim][OAc] | 293 | 15% CO2/85% N2 | 0.298 |
| [C4mim][OAc] | 293 | 15% CO2/2% SO2/83% N2 | 0.204 |
| [NH2emim][BF4] | 293 | 15% CO2/85% N2 | 0.290 |
| [NH2emim][BF4] | 293 | 15% CO2/2% SO2/83% N2 | 0.180 |
| [NH2emim][OAc] | 293 | 15% CO2/85% N2 | 0.291 |
| [NH2emim][OAc] | 293 | 15% CO2/2% SO2/83% N2 | 0.171 |
| Structural Parameters | CO2−[NH2emim][BF4] | SO2−[NH2emim][BF4] | [C4mim][OAc]−CO2 | [C4mim][OAc]−SO2 |
|---|---|---|---|---|
| C−O, Å | 1.19 | 1.21 | ||
| ∠O−C−O, ° | 166 | 146 | ||
| S−O, Å | 1.46 | 1.49 | ||
| ∠O−S−O, ° | 113.5 | 112.6 |
| OAc−CO2 | OAc−SO2 | OAc−CO2−SO2 | |
| ΔE, kJ/mol | −40.7 | −113.4 | −140.0 |
| ΔH, kJ/mol | −46.5 | −125.1 | −151.4 |
| ΔG, kJ/mol | −1.6 | −70.8 | −72.1 |
| net charge transfer, e | −0.510 | −0.382 | −0.035(CO2)/−0.316(SO2) |
| CO2−[NH2emim] | SO2−[NH2emim] | SO2−CO2−[NH2emim] | |
| ΔE, kJ/mol | −33.8 | −123.9 | −156.8 |
| ΔH, kJ/mol | −36.3 | −126.7 | −160.1 |
| ΔG, kJ/mol | −7.2 | −55.1 | −61.9 |
| net charge transfer, e | −0.312 | −0.399 | −0.308(CO2)/−0.334(SO2) |
| [C4mim][OAc]−CO2 | [C4mim][OAc]−SO2 | CO2−[NH2emim][BF4] | SO2−[NH2emim][BF4] | |
|---|---|---|---|---|
| ΔE, kJ/mol | −26.4 (−21.9) | −80.1 (−70.5) | −19.2 (−16.8) | −85.4 (−76.3) |
| ΔH, kJ/mol | −30.5 | −93.2 | −29.1 | −91.5 |
| ΔG, kJ/mol | −2.7 | −40.6 | 8.1 | −37.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Liu, Y.; Liu, G.; Pang, X. The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids. Molecules 2020, 25, 1034. https://doi.org/10.3390/molecules25051034
Wu G, Liu Y, Liu G, Pang X. The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids. Molecules. 2020; 25(5):1034. https://doi.org/10.3390/molecules25051034
Chicago/Turabian StyleWu, Guoqing, Ying Liu, Guangliang Liu, and Xiaoying Pang. 2020. "The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids" Molecules 25, no. 5: 1034. https://doi.org/10.3390/molecules25051034
APA StyleWu, G., Liu, Y., Liu, G., & Pang, X. (2020). The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids. Molecules, 25(5), 1034. https://doi.org/10.3390/molecules25051034






