Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence
Abstract
1. Introduction
2. Sources and Fate of Pharmaceuticals in the Environment
2.1. Sources
2.2. Consumption Patterns
2.3. Mechanism of Action, Metabolization and Excretion
3. Physicochemical Properties and Fate
3.1. Physicochemical Properties
3.2. Fate in Wastewater Treatment Plants
3.3. Fate in Surface Waters
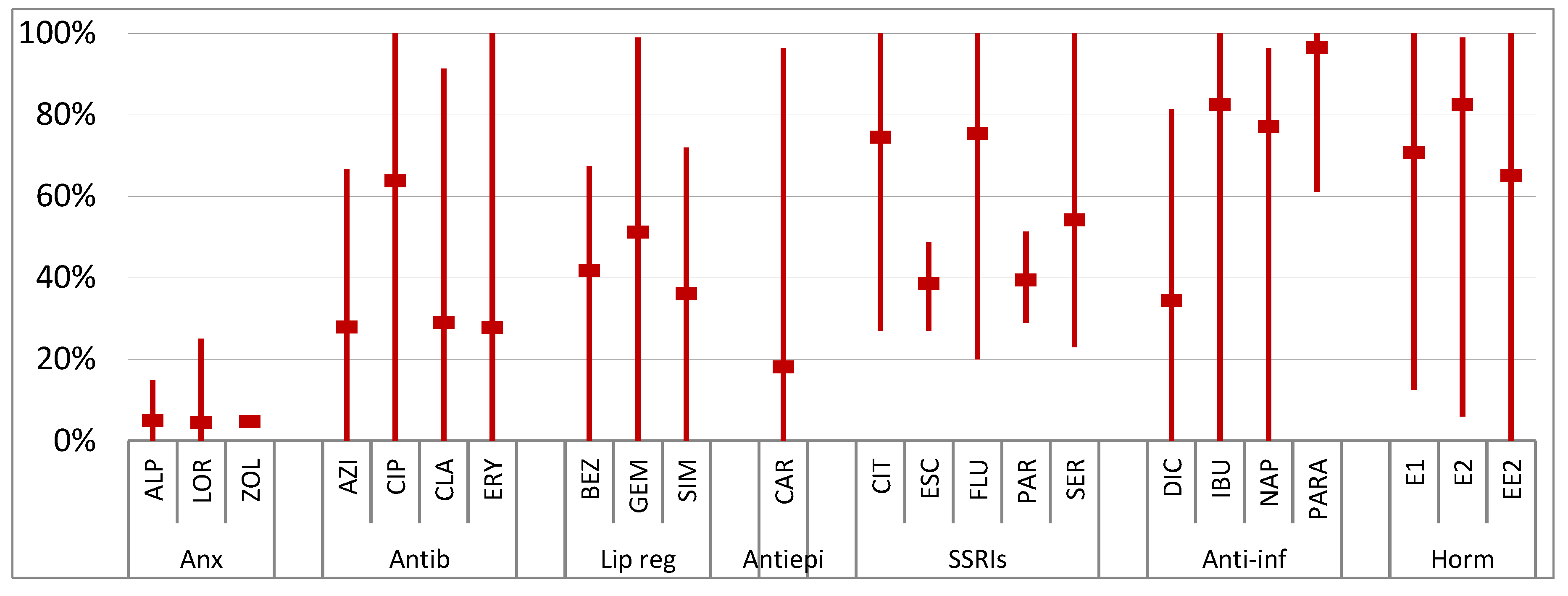
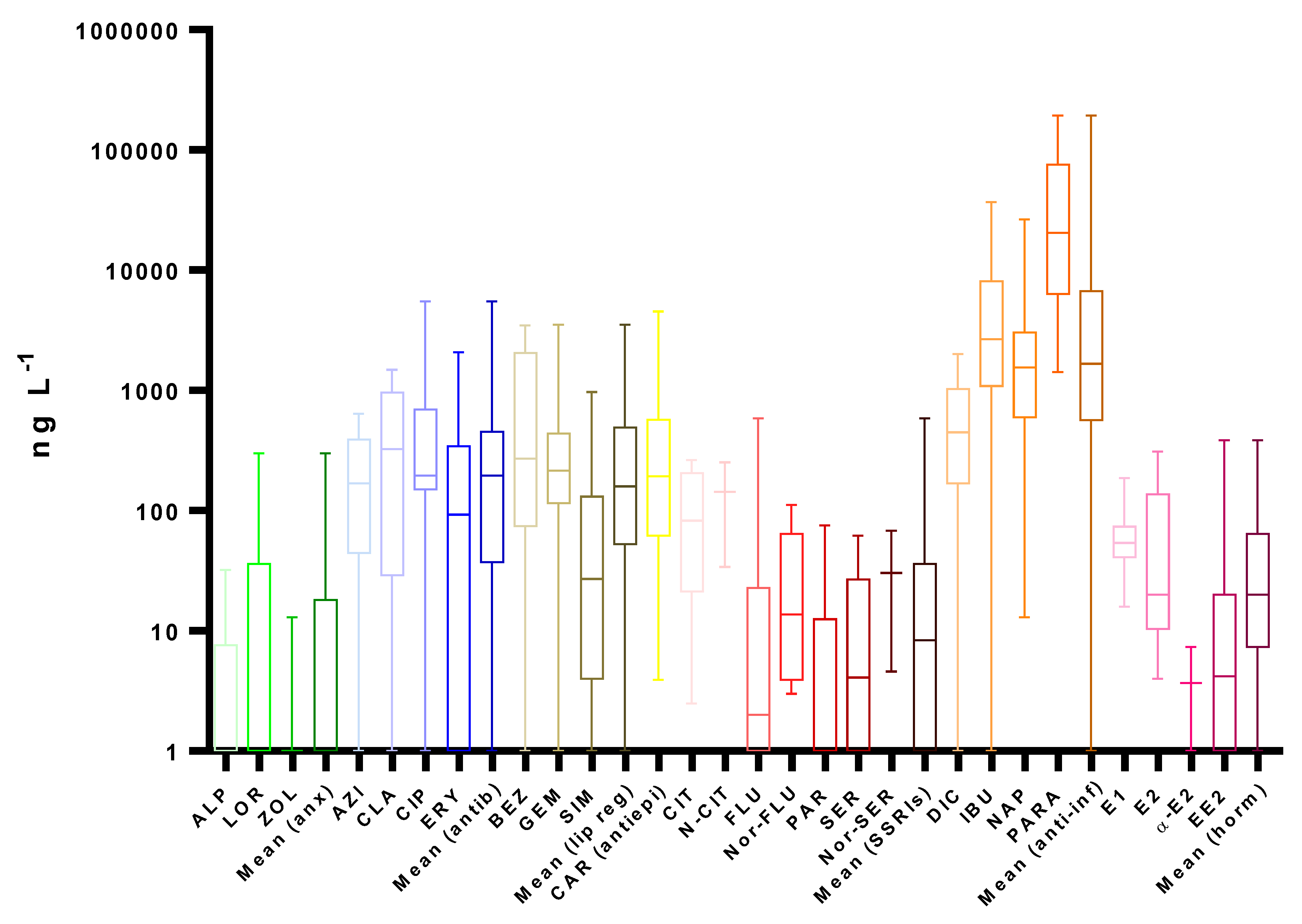
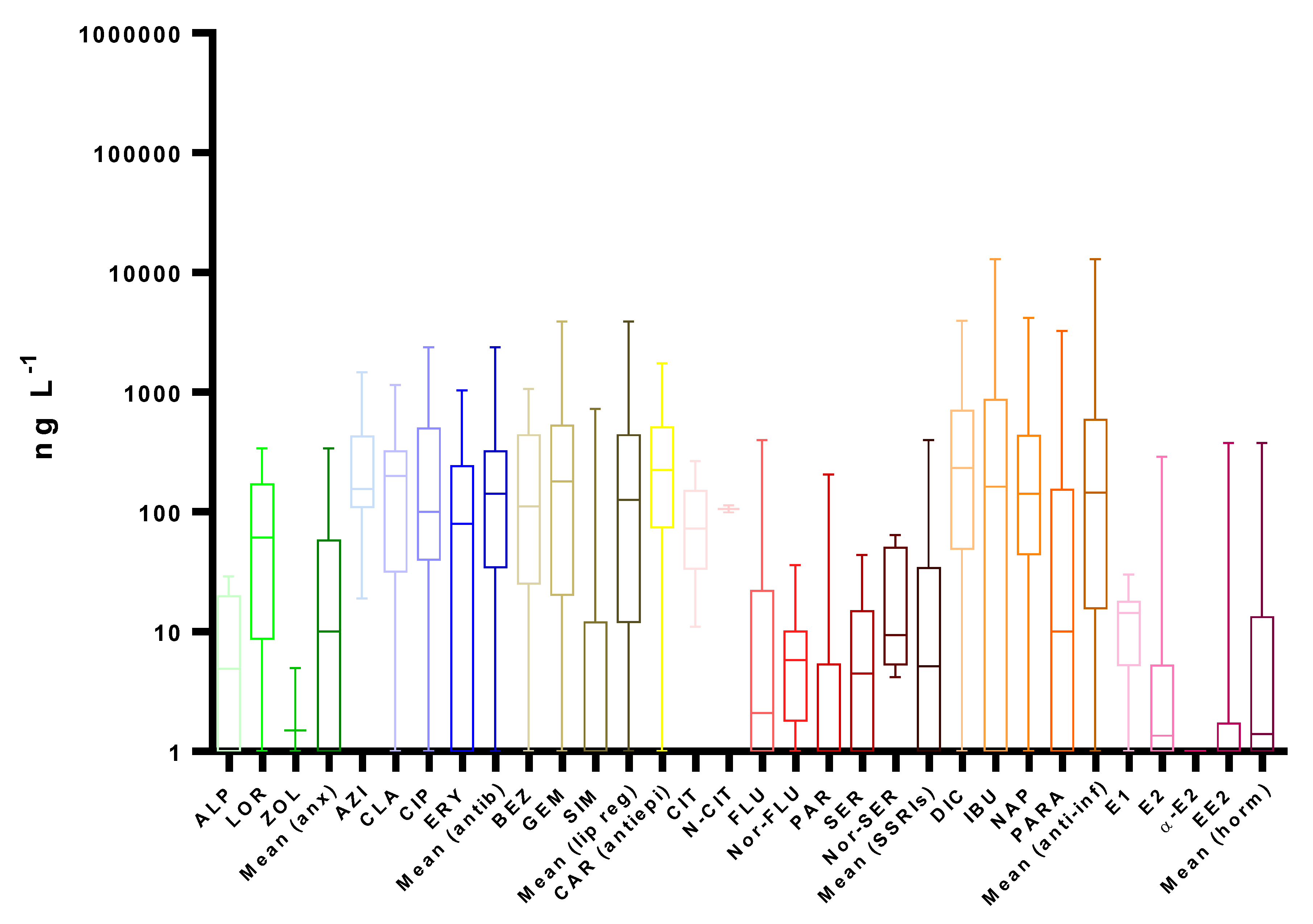
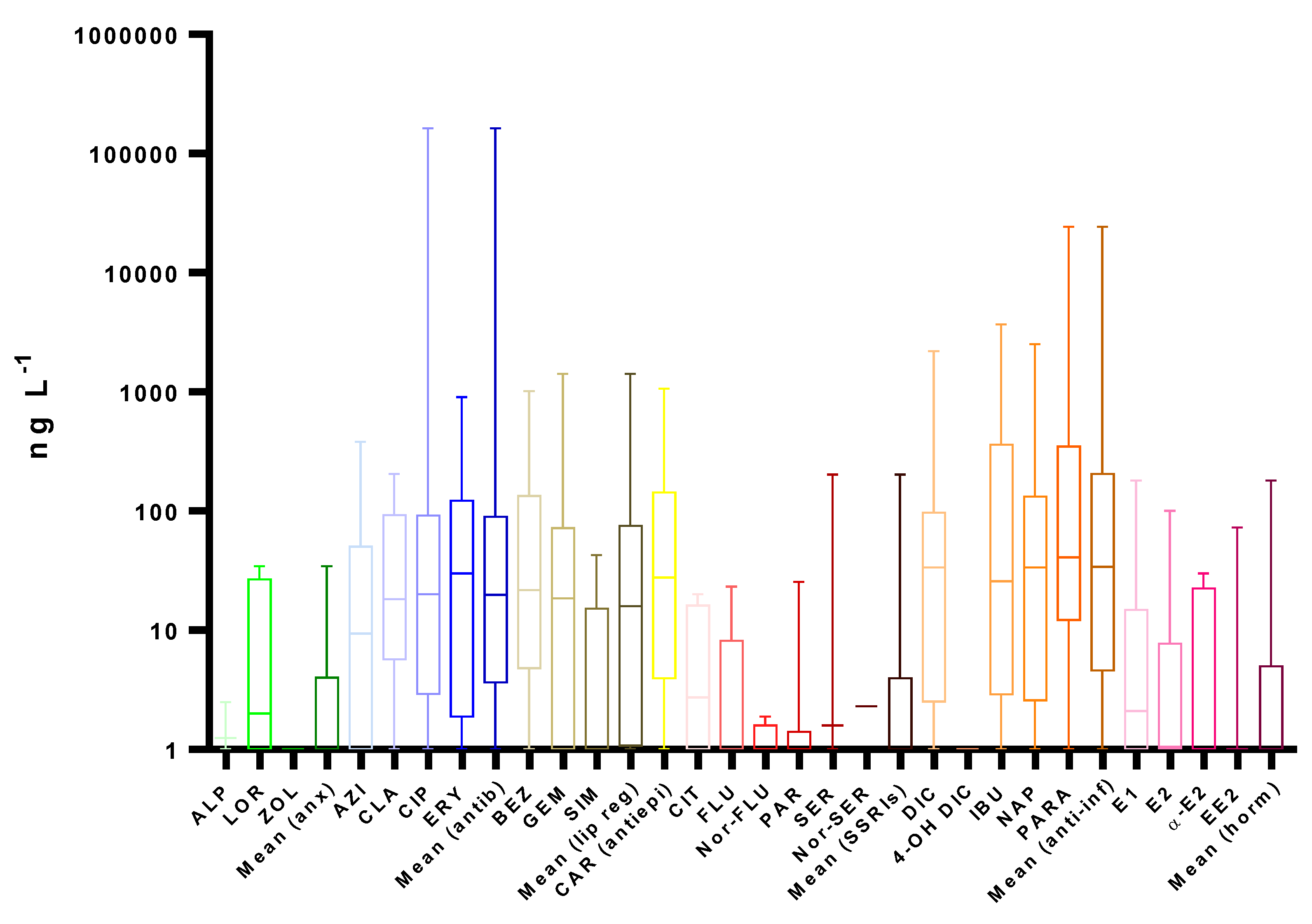
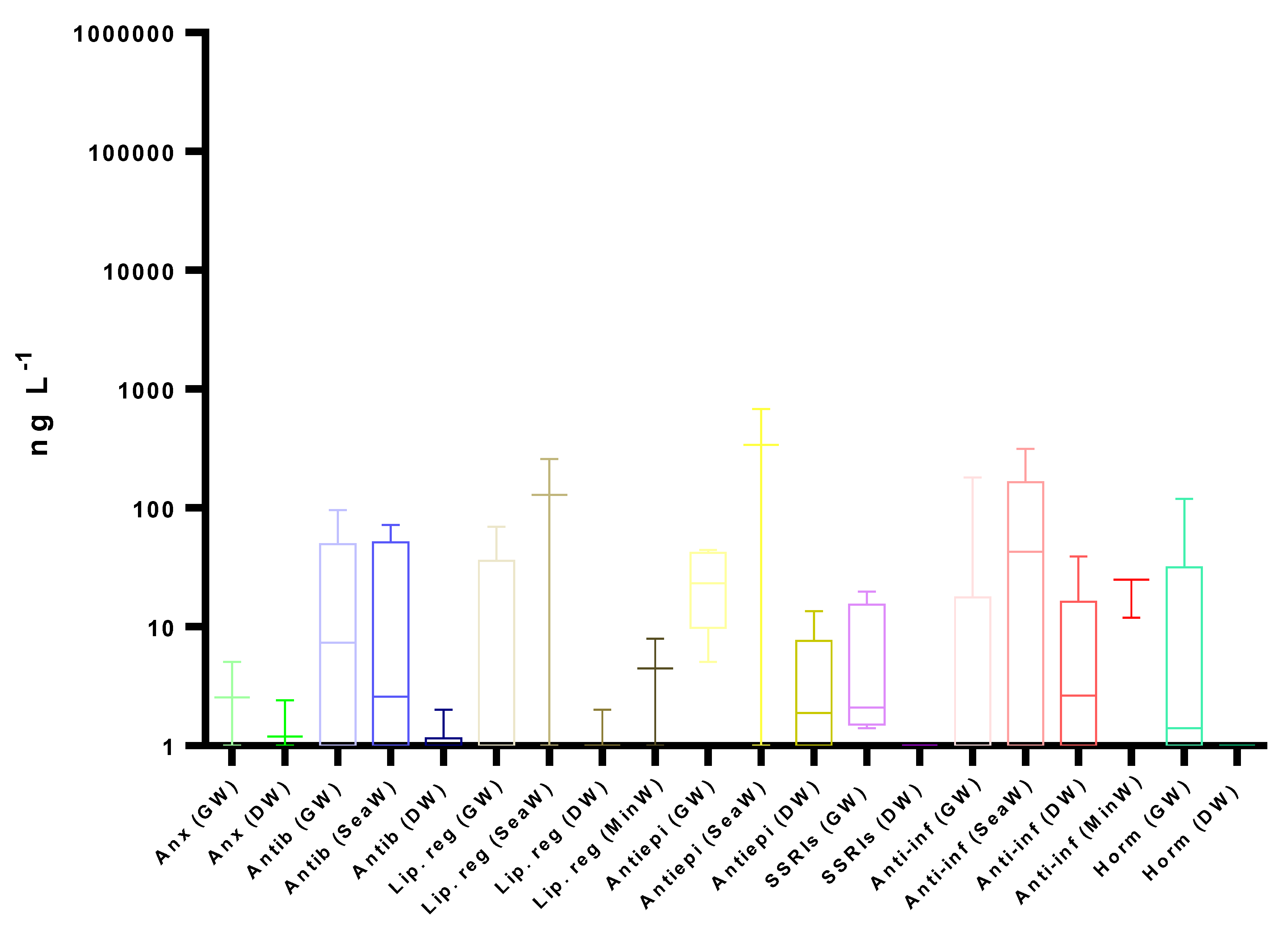
4. Occurrence
4.1. Wastewater
4.1.1. Wastewater Influents
4.1.2. Wastewater Effluents
4.2. Surface Water
4.3. Other Water Bodies
5. Final Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Barceló, D. LC-MS for identifying photodegradation products of pharmaceuticals in the environment. TrAC Trends Anal. Chem. 2007, 26, 486–493. [Google Scholar] [CrossRef]
- Seifrtová, M.; Pena, A.; Lino, C.M.; Solich, P. Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal. Bioanal. Chem. 2008, 391, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Focazio, M.J.; Kolpin, D.W.; Furlong, E.T. Occurrence of human pharmaceuticals in water resources of the United States: A review. In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Springer: Berlin/Heidelberg, Germany, 2004; pp. 91–105. [Google Scholar]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A. Occurrence and fate of pharmaceuticals in wastewater treatment processes; Universitat de Barcelona: Barcelona, Spain, 2012. [Google Scholar]
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Seifrtová, M.; Aufartová, J.; Vytlacilová, J.; Pena, A.; Solich, P.; Nováková, L. Determination of fluoroquinolone antibiotics in wastewater using ultra high-performance liquid chromatography with mass spectrometry and fluorescence detection. J. Sep. Sci. 2010, 33, 2094–2108. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Cunha, E.; Hajšlová, J.; Alpendurada, M.F. Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal. Bioanal. Chem. 2011, 399, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; Comero, S.; António, D.; Ghiani, M.; Lettieri, T.; Locoro, G.; Paracchini, B.; Tavazzi, S.; Gawlik, B.; et al. EU Wide Monitoring Survey on Waste Water Treatment Plant Effluents; European Comission Joint Research Centre Institute for Environment and Sustainability: Ispra, Italy, 2012; ISBN 978-92-79-26784-0. [Google Scholar]
- Salgado, R.; Noronha, J.P.; Oehmen, A.; Carvalho, G.; Reis, M.A.M. Analysis of 65 pharmaceuticals and personal care products in 5 wastewater treatment plants in Portugal using a simplified analytical methodology. Water Sci. Technol. 2010, 62, 2862. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef] [PubMed]
- European Commission Comission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing 495. Off. J. Eur. Union 2018, L141, 9–12.
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- ter Laak, T.L.; Van der Aa, M.; Houtman, C.J.; Stoks, P.G.; Van Wezel, A.P. Relating environmental concentrations of pharmaceuticals to consumption: A mass balance approach for the river Rhine. Environ. Int. 2010, 36, 403–409. [Google Scholar] [CrossRef]
- Bade, R.; Rousis, N.I.; Bijlsma, L.; Gracia-Lor, E.; Castiglioni, S.; Sancho, J.V.; Hernandez, F. Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS. Anal. Bioanal. Chem. 2015, 407, 8979–8988. [Google Scholar] [CrossRef]
- Altenburger, R.; Ait-Aissa, S.; Antczak, P.; Backhaus, T.; Barceló, D.; Seiler, T.-B.; Brion, F.; Busch, W.; Chipman, K.; de Alda, M.L.; et al. Future water quality monitoring — Adapting tools to deal with mixtures of pollutants in water resource management. Sci. Total Environ. 2015, 512–513, 540–551. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ruhoy, I.S. Environmental footprint of pharmaceuticals: the significance of factors beyond direct excretion to sewers. Environ. Toxicol. Chem. 2009, 28, 2495–2521. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Aceña, J.; Pérez, S.; López de Alda, M.; Barceló, D.; Gil, A.; Valcárcel, Y. Pharmaceuticals and iodinated contrast media in a hospital wastewater: A case study to analyse their presence and characterise their environmental risk and hazard. Environ. Res. 2015, 140, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Olsson, O.; Fiehn, R.; Herrel, M.; Kümmerer, K. The significance of different health institutions and their respective contributions of active pharmaceutical ingredients to wastewater. Environ. Int. 2015, 85, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: an ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2014, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Duarte, S.; Nunes, R.; Rocha, H.; Pena, A.; Meisel, L. Human and Veterinary Antibiotics Used in Portugal—A Ranking for Ecosurveillance. Toxics 2014, 2, 188–225. [Google Scholar] [CrossRef]
- Grung, M.; Källqvist, T.; Sakshaug, S.; Skurtveit, S.; Thomas, K.V. Environmental assessment of Norwegian priority pharmaceuticals based on the EMEA guideline. Ecotoxicol. Environ. Saf. 2008, 71, 328–340. [Google Scholar] [CrossRef]
- OECD Pharmaceutical consumption. Heal. glanceOECD Indic. 2012, 88–89.
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manage. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Jelic, A.; Petrović, M.; Barceló, D. Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: a case study of a catchment area in the Po Valley (Italy). Sci. Total Environ. 2014, 470–471, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, M.; Sacher, F.; Ter Laak, T.L. Prediction of concentration levels of metformin and other high consumption pharmaceuticals in wastewater and regional surface water based on sales data. Sci. Total Environ. 2013, 442, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Iii, C.E.G.; Kaye, A.M.; Pharm, D.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system – Mediated effects. Ochsner J. 2013, 214–223. [Google Scholar]
- Crestani, F.; Martin, J.R.; Möhler, H.; Rudolph, U. Mechanism of action of the hypnotic zolpidem in vivo. Br. J. Pharmacol. 2000, 131, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Blondeau, J.M. Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Ambrósio, A.F.; Soares-da-Silva, P.; Carvalho, C.M.; Carvalho, A.P. Mechanisms of Action of Carbamazepine and Its Derivatives, Oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002, 27, 121–130. [Google Scholar] [CrossRef]
- Kreke, N.; Dietrich, D.R. Physiological endpoints for potential SSRI interactions in fish. Crit. Rev. Toxicol. 2008, 38, 215–247. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E. Tools for evaluating selective serotonin re-uptake inhibitor residues as environmental contaminants. TrAC Trends Anal. Chem. 2010, 29, 832–847. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, D.M.; Oates, J.A.; Boutaud, O. New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin. Pharmacol. Ther. 2006, 79, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Hendry, L.B.; Mahesh, V.B. Emerging diversities in the mechanism of action of steroid hormones. J. Steroid Biochem. Mol. Biol. 1995, 52, 113–133. [Google Scholar] [CrossRef]
- Rivera, R.; Yacobson, I.; Grimes, D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999, 181, 1263–1269. [Google Scholar] [CrossRef]
- WHO. Pharmaceuticals in drinking water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Leclercq, M.; Mathieu, O.; Gomez, E.; Casellas, C.; Fenet, H.; Hillaire-Buys, D. Presence and fate of carbamazepine, oxcarbazepine, and seven of their metabolites at wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2009, 56, 408–415. [Google Scholar] [CrossRef]
- Straub, J. An Environmental Risk Assessment for Human-Use Trimethoprim in European Surface Waters. Antibiotics 2013, 2, 115–162. [Google Scholar] [CrossRef]
- Coetsier, C.M.; Spinelli, S.; Lin, L.; Roig, B.; Touraud, E. Discharge of pharmaceutical products (PPs) through a conventional biological sewage treatment plant: MECs vs PECs? Environ. Int. 2009, 35, 787–792. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. A critical evaluation of different parameters for estimating pharmaceutical exposure seeking an improved environmental risk assessment. Sci. Total Environ. 2017, 603C–604C, 226–236. [Google Scholar] [CrossRef]
- Fraser, A.D.; Bryan, W.; Isner, A.F. Urinary Screening for Alprazolam and its Major Metabolites by the Abbott ADx and TDx Analyzers with Confirmation by GC/MS. J. Anal. Toxicol. 1991, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Medical Products Agency–Sweden Medical Products Agency–Sweden. Available online: https://lakemedelsverket.se/english/ (accessed on 1 January 2015).
- Hempel, G.; Blaschke, G. Direct determination of zolpidem and its main metabolites in urine using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. B. Biomed. Appl. 1996, 675, 131–137. [Google Scholar] [CrossRef]
- Calamari, D.; Zuccato, E.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Strategic Survey of Therapeutic Drugs in the Rivers Po and Lambro in Northern Italy. Environ. Sci. Technol. 2003, 37, 1241–1248. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Fanelli, R. Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. J. Hazard. Mater. 2005, 122, 205–209. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Czech, B.; Buda, W. Photocatalytic treatment of pharmaceutical wastewater using new multiwall-carbon nanotubes/TiO2/SiO2 nanocomposites. Environ. Res. 2015, 137, 176–184. [Google Scholar] [CrossRef]
- Calisto, V.; Esteves, V.I. Psychiatric pharmaceuticals in the environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef]
- Rao, N. The clinical pharmacokinetics of escitalopram. Clin. Pharmacokinet. 2007, 46, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Martínez Bueno, M.J.; Lacorte, S.; Fernández-Alba, A.R.; Agüera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography-positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.; Gallagher, T.F. Excretion of estrogen metabolistes by humans II. The fate of large doses of estradiol-17beta after intramuscular and oral administration. J. Biol. Chem. 1955, 214, 315–364. [Google Scholar]
- Johnson, A.C.; Williams, R.J. A Model To Estimate Influent and Effluent Concentrations of Estradiol, Estrone, and Ethinylestradiol at Sewage Treatment Works. Environ. Sci. Technol. 2004, 38, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Keller, V.D.J.; Straub, J.O.; Monteiro, S.C.; Fussell, R.; Williams, R.J. Exploiting monitoring data in environmental exposure modelling and risk assessment of pharmaceuticals. Environ. Int. 2014, 73, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Jones-Lepp, T.L.; Sanchez, C.; Alvarez, D.A.; Wilson, D.C.; Taniguchi-Fu, R.L. Point sources of emerging contaminants along the Colorado River Basin: Source water for the arid Southwestern United States. Sci. Total Environ. 2012, 430, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodriguez-Mozaz, S.; Nannou, C.; Nakis, L.; Ruhí, A.; Acuña, V.; Sabater, S.; Barcelo, D. Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in biofilm from a waste water treatment plant-impacted river. Sci. Total Environ. 2016, 540, 241–249. [Google Scholar] [CrossRef]
- Cunningham, V.L.; Binks, S.P.; Olson, M.J. Human health risk assessment from the presence of human pharmaceuticals in the aquatic environment. Regul. Toxicol. Pharmacol. 2009, 53, 39–45. [Google Scholar] [CrossRef]
- Jelic, A.; Rodriguez-Mozaz, S.; Barceló, D.; Gutierrez, O. Impact of in-sewer transformation on 43 pharmaceuticals in a pressurized sewer under anaerobic conditions. Water Res. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment--a review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants-conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Marques, R.; Noronha, J.P.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant. Environ. Sci. Pollut. Res. Int. 2012, 19, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Gao, P.; Ding, Y.; Li, H.; Xagoraraki, I. Occurrence of pharmaceuticals in a municipal wastewater treatment plant: mass balance and removal processes. Chemosphere 2012, 88, 17–24. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Callejón, M.; Alonso, E. Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J. Hazard. Mater. 2009, 164, 1509–1516. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Jones, V.; Comber, S.; Scrimshaw, M.D.; Coello-Garcia, T.; Cartmell, E.; Lester, J.; Ellor, B. Performance of UK wastewater treatment works with respect to trace contaminants. Sci. Total Environ. 2013, 456–457, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69C, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E.; Pérez, S.; Petrović, M.; Barceló, D. Metabolism studies of diclofenac and clofibric acid in activated sludge bioreactors using liquid chromatography with quadrupole – time-of-flight mass spectrometry. J. Hydrol. 2009, 372, 109–117. [Google Scholar] [CrossRef]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 2014, 476–477, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Subedi, B.; Kannan, K. Occurrence and fate of select psychoactive pharmaceuticals and antihypertensives in two wastewater treatment plants in New York State, USA. Sci. Total Environ. 2015, 514, 273–280. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Lucas, D.; Gros, M.; Rodríguez-Mozaz, S.; Barceló, D.; Caminal, G.; Vicent, T. Identification of some factors affecting pharmaceutical active compounds (PhACs) removal in real wastewater. Case study of fungal treatment of reverse osmosis concentrate. J. Hazard. Mater. 2015, 283, 663–671. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodriguez-Roda, I.; Rodriguez-Mozaz, S.; Barceló, D. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Boix, C.; Ibáñez, M.; Sancho, J.V.; Parsons, J.R.; de Voogt, P.; Hernández, F. Biotransformation of pharmaceuticals in surface water and during waste water treatment: Identification and occurrence of transformation products. J. Hazard. Mater. 2016, 302, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sobek, A.; Radke, M. Fate of pharmaceuticals and their transformation products in four small european rivers receiving treated wastewater. Environ. Sci. Technol. 2016, 50, 5614–5621. [Google Scholar] [CrossRef] [PubMed]
- Schaar, H.; Clara, M.; Gans, O.; Kreuzinger, N. Micropollutant removal during biological wastewater treatment and a subsequent ozonation step. Environ. Pollut. 2010, 158, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Takada, H.; Correa, J.A.; El Saadi, K.; Koike, T.; Onwona-Agyeman, S.; Ofosu-Anim, J.; Sabi, E.B.; Wasonga, O.V.; Mghalu, J.M.; et al. Global occurrence of anti-infectives in contaminated surface waters: Impact of income inequality between countries. Environ. Int. 2015, 80, 89–97. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed—Analysis of their respective contributions. Sci. Total Environ. 2013, 454–455, 411–425. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Assessing environmental risk of pharmaceuticals in Portugal: an approach for the selection of the Portuguese monitoring stations in line with Directive 2013/39/EU. Chemosphere 2016, 144, 2507–2515. [Google Scholar] [CrossRef]
- Petrovic, M.; Gros, M.; Barcelo, D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A 2006, 1124, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Lee, M.Y.; Lai, W.W.P.; Lee, C.H.; Lin, A.Y.C.; Lin, C.F.; Lin, J.G. Removal of pharmaceuticals and organic matter from municipal wastewater using two-stage anaerobic fluidized membrane bioreactor. Bioresour. Technol. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.H.; Wennberg, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A.V. Screening of Human Antibiotic Substances and Determination of Weekly Mass Flows in Five Sewage Treatment Plants in Sweden. Environ. Sci. Technol. 2005, 39, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Lee, H.-B.; Peart, T.E.; Svoboda, M.L. Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2005, 1094, 122–129. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J. Hazard. Mater. 2013. [Google Scholar] [CrossRef]
- Styrishave, B.; Halling-Sørensen, B.; Ingerslev, F. Environmental risk assessment of three selective serotonin reuptake inhibitors in the aquatic environment: a case study including a cocktail scenario. Environ. Toxicol. Chem. 2011, 30, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Vasskog, T.; Berger, U.; Samuelsen, P.-J.; Kallenborn, R.; Jensen, E. Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J. Chromatogr. A 2006, 1115, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Vasskog, T.; Anderssen, T.; Pedersen-Bjergaard, S.; Kallenborn, R.; Jensen, E. Occurrence of selective serotonin reuptake inhibitors in sewage and receiving waters at Spitsbergen and in Norway. J. Chromatogr. A 2008, 1185, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of Basic Antidepressants and Their N-Desmethyl Metabolites in Raw Sewage and Wastewater Using Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. A one-year follow-up analysis of antidepressants in Portuguese wastewaters: occurrence and fate, seasonal influence, and risk assessment. Sci. Total Environ. 2014, 490, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Yu, T.-H.; Lateef, S.K. Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. J. Hazard. Mater. 2009, 167, 1163–1169. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.; Kim, H.S.; Kim, P. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, S.; Duennbier, U.; Heberer, T. Determination of estrogenic steroids in surface water and wastewater by liquid chromatography-electrospray tandem mass spectrometry. J. Sep. Sci. 2005, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Boisvert, M.; Fayad, P.B.; Sauvé, S. Development of a new multi-residue laser diode thermal desorption atmospheric pressure chemical ionization tandem mass spectrometry method for the detection and quantification of pesticides and pharmaceuticals in wastewater samples. Anal. Chim. Acta 2012, 754, 75–82. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multianalyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 4229–4245. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef]
- Qian, F.; He, M.; Song, Y.; Tysklind, M.; Wu, J. A bibliometric analysis of global research progress on pharmaceutical wastewater treatment during 1994-2013. Environ. Earth Sci. 2015, 4995–5005. [Google Scholar] [CrossRef]
- Grujić, S.; Vasiljević, T.; Laušević, M. Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography-ion trap-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4989–5000. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, A.B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Paíga, P.; Araújo, A.N.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Development of a simple analytical method for the simultaneous determination of paracetamol, paracetamol-glucuronide and p-aminophenol in river water. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2013, 930, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Shiraishi, H.; Yasuda, M.; Shinoda, A.; Suzuki, H.; Morita, M. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2003, 984, 195–202. [Google Scholar] [CrossRef]
- Al-Qaim, F.F.; Abdullah, M.P.; Othman, M.R.; Latip, J.; Zakaria, Z. Multi-residue analytical methodology-based liquid chromatography-time-of-flight-mass spectrometry for the analysis of pharmaceutical residues in surface water and effluents from sewage treatment plants and hospitals. J. Chromatogr. A 2014, 1345, 139–153. [Google Scholar] [CrossRef] [PubMed]
- McEachran, A.D.; Shea, D.; Bodnar, W.; Nichols, E.G. Pharmaceutical occurrence in groundwater and surface waters in forests land-applied with municipal wastewater. Environ. Toxicol. Chem. 2016, 35, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Acuña, V.; Schiller, D.; García-Galán, M.J.; Rodríguez-Mozaz, S.; Corominas, L.; Petrovic, M.; Poch, M.; Barceló, D.; Sabater, S. Occurrence and in-stream attenuation of wastewater-derived pharmaceuticals in Iberian rivers. Sci. Total Environ. 2015, 503–504, 133–141. [Google Scholar] [CrossRef]
- Navarro-Ortega, A.; Acuña, V.; Bellin, A.; Burek, P.; Cassiani, G.; Choukr-Allah, R.; Dolédec, S.; Elosegi, A.; Ferrari, F.; Ginebreda, A.; et al. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ. 2015, 503–504, 3–9. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Takada, H.; Mutoh, K.; Hosoda, H.; Harada, A.; Nakada, N. Nationwide monitoring of selected antibiotics: Distribution and sources of sulfonamides, trimethoprim, and macrolides in Japanese rivers. Sci. Total Environ. 2011, 409, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Vergeynst, L.; Haeck, A.; De Wispelaere, P.; Van Langenhove, H.; Demeestere, K. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography-magnetic sector mass spectrometry: Method quality assessment and application in a Belgian case study. Chemosphere 2015, 119, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- YUAN, S.-L.; LI, X.-F.; JIANG, X.-M.; ZHANG, H.-X.; ZHENG, S.-K. Simultaneous determination of 13 psychiatric pharmaceuticals in sewage by automated solid phase extraction and liquid chromatography-mass spectrometry. Chinese J. Anal. Chem. 2013, 41, 49–56. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.P.; Mexia, J.T.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludge plant. Environ. Pollut. 2011, 159, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Ginebreda, A.; Jelić, A.; Petrović, M.; López de Alda, M.; Barceló, D. New indexes for compound prioritization and complexity quantification on environmental monitoring inventories. Environ. Sci. Pollut. Res. Int. 2012, 19, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.H.; Östman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Treguer, R.J.F.; Magruder, C.; Royer, L.S.; Klaper, R.D. Evaluation of a model for the removal of pharmaceuticals, personal care products, and hormones from wastewater. Sci. Total Environ. 2013, 444, 515–521. [Google Scholar] [CrossRef]
- Sim, W.; Lee, J.; Oh, J. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 10298–10308. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.; Ternes, T.A.; Wilken, R.D.; Rodrigues, S.V.; Baumann, W. Polar drug residues in sewage and natural waters in the state of Rio de Janeiro, Brazil. Sci. Total Environ. 1999, 225, 135–141. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.P. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Miao, X.-S.; Metcalfe, C.D. Determination of cholesterol-lowering statin drugs in aqueous samples using liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2003, 998, 133–141. [Google Scholar] [CrossRef]
- Koutsouba, V.; Heberer, T.; Fuhrmann, B.; Schmidt-Baumler, K.; Tsipi, D.; Hiskia, A. Determination of polar pharmaceuticals in sewage water of Greece by gas chromatography-mass spectrometry. Chemosphere 2003, 51, 69–75. [Google Scholar] [CrossRef]
- Hernando, M.D.; Heath, E.; Petrovic, M.; Barceló, D. Trace-level determination of pharmaceutical residues by LC-MS/MS in natural and treated waters. A pilot-survey study. Anal. Bioanal. Chem. 2006, 385, 985–991. [Google Scholar] [CrossRef]
- Pailler, J.-Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef]
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013, 244–245, 259–267. [Google Scholar] [CrossRef]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Joshua, D.I.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products including psychoactives, antihypertensives, and antibiotics in two sewage treatment plants in southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Nebot, C.; Falcon, R.; Boyd, K.G.; Gibb, S.W. Introduction of human pharmaceuticals from wastewater treatment plants into the aquatic environment: a rural perspective. Environ. Sci. Pollut. Res. 2015, 14, 10559–10568. [Google Scholar] [CrossRef] [PubMed]
- Kostich, M.S.; Batt, A.L.; Lazorchak, J.M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 2014, 184, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187C, 193–201. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Hilton, M.J.; Thomas, K.V. Determination of selected human pharmaceutical compounds in effluent and surface water samples by high-performance liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2003, 1015, 129–141. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Agüera, A.; Hernando, M.D.; Gómez, M.J.; Fernández-Alba, A.R. Evaluation of various liquid chromatography-quadrupole-linear ion trap-mass spectrometry operation modes applied to the analysis of organic pollutants in wastewaters. J. Chromatogr. A 2009, 1216, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Verenitch, S.S.; Lowe, C.J.; Mazumder, A. Determination of acidic drugs and caffeine in municipal wastewaters and receiving waters by gas chromatography-ion trap tandem mass spectrometry. J. Chromatogr. A 2006, 1116, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Codru, N.; Dziewulski, D.M.; Wilson, L.R.; Xue, J.; Yun, S.; Braun-Howland, E.; Minihane, C.; Kannan, K. A pilot study on the assessment of trace organic contaminants including pharmaceuticals and personal care products from on-site wastewater treatment systems along Skaneateles Lake in New York State, USA. Water Res. 2014, 72, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, A.; Svanfelt, J.; Kronberg, L.; Prévost, M.; Weyhenmeyer, G.A. Seasonal variations in the occurrence and fate of basic and neutral pharmaceuticals in a Swedish river-lake system. Chemosphere 2010, 80, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Crouse, B.A.; Ghoshdastidar, A.J.; Tong, A.Z. The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ. Res. 2012, 112, 92–99. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Ros, O.; Kortazar, L.; Fernández, L.A.; Olivares, M.; Zuloaga, O.; Prieto, A.; Etxebarria, N. Occurrence of emerging pollutants in estuaries of the Basque Country: Analysis of sources and distribution, and assessment of the environmental risk. Water Res. 2018, 147, 152–163. [Google Scholar] [CrossRef]
- Chiffre, A.; Degiorgi, F.; Buleté, A.; Spinner, L.; Badot, P.M. Occurrence of pharmaceuticals in WWTP effluents and their impact in a karstic rural catchment of Eastern France. Environ. Sci. Pollut. Res. 2016, 23, 25427–25441. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Tsai, Y.T. Occurrence of pharmaceuticals in Taiwan’s surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities. Sci. Total Environ. 2009, 407, 3793–3802. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jang, H.-S.; Kim, J.-G.; Ishibashi, H.; Hirano, M.; Nasu, K.; Ichikawa, N.; Takao, Y.; Shinohara, R.; Arizono, K. Occurrence of Pharmaceutical and Personal Care Products (PPCPs) in Surface Water from Mankyung River, South Korea. J. Heal. Sci. 2009, 55, 249–258. [Google Scholar] [CrossRef]
- Lv, M.; Sun, Q.; Hu, A.; Hou, L.; Li, J.; Cai, X.; Yu, C.-P. Pharmaceuticals and personal care products in a mesoscale subtropical watershed and their application as sewage markers. J. Hazard. Mater. 2014, 280, 696–705. [Google Scholar] [CrossRef]
- European Commission Commission implementing decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015.
- European Parliament Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, L 226, 1–17.
- Gonçalves, C.M.O.; Sousa, M.A.D.; Alpendurada, M.D.F.P. Analysis of acidic, basic and neutral pharmaceuticals in river waters: clean-up by 1°, 2° amino anion exchange and enrichment using an hydrophilic adsorbent. Int. J. Environ. Anal. Chem. 2013, 93, 1–22. [Google Scholar]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE 2013, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Petrović, M.; Barceló, D. Direct analysis of pharmaceuticals, their metabolites and transformation products in environmental waters using on-line TurboFlowTM chromatography-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1252, 115–129. [Google Scholar] [CrossRef]
- Yang, L.; Luan, T.; Lan, C. Solid-phase microextraction with on-fiber silylation for simultaneous determinations of endocrine disrupting chemicals and steroid hormones by gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1104, 23–32. [Google Scholar] [CrossRef]
- López-Roldán, R.; de Alda, M.L.; Gros, M.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography-triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography-time of flight-mass spectrometry. Chemosphere 2010, 80, 1337–1344. [Google Scholar] [CrossRef]
- Madureira, T.V.; Barreiro, J.C.; Rocha, M.J.; Rocha, E.; Cass, Q.B.; Tiritan, M.E. Spatiotemporal distribution of pharmaceuticals in the Douro River estuary (Portugal). Sci. Total Environ. 2010, 408, 5513–5520. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.-F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ. Chem. Lett. 2009, 9, 103–114. [Google Scholar] [CrossRef]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Managaki, S.; Murata, A.; Takada, H.; Bui, C.T.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- Hoa, P.T.P.; Managaki, S.; Nakada, N.; Takada, H.; Shimizu, A.; Anh, D.H.; Viet, P.H.; Suzuki, S. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 2011, 409, 2894–2901. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II) Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Brown, L.E.; Kay, P. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef]
- Ginebreda, A.; Muñoz, I.; Alda, M.L.; Brix, R.; López-Doval, J.; Barceló, D. Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Witter, J.D.; Spongberg, A.L.; Wang, K.; Wang, D.; Liu, J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze river, China. Ecotoxicol. Environ. Saf. 2014, 106, 19–26. [Google Scholar] [CrossRef]
- de Gaffney, V.J.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Chmielova, D.; Lino, C.M.; Solich, P. Determination of fluoroquinolone antibiotics in surface waters from Mondego River by high performance liquid chromatography using a monolithic column. J. Sep. Sci. 2007, 30, 2924–2928. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, S.; Pileggi, V.; Yang, P.; Hao, C.; Zhao, X.; Rocks, C.; Thach, S.; Cheung, P.; Whitehead, B. Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Sci. Total Environ. 2011, 409, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Ginebreda, A.; Kuzmanovic, M.; Guasch, H.; de Alda, M.L.; López-Doval, J.C.; Muñoz, I.; Ricart, M.; Romaní, A.M.; Sabater, S.; Barceló, D. Assessment of multi-chemical pollution in aquatic ecosystems using toxic units: compound prioritization, mixture characterization and relationships with biological descriptors. Sci. Total Environ. 2014, 468–469, 715–723. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, C.M.; Kooij, P.J.F.; de Voogt, P.; ter Laak, T.L. Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci. Total Environ. 2012, 427–428, 70–77. [Google Scholar] [CrossRef]
- Kunkel, U.; Radke, M. Fate of pharmaceuticals in rivers: Deriving a benchmark dataset at favorable attenuation conditions. Water Res. 2012, 46, 5551–5565. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Zhao, J.L.; Ying, G.G.; Wang, L.; Yang, J.F.; Yang, X.B.; Yang, L.H.; Li, X. Determination of phenolic endocrine disrupting chemicals and acidic pharmaceuticals in surface water of the Pearl Rivers in South China by gas chromatography-negative chemical ionization-mass spectrometry. Sci. Total Environ. 2009, 407, 962–974. [Google Scholar] [CrossRef]
- Schriks, M.; Heringa, M.B.; Van der Kooi, M.M.E.; de Voogt, P.; Van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 2010, 44, 461–476. [Google Scholar] [CrossRef]
- Weigel, S.; Kallenborn, R.; Hühnerfuss, H. Simultaneous solid-phase extraction of acidic, neutral and basic pharmaceuticals from aqueous samples at ambient (neutral) pH and their determination by gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1023, 183–195. [Google Scholar] [CrossRef]
- Vulliet, E.; Wiest, L.; Baudot, R.; Grenier-Loustalot, M.F. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2008, 1210, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Na, T.W.; Kang, T.W.; Lee, K.H.; Hwang, S.H.; Jung, H.J.; Kim, K. Distribution and ecological risk of pharmaceuticals in surface water of the Yeongsan river, Republic of Korea. Ecotoxicol. Environ. Saf. 2019, 181, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.G.; Katz, D.R.; Sullivan, J.C.; Shapley, D.; Lipscomb, J.; Epstein, J.; Juhl, A.R.; Knudson, C.; O’Mullan, G.D. Spatial patterns of pharmaceuticals and wastewater tracers in the Hudson River Estuary. Water Res. 2018, 137, 335–343. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Caçador, I.; Freitas, A.; Vila Pouca, A.S.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F.; et al. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef]
- Ivešić, M.; Krivohlavek, A.; Žuntar, I.; Tolić, S.; Šikić, S.; Musić, V.; Pavlić, I.; Bursik, A.; Galić, N. Monitoring of selected pharmaceuticals in surface waters of Croatia. Environ. Sci. Pollut. Res. 2017, 24, 23389–23400. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J.; Chitongo, R. Determination of selected steroid hormones in some surface water around animal farms in Cape Town using HPLC-DAD. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Torres-Padrón, M.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics 2013, 2, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Magnér, J.; Filipovic, M.; Alsberg, T. Application of a novel solid-phase-extraction sampler and ultra-performance liquid chromatography quadrupole-time-of-flight mass spectrometry for determination of pharmaceutical residues in surface sea water. Chemosphere 2010, 80, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Lange, F.T.; Brauch, H.-J.; Blankenhorn, I. Pharmaceuticals in groundwaters. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A. Occurrence, Treatment, and Toxicological Relevance of EDCs and Pharmaceuticals in Water. Ozone Sci. Eng. 2008, 30, 65–69. [Google Scholar] [CrossRef]
| Therapeutic Group | Compound and Chemical Structure | |||
|---|---|---|---|---|
| Anxiolytics and Hypnotics (Anx) | Alprazolam (ALP) | Lorazepam (LOR) | Zolpidem (ZOL) | |
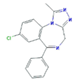 | 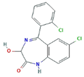 | 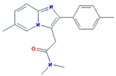 | ||
| Antibiotics (Antib) | Azithromycin (AZI) | Ciprofloxacin (CIP) | Clarithromycin (CLA) | Erythromycin (ERY) |
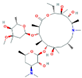 |  |  | 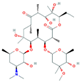 | |
| Lipid Regulators (Lip Reg) | Bezafibrate (BEZ) | Gemfibrozil(GEM) | Simvastatin (SIM) | |
 | 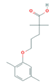 | 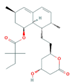 | ||
| Antiepileptic (Antiepi) | Carbamazepine (CAR) | |||
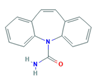 | ||||
| Selective Serotonin Reuptake Inhibitors (SSRIs) | Citalopram (CIT) | Desmethylcitalopram (N-Cit) (metabolite) | Escitalopram (ESC) | Fluoxetine (FLU) |
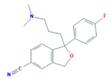 | 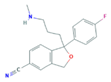 | 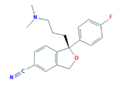 | 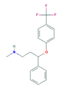 | |
| Norfluoxetine (Nor-FLU) (metabolite) | Paroxetine (PAR) | Sertraline (SER) | Desmethylsertraline (Nor-SER) (metabolite) | |
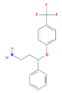 | 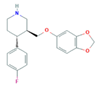 | 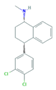 | 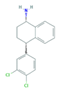 | |
| Anti-Inflammatories (Anti-inf) | Diclofenac (DIC) | 4-hydroxydiclofenac (4-OH-DIC) (metabolite) | Ibuprofen(IBU) | Naproxen (NAP) |
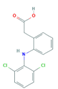 | 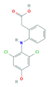 | 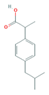 | 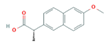 | |
| Paracetamol (PARA) | 4-aminophenol (4-PARA) (transformation product) | |||
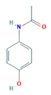 |  | |||
| Hormones (Horm) | Estrone (E1) (natural hormone/metabolite) | 17β-estradiol (E2) | 17α-ethinylestradiol (EE2) | |
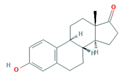 | 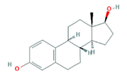 | 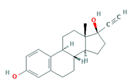 | ||
| Therapeutic Group | Pharmaceutical | DDD 1000 inh−1 d−1 | mg inh−1 y−1 | kg y−1 | Year | Country | Reference |
|---|---|---|---|---|---|---|---|
| Anx | ALP | 17.64 a | 6.4 a | 302 a | 2010 | Spain | [27] |
| NA | 2.9 | 178 | 2004 | France | |||
| LOR | 19.67 a | 17.9 | 844 | 2010 | Spain | [27] | |
| NA | 9.6 | 585 | 2004 | France | |||
| 13.3 | NA | 709 | 2010 | Italy | [5] | ||
| Antib | AZI | 0.9 a | 98.6 | 4634 a | 2010 | Spain | [27] |
| NA | 67.1 | 4073 | 2004 | France | |||
| NA | NA | 13870 | 2010 | Italy | [34] | ||
| 1.3 | NA | 13870 | 2010 | Italy | [5] | ||
| CIP | 1.1 a | 401.5 | 18870 a | 2010 | Spain | [27] | |
| NA | 200.7 | 12186 | 2004 | France | |||
| NA | NA | 21672 | 2010 | Italy | [34] | ||
| 1.0 | NA | 21672 | 2010 | Italy | [5] | ||
| CLA | 0.6 a | 231.0 | 10864 a | 2010 | Spain | [27] | |
| NA | 150 | 12360 | 2010 | Germany | |||
| NA | 232.9 | 1700 | 2010 | Switzerland | |||
| NA | 276.1 | 16889 | 2010 | France | |||
| NA | NA | 64470 | 2010 | Italy | [34] | ||
| 3.0 | NA | 64470 | 2010 | Italy | [5] | ||
| ERY | 0.1 a | NA | 1716a | 2010 | Spain | [27] | |
| NA | NA | 0.12 | 2010 | Italy | [34] | ||
| Lip reg | BEZ | 0.6 a | 133.0 a | 6178 a | 2010 | Spain | [27] |
| NA | 475.2 | 39158 | 2010 | Germany | |||
| NA | 215.6 | 1574 | 2010 | Switzerland | |||
| NA | 343.4 | 20852 | 2004 | France | |||
| NA | 66.7 | NA | 2005 | Sweden | |||
| NA | NA | 7600 | 2001 | Italy | [5] | ||
| SIM | NA | 282.7 a | 13340 a | 2010 | Spain | [27] | |
| NA | 114.3 | 6943 | 2004 | France | |||
| Antiepi | CAR | 1.2 a | 438.0 | 20595 | 2010 | Spain | [27] |
| NA | 1010.9 | 83299 | 2010 | Germany | |||
| NA | 857.5 | 6260 | 2010 | Switzerland | |||
| NA | 554.3 | 33364 | 2010 | France | |||
| NA | 463.0 | 820 | 2005 | Sweden | |||
| NA | NA | 31190 | 2010 | Italy | [34] | ||
| NA | 0.61–0.98 | NA | 2010 | Europe | [35] | ||
| NA | NA | 31190 | 2010 | Italy | [5] | ||
| NA | NA | 88000 | 2001 | Germany | [1] | ||
| SSRIs | ESC | 0.01 a | 38.8 | 1824 a | 2010 | Spain | [27] |
| NA | 0.08 | 4.6 | 2004 | France | |||
| FLU | 0.02 a | 62.0 | 2914 a | 2010 | Spain | [27] | |
| NA | 61.6 | 3740 | 2004 | France | |||
| PAR | 0.02 a | 69.4 | 3264 a | 2010 | Spain | [27] | |
| NA | 90.8 | 5515 | 2004 | France | |||
| SER | 0.05 a | 102.1 | 4800 a | 2010 | Spain | [27] | |
| NA | 102.5 | 6224 | 2004 | France | |||
| Anti-inf | DIC | 7.9 a | 369.9 | 17395 a | 2010 | Spain | [27] |
| NA | 953.6 | 78579 | 2010 | Germany | |||
| NA | 934.1 | 6819 | 2010 | Switzerland | |||
| NA | 370.1 | 22640 | 2010 | France | |||
| NA | 375.9 | NA | 2005 | Sweden | |||
| NA | 60–880 | NA | 2009 | Europe | [35] | ||
| 4.5 | NA | 9602 | 2010 | Italy | [5] | ||
| NA | NA | 345000 | 2001 | Germany | [1] | ||
| IBU | NA | 4647.5 | 218527 | 2010 | Spain | [27] | |
| NA | 3043.6 | 250792 | 2010 | Germany | |||
| NA | 3078.2 | 22471 | 2010 | Switzerland | |||
| NA | 953.8 | 58353 | 2010 | France | |||
| NA | NA | 7864 | 2005 | Sweden | |||
| NA | NA | 622000 | 2001 | Germany | [1] | ||
| NAP | 5.15 a | 1205.9 | 56700 a | 2010 | Spain | [27] | |
| NA | 614.7 | 37332 | 2004 | France | |||
| PARA | NA | 22667.7 | 1065835 | 2010 | Spain | [27] | |
| NA | 54389.5 | 3303077 | 2004 | France | |||
| NA | NA | 836000 | 2001 | Germany | [1] | ||
| Horm | E2 | 0.894 a | 12.6 a | 2010 | Spain | [27] | |
| EE2 | 1.1969 a | 0.03 | 1.2 a | 2010 | Spain | [27] | |
| NA | 0.58 | 48.2 | 2001 | Germany | |||
| NA | 0.54 | 4.0 | 2000 | Switzerland | |||
| NA | 0.11 | NA | 2005 | Sweden |
| Therapeutic Group | Pharmaceutical | Excretion Results | References |
|---|---|---|---|
| Anx | ALP | 20 | [55] |
| LOR | 72.5 | [56] | |
| ZOL | 0.75 | [57] | |
| Antib | AZI | 12 | [56] |
| CIP | 60/83.7 | [1] | |
| 70 | [5] | ||
| 70 | [56] | ||
| CLA | 25 | [58] | |
| 25 | [25] | ||
| ERY | 25 | [49] | |
| 10 | [58] | ||
| 5 | [59] | ||
| Lip reg | BEZ | 72 | [60] |
| 69 | [5] | ||
| 47.5 | [1] | ||
| 50 | [61] | ||
| 45 | [62] | ||
| GEM | 50 | [63] | |
| SIM | 12.5 | [1] | |
| 12.5 | [62] | ||
| Antiepi | CAR | 33 | [25] |
| 5 | [64] | ||
| 3 | [29] | ||
| 3 | [59] | ||
| SSRIs | CIT | 23 | [56] |
| 12/20 | [65] | ||
| ESC | 9 | [66] | |
| FLU | 5/10/11 | [65] | |
| 10 | [28] | ||
| SER | 0.2 | [56] | |
| 0.2 | [28] | ||
| 0.2 | [65] | ||
| PAR | 3 | [56] | |
| 3 | [28] | ||
| 3 | [65] | ||
| Anti-inf | DIC | 39 | [5] |
| 15 | [1] | ||
| 15 | [63] | ||
| 15 | [60] | ||
| 12.5 | [62] | ||
| IBU | 15 | [67] | |
| 10 | [68] | ||
| 10 | [61] | ||
| 5 | [1] | ||
| NAP | 10 | [25] | |
| <1 | [59] | ||
| PARA | 80 | [69] | |
| 75 | [56] | ||
| Horm | E2 | 5.6 | [70] |
| EE2 | 22/26/27/35/42/53/66/68 | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules 2020, 25, 1026. https://doi.org/10.3390/molecules25051026
Pereira A, Silva L, Laranjeiro C, Lino C, Pena A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules. 2020; 25(5):1026. https://doi.org/10.3390/molecules25051026
Chicago/Turabian StylePereira, André, Liliana Silva, Célia Laranjeiro, Celeste Lino, and Angelina Pena. 2020. "Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence" Molecules 25, no. 5: 1026. https://doi.org/10.3390/molecules25051026
APA StylePereira, A., Silva, L., Laranjeiro, C., Lino, C., & Pena, A. (2020). Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules, 25(5), 1026. https://doi.org/10.3390/molecules25051026






