Laser-Plasma Spectroscopy of Hydroxyl with Applications
Abstract
1. Introduction
2. Mini-Summary of OH Literature and Applications
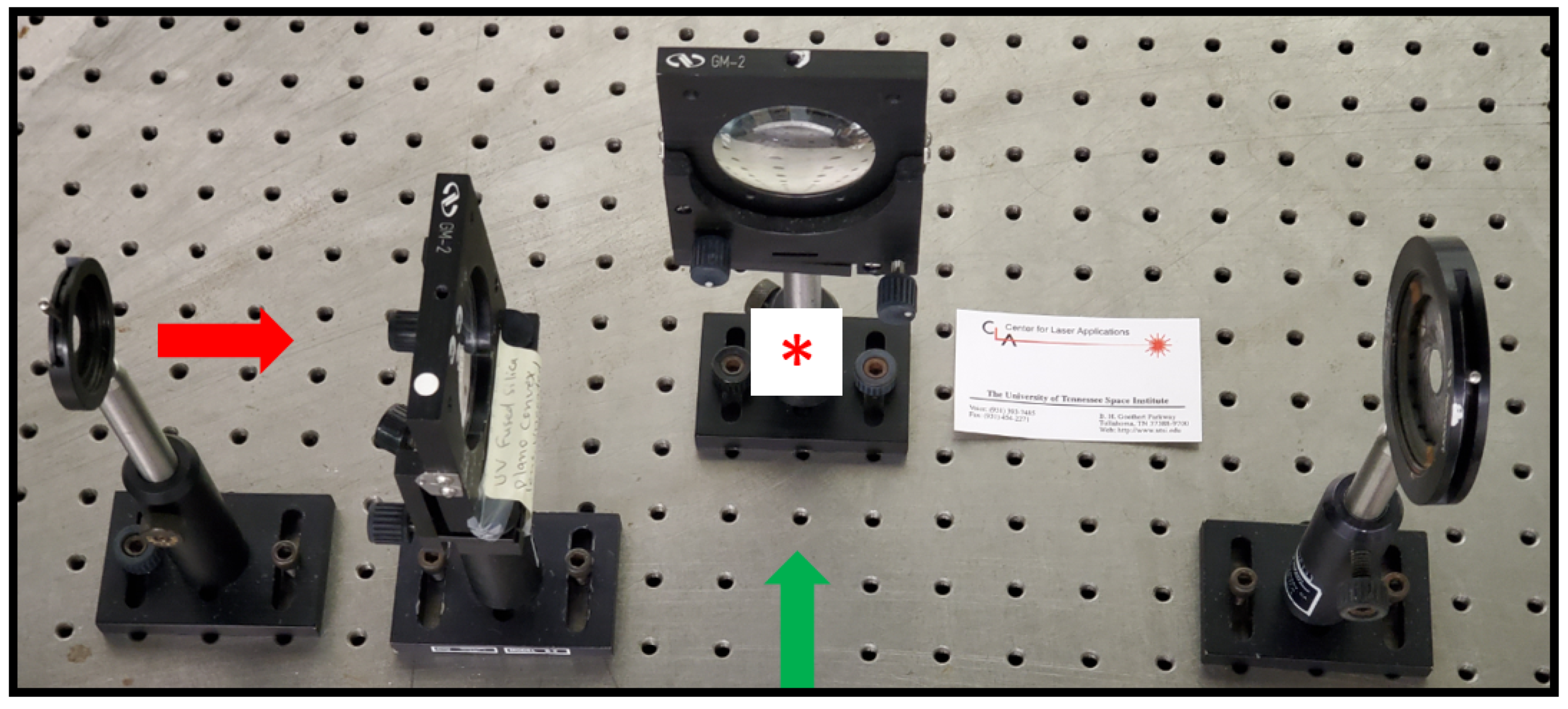
3. Experimental Details
4. Results and Discussion
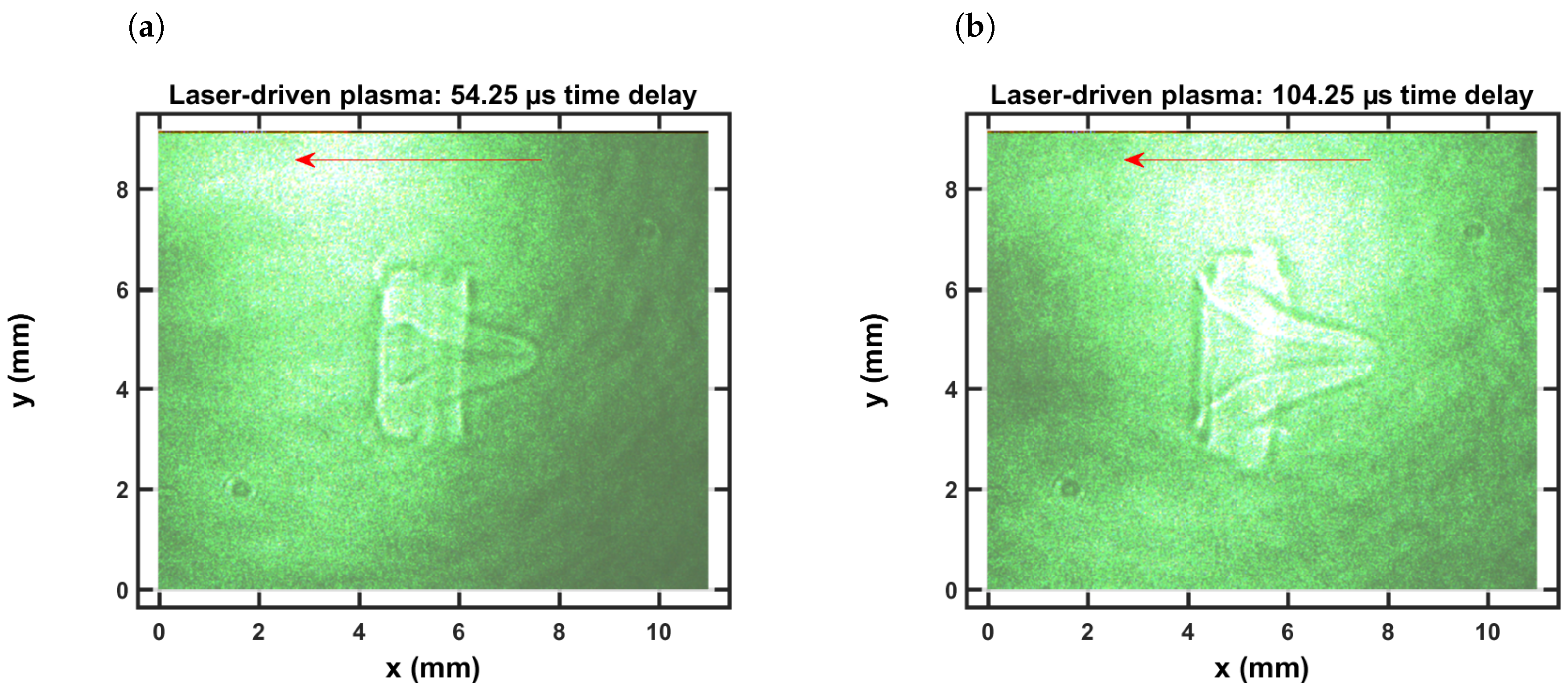
4.1. Shadowgraphs
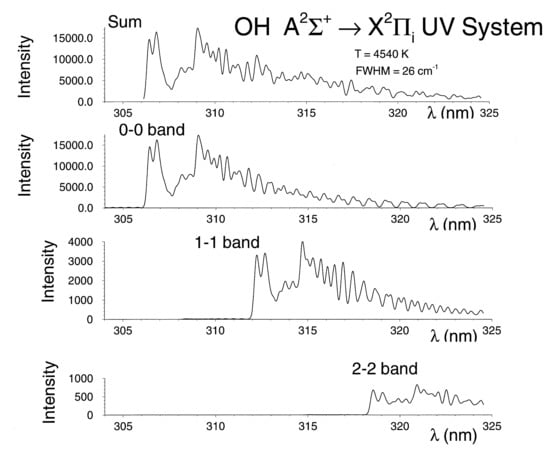
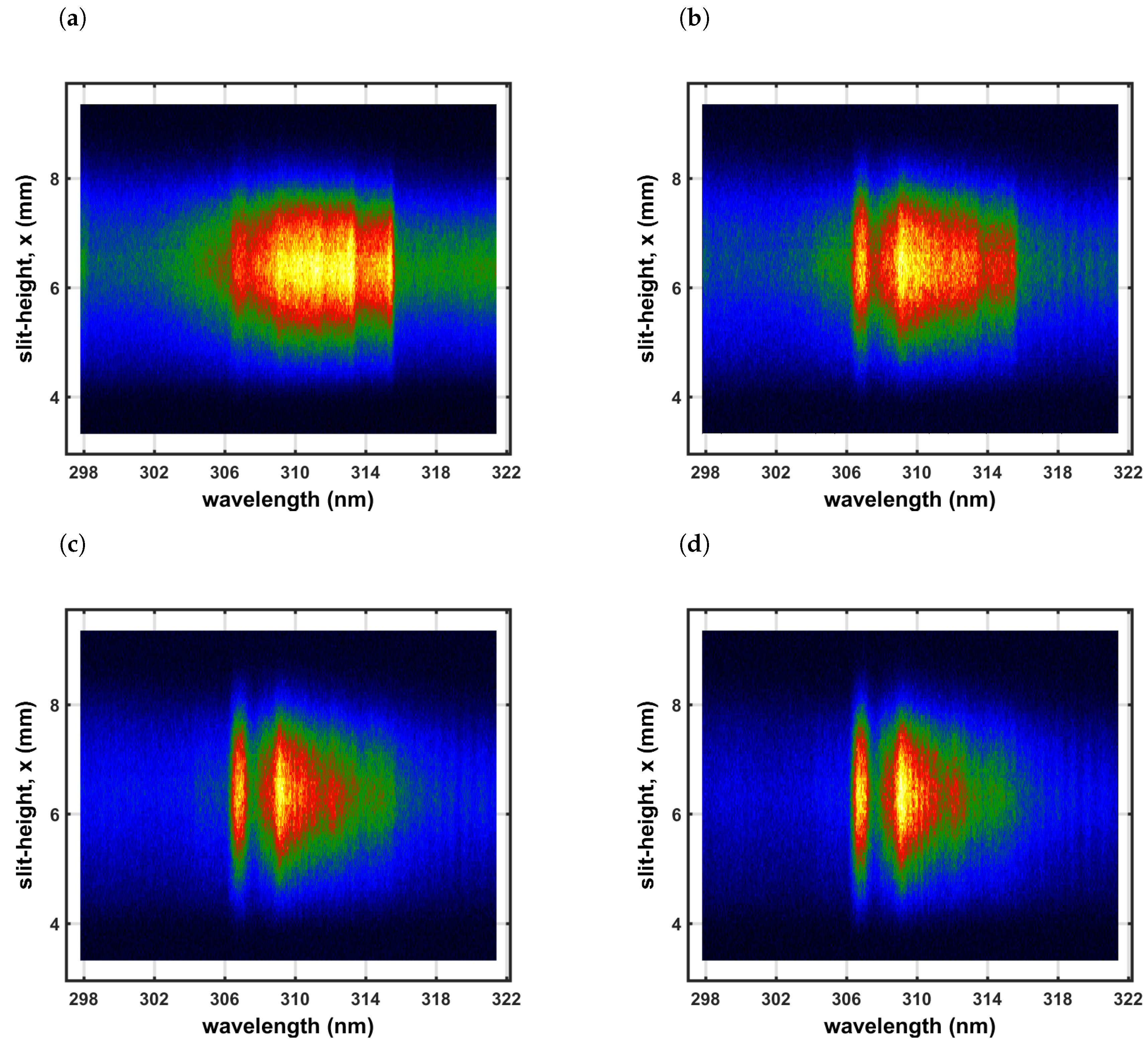
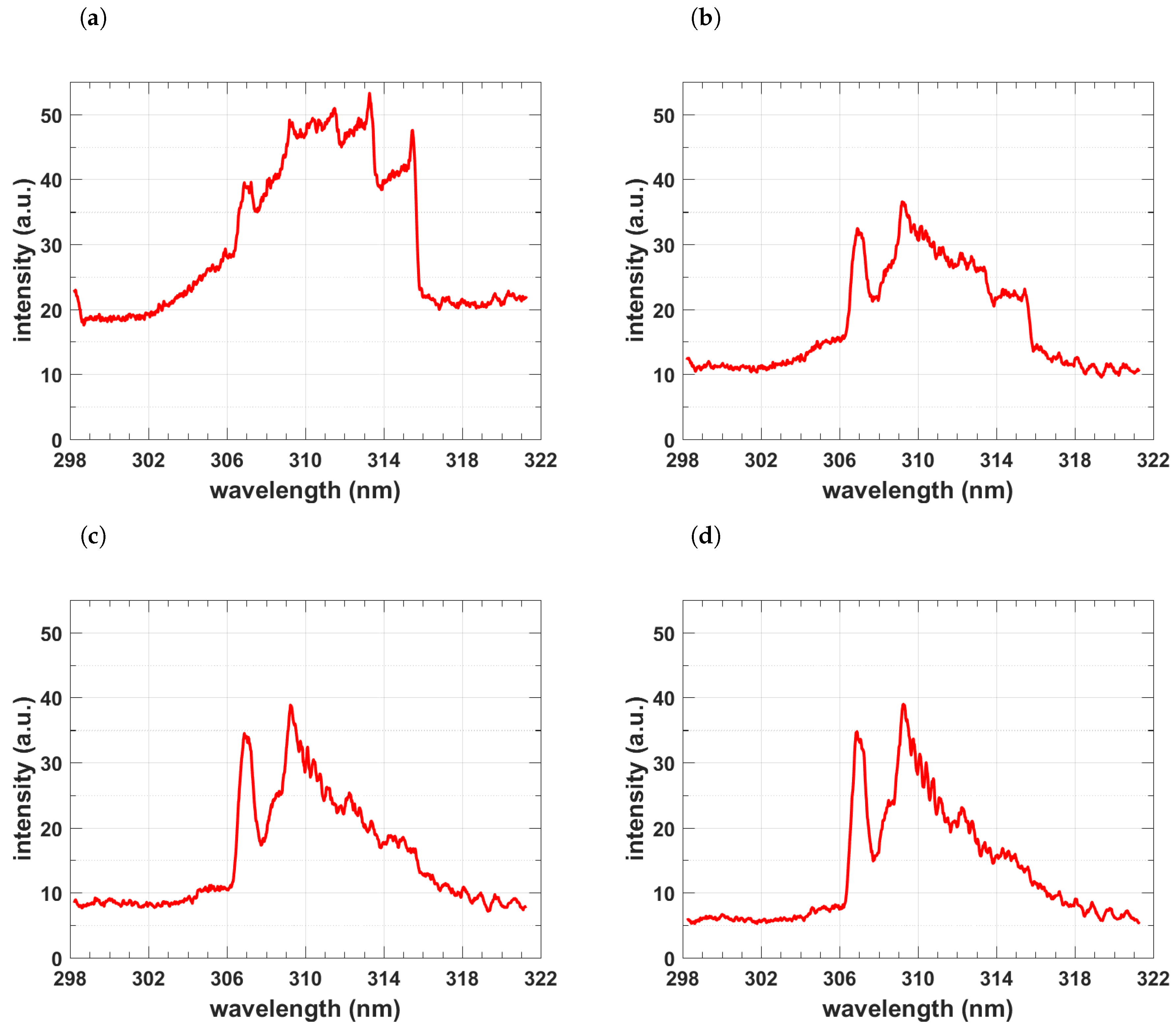
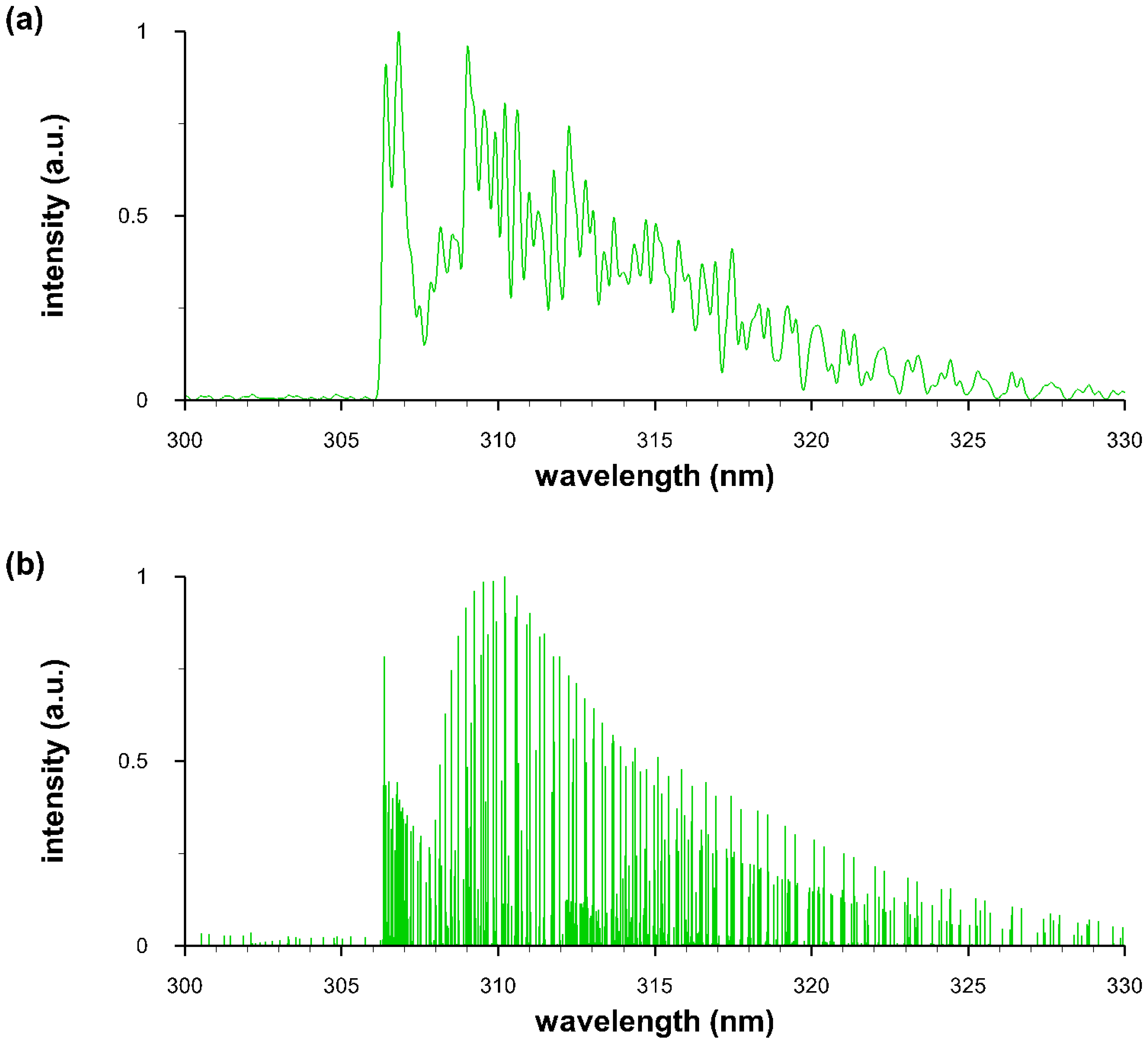
4.2. Measurement of OH Spectra
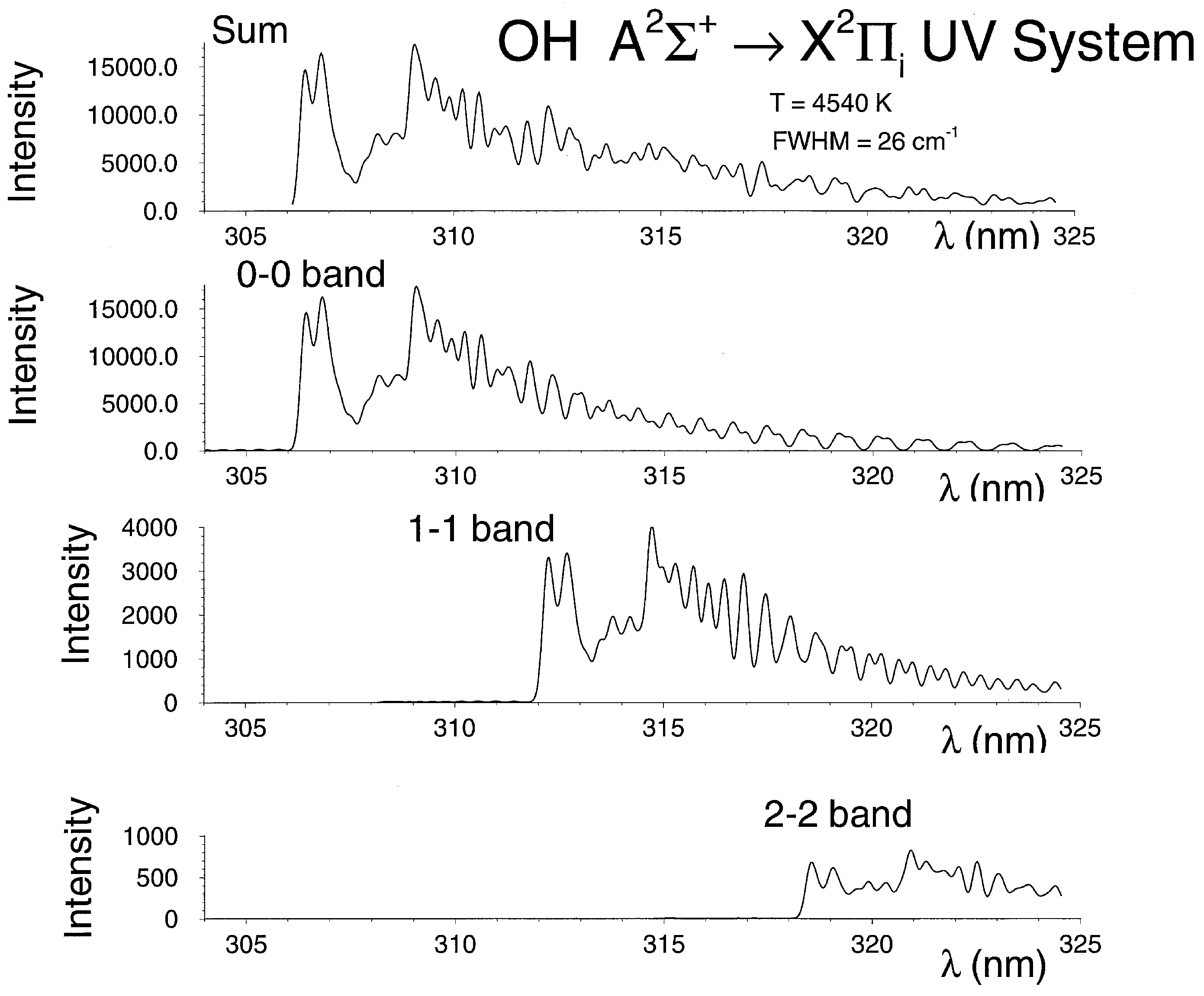
4.3. Computation of OH Spectra
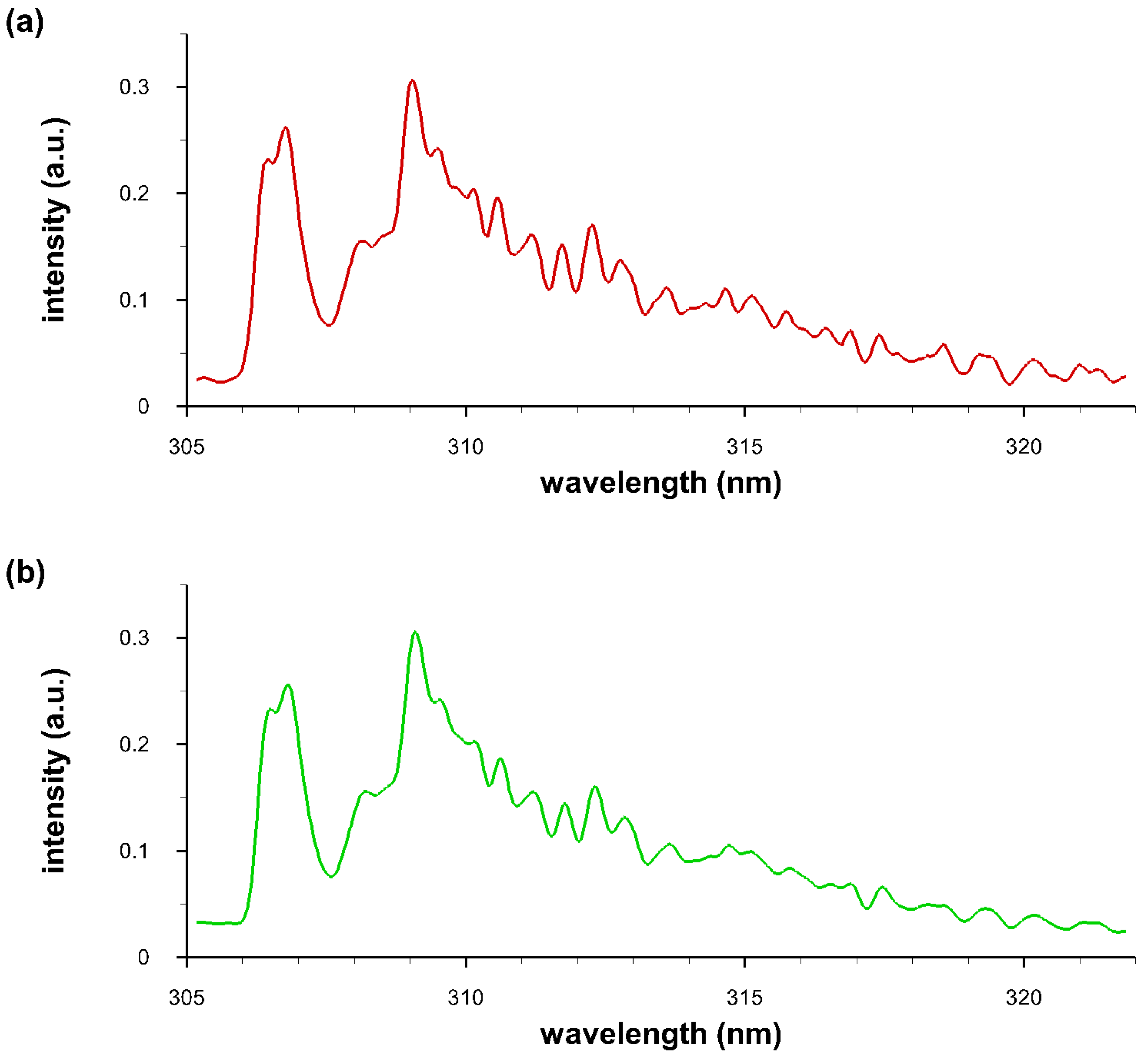
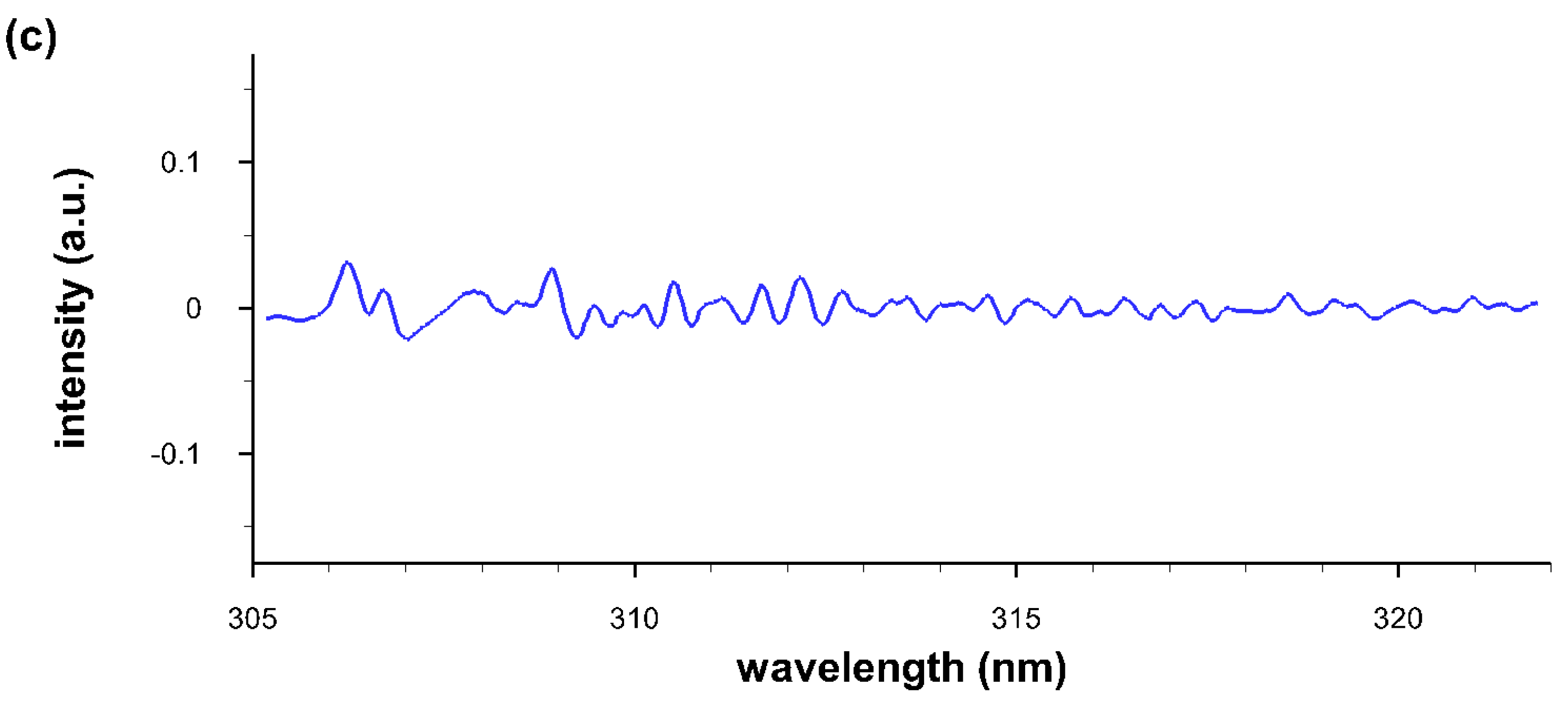
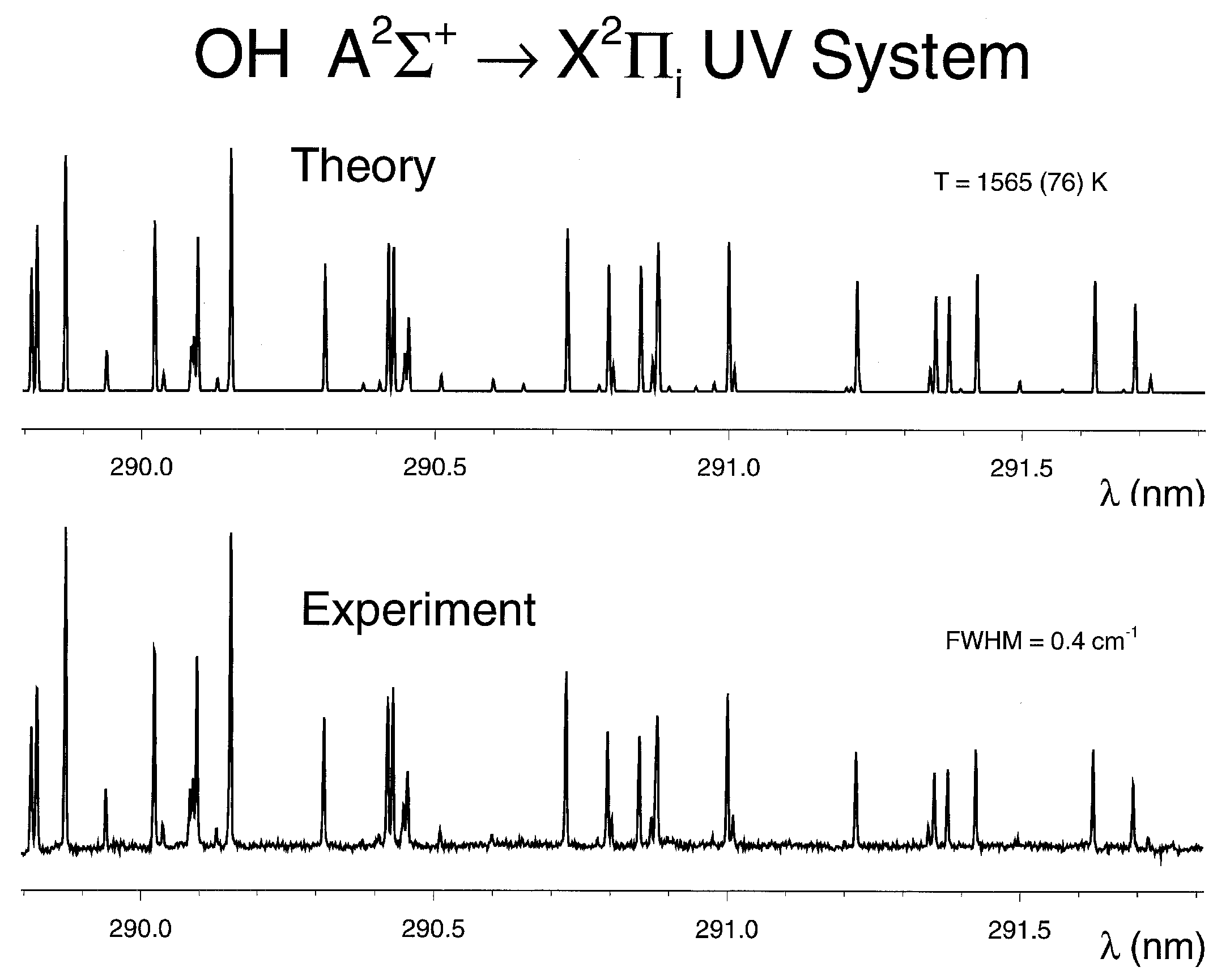
4.4. Comparisons of Recorded and Computed OH Spectra
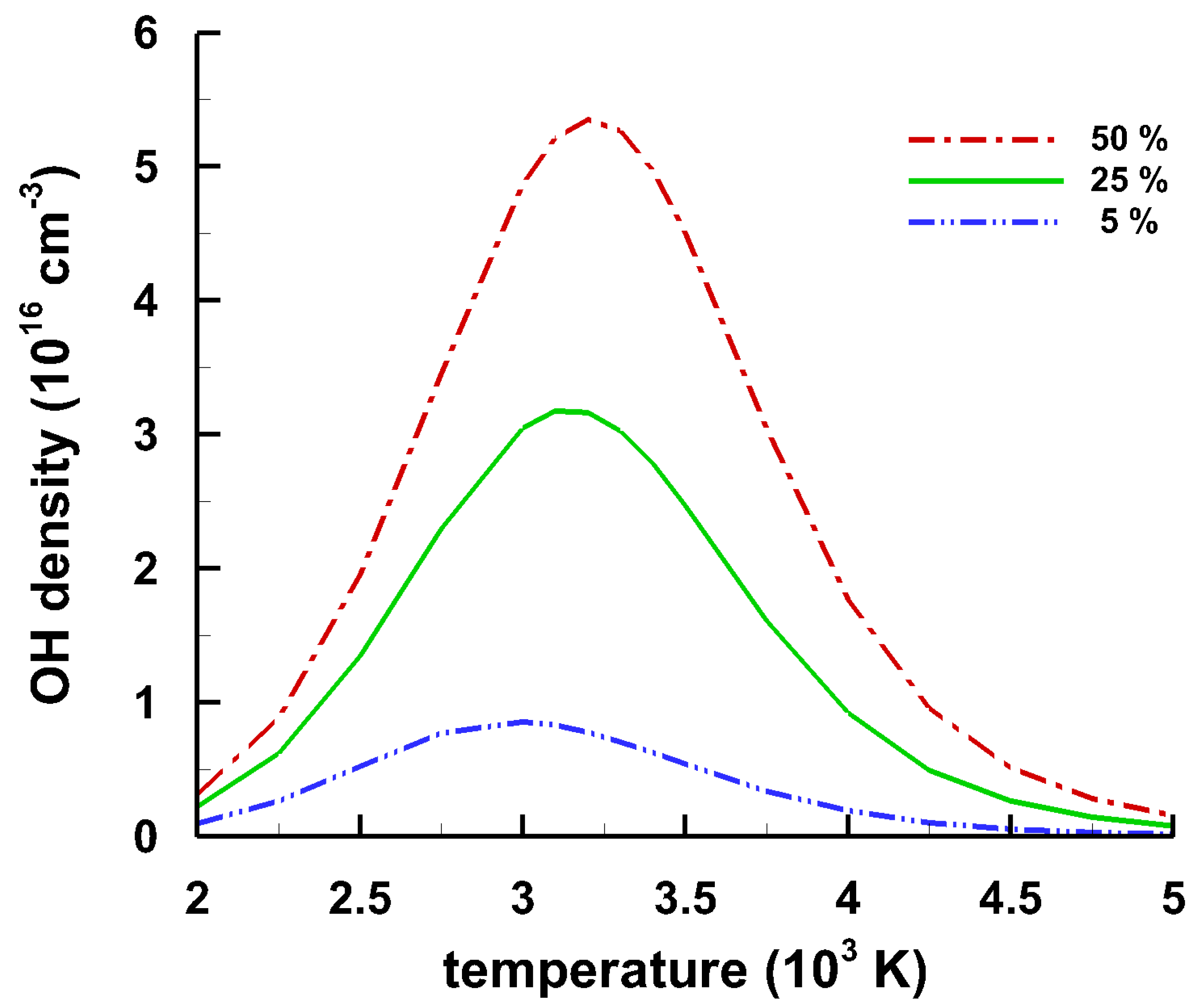
4.5. Temperature and Density of OH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Hydroxyl Line Strengths
Appendix A.1. Overview
Appendix A.2. Example Data File and Fitted Spectra
References
- Kunze, H.-J. Introduction to Plasma Spectroscopy; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Fujimoto, T. Plasma Spectroscopy; Clarendon Press: Oxford, UK, 2004. [Google Scholar]
- Ochkin, V.N. Spectroscopy of Low Temperature Plasma; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasmas. Fundamentals and Applications; Plenum Press: London, UK, 1994. [Google Scholar]
- Parigger, C.G.; Guan, G.; Hornkohl, J.O. Measurement and analysis of OH emission spectra following laser-induced optical breakdown in air. Appl. Opt. 2003, 42, 5986–5991. [Google Scholar] [CrossRef]
- Parigger, C.G. Laser-induced breakdown in gases: Experiments and simulation. In Laser Induced Breakdown Spectroscopy (LIBS): Fundamentals and Applications; Miziolek, A.W., Palleschi, V., Schechter, I., Eds.; Cambridge Univ. Press: New York, NY, USA, 2006; pp. 171–193. [Google Scholar]
- Chen, Y.-L.; Lewis, J.W.L. Visualization of laser-induced breakdown and ignition. Opt. Express 2001, 9, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, Y.-L.; Lewis, J.W.L. Time-resolved temperature images of laser-ignition using OH two-line laser-induced fluorescence (LIF) thermometry. Int. Flame Res. Found. Combust. J. 2005, 8, 200508. [Google Scholar]
- Parigger, C.G.; Hornkohl, J.O. Quantum Mechanics of the Diatomic Molecule with Applications; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Parigger, C.G.; Woods, A.C.; Surmick, D.M.; Gautam, G.; Witte, M.J.; Hornkohl, J.O. Computation of diatomic molecular spectra for selected transitions of aluminum monoxide, cyanide, diatomic carbon, and titanium monoxide. Spectrochim. Acta Part B At. Spectrosc. 2015, 107, 132–138. [Google Scholar] [CrossRef]
- Luque, L.; Crosley, D.R. LIFBASE: Database and Spectral Simulation for Diatomic Molecules (Version 2.1.1). 2013. Available online: https://www.sri.com/cem/lifbase (accessed on 3 September 2019).
- Parigger, C.G.; Surmick, D.M.; Helstern, C.M.; Gautam, G.; Bol’shakov, A.A. Molecular Laser-Induced Breakdown Spectroscopy. In Laser Induced Breakdown Spectroscopy, 2nd ed.; Singh, J.P., Thakur, S.N., Eds.; Elsevier: New York, NY, USA; Amsterdam, The Netherlands, 2020; Chapter 7. [Google Scholar]
- Yousefi, M.; Bernath, P.F.; Hodges, J.; Masseron, T. A new line list for the A2Σ+ − X2Π electronic transition of OH. J. Quant. Spectrosc. Radiat. Transf. 2018, 217, 416–424. [Google Scholar] [CrossRef]
- Western, C.M. PGOPHER: A program for simulating rotational, vibrational and electronic spectra. J. Quant. Spectrosc. Radiat. Transf. 2017, 186, 221–242. [Google Scholar] [CrossRef]
- Tennyson, J. The ExoMol Project: Molecular Opacity Calculations at University College London, Workshop on Astrophysical Opacities. In Astronomical Society of the Pacific (ASP) Conference Series 515, Michigan University, Kalamazoo, MI, USA, 1–4 August 2017; Mendoza, C., Sylvaine, T.-C., Colgan, J., Eds.; Astronomical Society of the Pacific: San Francisco, CA, USA, 2018; Volume 137. [Google Scholar]
- Tennyson, J.; Yurchenko, S.N.; Al-Refaie, A.F.; Barton, E.J.; Chubb, K.L.; Coles, P.A.; Diamantopoulou, S.; Gorman, M.N.; Hill, C.; Lam, A.Z.; et al. The ExoMol database: Molecular line lists for exoplanet and other hot atmospheres. arXiv 2018, arXiv:1603.05890. [Google Scholar] [CrossRef]
- Chernyak, V.Y.; Nedybaliuk, O.A.; Martysh, E.V.; Iukhymenko, V.V.; Prysiazhnevych, I.Y.; Solomenko, O.V.; Veremii, I.P.; Shevchenko, T. Low temperature plasma and plasma technologies: Plasma catalysis of chemical reactions. Prob. At. Sci. Technol. Ser. Plasma Phys. 2014, 6, 124–129. [Google Scholar]
- Srivastava, N.; Wang, C.J. Effect of N2 and O2 on OH radical production in an atmospheric helium microwave plasma jet. Plasma Sci. Technol. 2019, 21, 115401. [Google Scholar] [CrossRef]
- Vorać, J.; Synek, P.; Procházka, V.; Hoder, T. State-by-state emission spectra fitting for non-equilibrium plasmas: OH spectra of surface barrier discharge at argon/water interface. J. Phys. D Appl. Phys. 2017, 50, 294002. [Google Scholar] [CrossRef]
- Ribarov, L.A.; Hu, S.; Wehrmeyer, J.A.; Pitz, W. Hydroxyl tagging velocimetry method optimization: Signal intensity and spectroscopy. Appl. Opt. 2005, 44, 6616–6626. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kakuho, A.; Hishinuma, H.; Urushiahara, T.; Takagi, Y.; Horie, K.; Asano, M.; Ogata, E.; Yamasita, T. Development of a New Compound Fuel and Fluorescent Tracer Combination for Use with Laser Induced Fluorescence. Society of Automotive Engineering (SAE) transactions. Int. J. Engine Res. 1995, 104, 2287–2300. [Google Scholar]
- Ahmed, A.M.; Mancarella, S.; Desgroux, P.; Gasnot, L.; Pauwels, J.-F.; El Bakali, A. Experimental and numerical study on rich methane/hydrogen/air laminar premixed flames at atmospheric pressure: Effect of hydrogen addition to fuel on soot gaseous precursors. Int. J. Hydrogen Energy 2016, 41, 6929–6942. [Google Scholar] [CrossRef]
- Fuchs, H. Measurement of Peroxy Radicals Using Laser-Induced Fluorescence Technique; Forschungszentrum Jülich GmbH, Environment: Jülich, Germany, 2006; Volume 72. [Google Scholar]
- Wang, F.; Hu, R.; Chen, H.; Xie, O.; Wang, Y.; Li, Z.; Jin, H.; Liu, J.; Liu, W. Development of a field system for measurement of tropospheric OH radical using laser-induced fluorescence technique. Opt. Express 2019, 27, A419–A435. [Google Scholar] [CrossRef]
- Wang, Y.; Jain, A.; Kulatilaka, W. Hydroxyl radical planar imaging in flames using femtosecond laser pulses. Appl. Phys. B Lasers Opt. 2019, 125, 90. [Google Scholar] [CrossRef]
- Malmqvist, E.; Jonsson, M.; Larsson, K.; Alden, M.; Bood, J. Two-dimensional OH-thermometry in reacting flows using photofragmentation laser-induced florescence. Combust. Flame 2016, 196, 297–306. [Google Scholar] [CrossRef]
- Kiefer, J.; Meyerhoefer, A.; Seeger, T.; Leipertz, A.; Li, Z.S.; Aldén, M. OH-thermometry using laser polarization spectroscopy and laser-induced fluorescence spectroscopy in the OH A-X (1,0) band. J. Raman Spectrosc. 2009, 40, 828–835. [Google Scholar] [CrossRef]
- Abrams, M.C.; Davis, S.P.; Rao, M.L.P.; Engleman, R., Jr.; Brault, J.W. High-resolution Fourier transform spectroscopy of the Meinel system of OH. Astrophys. J. Suppl. Ser. 1994, 93, 351–395. [Google Scholar] [CrossRef]
- Stark, G.; Brault, J.W.; Abrahms, M.C. Fourier-transform spectra of the A2Σ+ − X2ΠΔv = 0 bands of OH and OD. J. Opt. Soc. Am. B Opt. Phys. 1994, 11, 3–32. [Google Scholar] [CrossRef]
- Varberg, T.D.; Evenson, K.M. The rotational spectrum of OH in the v = 0–3 levels of its ground-state. J. Mol. Spectrosc. 1993, 157, 55–67. [Google Scholar] [CrossRef]
- Becker, K.H.; Haaks, D.; Tatarczyk, T. The Natural lifetime of OH (2Σ+, v = 0, N = 2, J = 32) and its quenching by atomic hydrogen. Chem. Phys. Lett. 1974, 25, 564–567. [Google Scholar] [CrossRef]
- Brockhinke, A.; Lenhard, U.; Bülter, A.; Kohse-Höinghaus, K. Energy transfer in the OH A2Σ+ state: The role of polarization and of multi-quantum energy transfer. Phys. Chem. Chem. Phys. 2005, 7, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.H.; Silver, J.A.; Kinsey, J.L. Direct measurement of the radiative lifetime of the A2Σ+ (v′ = 0, K′ = 1, J′ = 32) state of OH and OD. Chem. Phys. Lett. 1974, 28, 418–421. [Google Scholar] [CrossRef]
- Burris, J.; Butler, J.J.; Mcgee, T.J.; Heaps, W.S. Collisional deactivation rates for A2Σ+ (v′ = 1) state of OH. Chem. Phys. 1988, 124, 251–258. [Google Scholar] [CrossRef]
- Cattolica, R.J.; Mataga, T.G. Rotational-level-dependent quenching of OH A2Σ+ (v′ = 1) by collisions with H2O in a low-pressure flame. Chem. Phys. Lett. 1991, 182, 623–631. [Google Scholar] [CrossRef]
- Cleveland, C.B.; Wiesenfeld, J.R. Electronic quenching of highly rotationally excited OH (A2Σ+, v′=0, 1) by H2O. Chem. Phys. Lett. 1988, 144, 479–485. [Google Scholar] [CrossRef]
- Copeland, R.A.; Chalamala, B.R.; Coxon, J.A. Laser-Induced Fluorescence of the B2Σ+ − X2Π System of OH: Molecular constants for the B2Σ+ (v = 0, 1) and X2Π(v = 7 − 9,11 − 3). J. Mol. Spectrosc. 1993, 161, 243–252. [Google Scholar] [CrossRef]
- Copeland, R.A.; Wiese, M.L.; Crosley, D.R. Vibrational-Energy transfer and quenching of OH (A2Σ+, v′ = 1). J. Phys. Chem. 1988, 92, 5710–5715. [Google Scholar] [CrossRef]
- Crosley, D.R.; Lengel, R.K. Relative transition-probabilities and electronic-transition moment in A-X system of OH. J. Quant. Spectrosc. Radiat. Transfer 1975, 15, 579–591. [Google Scholar] [CrossRef]
- Derro, E.L.; Pollack, I.B.; Dempsey, L.P.; Greenslade, M.F.; Lei, Y.; Radenović, D.Ć.; Lester, M.I. Fluorescence-dip infrared spectroscopy and predissociation dynamics of OH A2Σ+(v = 4) radicals. J. Chem. Phys. 2005, 122, 244313. [Google Scholar] [CrossRef]
- German, K.R. Direct measurment of radiative lifetime of A2Σ+(v′ = 0) states of OH and OD. J. Chem. Phys. 1975, 62, 2584–2587. [Google Scholar] [CrossRef]
- German, K.R. Collision and quenching cross-sections in A2Σ+ state of OH and OD. J. Chem. Phys. 1976, 64, 4065–4068. [Google Scholar] [CrossRef]
- Hogan, P.; Davis, D.D. Comments on “Electronic quenching and vibrational-relaxation of OH (A2Σ+v′ = 1) state”. J. Chem. Phys. 1976, 64, 3901. [Google Scholar] [CrossRef]
- Hogan, P.; Davis, D.D. Electronic quenching and vibrational-relaxation of OH (A2Σ+v′ = 1) state. J. Chem. Phys. 1975, 62, 4574–4576. [Google Scholar] [CrossRef]
- Huber, K.P.; Holland, F.; Coxon, J.A. Jet emission-spectroscopy of OH and OD near 1850-A—1st observation of A2Π − 2Π electronic-transition of OD. J. Chem. Phys. 1992, 96, 1005–1015. [Google Scholar] [CrossRef]
- Jorg, A.; Espositi, D.; Werner, H.J. Rotational energy-transfer in OH (A2Σ+, v′ = 0)—A method for the direct determination of state-to-state transfer-coefficients. J. Chem. Phys. 1990, 93, 6453–6462. [Google Scholar] [CrossRef]
- Kienle, R.; Li, M.P.; Kohse-Höinghaus, K. A detailed rate equation model for the simulation of energy transfer in OH laser-induced fluorescence. Appl. Phys. B Lasers Opt. 1996, 62, 583–599. [Google Scholar] [CrossRef]
- McDermid, I.S.; Laudenslager, J.B. Radiative lifetime and quenching rate coefficients for directly excited rotational levels of OH (A2Σ+, v = O). J. Chem. Phys. 1982, 76, 1824–1831. [Google Scholar] [CrossRef]
- Rahmann, U.; Kreutner, W.; Kohse-Höinghaus, K. Rate-equation modeling of single- and multiple-quantum vibrational energy transfer of OH (A2Σ+, v′ = 0 to 3). Appl. Phys. B Lasers Opt. 1999, 69, 61–70. [Google Scholar] [CrossRef]
- Bormann, F.C.; Nielsen, T.; Burrows, M.; Andresen, P. Single-pulse collision-insensitive picosecond planar laser-induced fluorescence of OH A2Σ+ (v′ = 2) in atmospheric-pressure flames. Appl. Phys. B Lasers Opt. 1996, 62, 601–607. [Google Scholar] [CrossRef]
- Bormann, F.C.; Nielsen, T.; Burrows, M.; Andresen, P. Picosecond planar laser-induced fluorescence measurements of OH A2Σ+ (v′ = 2) lifetime and energy transfer in atmospheric pressure flames. Appl. Opt. 1997, 36, 6129–6140. [Google Scholar] [CrossRef]
- Brockhinke, A.; Kreutner, W.; Rahmann, U.; Kohse-Höinghaus, K.; Settersten, T.B.; Linne, M.A. Time-, wavelength-, and polarization-resolved measurements of OH (A2Σ+) picosecond laser-induced fluorescence in atmospheric- pressure flames. Appl. Phys. B Lasers Opt. 1999, 69, 477–485. [Google Scholar] [CrossRef]
- Brophy, J.H.; Silver, J.A.; Kinsey, J.L. Vibrationally excited OH from the reaction of H with NO2 observed by laser-induced fluorescence. J. Chem. Phys. 1975, 62, 3820–3822. [Google Scholar] [CrossRef]
- Cattolica, R. OH rotational temperature from 2-line laser-escited fluorescence. Appl. Opt. 1981, 20, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, R.; Xie, P.; Xing, X.; Ling, L.; Li, Z.; Wang, F.; Wang, Y.; Liu, J.; Liu, W. A hydroxyl radical detection system using gas expansion and fast gating laser-induced fluorescence techniques. J. Environ. Sci. 2018, 65, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.; Friedman, J.; Renksizbulut, M. Planar temperature imaging using thermally assisted laser induced fluorescence of OH in a methane-air flame. Exp. Therm. Fluid Sci. 2007, 31, 221–236. [Google Scholar] [CrossRef]
- Crosley, D.R.; Smith, G.P. Vibrationa energy-transfer in laser-excited A2Σ+ OH as flame thermometer. Appl. Opt. 1980, 19, 517–520. [Google Scholar] [CrossRef]
- Dilecce, G.; Martini, L.M.; Cepelli, M.; Scotoni, M.; Tosi, P. Progress on laser induced fluorescence in a collisional environment: The case of OH molecules in ns pulsed discharges. Plasma Sources Sci. Technol. 2019, 28, 025012. [Google Scholar] [CrossRef]
- Dreizler, A.; Tadday, R.; Monkhouse, P.; Wolfrum, J. Time and Spatially Resolved LIF of OH A2Σ+ (v′ = 1) in Atmospheric-Pressure Flames using Picosecond Excitation. Appl. Phys. B Photophys. Laser Chem. 1993, 57, 85–87. [Google Scholar] [CrossRef]
- Kienle, R.; Lee, M.P.; Kohse-Höinghaus, K. A scaling formalism for the representation of rotational energy transfer in OH (A (2)Sigma (+)) in combustion experiments. Appl. Phys. B Lasers Opt. 1996, 63, 403–418. [Google Scholar]
- Killinger, D.K.; Hanabusa, M. Intensity and pressure-dependence of resonance fluorescence of OH induced by a tunable uv laser. Phys. Rev. A 1976, 13, 2145–2152. [Google Scholar] [CrossRef]
- Kollner, M.; Monkhouse, P. Time-resolved LIF of OH in the flame front of premixed and diffusion flames at atmospheric pressure. Appl. Phys. B Lasers Opt. 1995, 61, 499–503. [Google Scholar] [CrossRef]
- Mather, J.H.; Stevens, P.S.; Brune, W.H. OH and HO2 measurements using laser-induced fluorescence. J. Geophys. Res. Atmos. 1997, 102, 6427–6436. [Google Scholar] [CrossRef]
- Nielsen, T.; Bormann, F.; Burrows, M.; Andresen, P. Picosecond laser-induced fluorescence measurement of rotational energy transfer of OH A2Σ+(v′ = 2) in atmospheric pressure flames. Appl. Opt. 1997, 36, 7960–7969. [Google Scholar] [CrossRef] [PubMed]
- Sappey, A.D.; Copeland, R.A. Laser double-resonance study of OH (X2Πi, v = 12). J. Mol. Spectrosc. 1990, 143, 160–168. [Google Scholar] [CrossRef]
- Sick, V.; Wermuth, N. Single-shot imaging of OH radicals and simultaneous OH radical/acetone imaging with a tunable Nd:YAG laser. Appl. Phys. B Lasers Opt. 2004, 79, 139–143. [Google Scholar] [CrossRef][Green Version]
- Stone, D.; Whalley, L.K.; Ingham, T.; Edwards, P.M.; Cryer, D.R.; Brumby, C.A.; Seakins, P.W.; Heard, D.E. Measurement of OH reactivity by laser flash photolysis coupled with laser-induced fluorescence spectroscopy. Atmos. Meas. Tech. 2016, 9, 2827–2844. [Google Scholar] [CrossRef]
- Zizak, G.; Lanauze, J.A.; Winefordner, J.D. An experimental study of the excited state rotational population of OH in flames using laser induced fluorescence. Combust. Flame 1986, 65, 203–214. [Google Scholar] [CrossRef]
- Graña-Otero, J.; Mahmoudi, S. Excited OH kinetics and distribution in H2 premixed flames. Fuel 2019, 255, 1115750. [Google Scholar] [CrossRef]
- Greenslade, M.E.; Letser, M.I. (2 + 1) resonance-enhanced ionization spectroscopy of a state-selected beam of OH radicals. J. Chem. Phys. 2005, 123, 074309. [Google Scholar] [CrossRef]
- Jeffries, J.B.; Kohse-Höinghaus, K.; Smith, G.P.; Copeland, R.A.; Crosley, D.R. Rotational-level-dependent quenching of OH (A2Σ+) at flame temperatures. Chem. Phys. Lett. 1988, 152, 160–166. [Google Scholar] [CrossRef]
- Neuber, A.A.; Janicka, A.; Hassel, E.P. Thermally assisted fluorescence of laser-excited OH A2Σ+ as a flame diagnostic tool. Appl. Opt. 1996, 35, 4033–4040. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.L.; Hanson, R.K. Temperature imaging in a supersonic free jet of combustion gases with two-line OH fluorescence. Appl. Opt. 1996, 35, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Crosley, D.R. Vibrational-energy transfer in A2Σ+ OH in flames. Appl. Opt. 1983, 22, 1428–1430. [Google Scholar] [CrossRef]
- Carlone, C.; Dalby, F.W. Spectrum of the hydroxyl radical. Can. J. Phys. 1969, 47, 1945–1957. [Google Scholar] [CrossRef]
- Copeland, R.A.; Jeffries, J.B.; Crosley, D.R. The OH A2Σ+ − X2Πi(4, 2) band-line positions and linewidths. J. Mol. Spectrosc. 1990, 143, 183–185. [Google Scholar] [CrossRef]
- Coxon, J.A.; Foster, S.C. Rotational analysis of hydroxyl vibration-rotation emission bands: Molecular constants for OH X2Π, 6 ≤ v ≤ 10. Can. J. Phys. 1980, 60, 41–48. [Google Scholar] [CrossRef]
- Coxon, J.A.; Sappey, A.D.; Copeland, R.A. Molecular constants and term values for the hydroxyl radical, OH: The X2Π (v = 8, 12), A2Σ+ (v = 4–9), B2Σ+ (v = 0, 1), and C2Σ+ (v = 0, 1) states. J. Mol. Spectrosc. 1991, 145, 41–55. [Google Scholar] [CrossRef]
- Dieke, G.H.; Crosswhite, H.M. The ultraviolet bands of OH - fundamental data. J. Quant. Spectrosc. Radiat. Transfer 1962, 2, 97–199. [Google Scholar] [CrossRef]
- Dyer, M.J.; Knutsen, K.; Copeland, R.A. Energy transfer in the ground state of OH: Measurements of OH (v=8, 10, 11) removal. J. Chem. Phys. 1997, 107, 7809–7815. [Google Scholar] [CrossRef]
- Gillis, J.R.; Goldman, A.; Stark, G.; Rinsland, C.P. Line parameters for the A2Σ+ − X2Π bands of OH. J. Quant. Spectrosc. Radiat. Transfer 2001, 68, 225–230. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lipson, J.B.; Field, R.W.; Dood, J.R. Detection of OH (X, v″, J″) via the B2Σ+ − X2Π transition and properties of the B2Σ+ state. J. Phys. Chem. A 2001, 105, 6030–6037. [Google Scholar] [CrossRef]
- Langhoff, S.R.; Partridge, H. Theoretical-study of the lambda-doubling parameters for X 2Π OH. J. Mol. Spectros. 1984, 105, 261–275. [Google Scholar] [CrossRef]
- Langhoff, S.R.; Sink, M.L.; Pritchard, R.H.; Kern, C.W. Theoretical-study of the spin-orbit coupling in the X2Π state of OH. J. Mol. Spectrosc. 1982, 96, 200–218. [Google Scholar] [CrossRef]
- Lengel, R.K.; Crosley, D.R. Rotational dependence of vibrational-relaxation in A2Σ+ OH. Chem. Phys. Lett. 1975, 32, 261–264. [Google Scholar] [CrossRef]
- Lengel, R.K.; Crosley, D.R. Comments on “Electronic quenching and vibrational-relaxation of OH (A2Σ+,v′ = 1) state”. J. Chem. Phys. 1976, 64, 3900–3901. [Google Scholar] [CrossRef]
- Lengel, R.K.; Crosley, D.R. Energy-transfer in 2Σ+ OH I. Rotational. J. Chem. Phys. 1977, 67, 2085–2101. [Google Scholar] [CrossRef]
- Lengel, R.K.; Crosley, D.R. Energy-transfer in 2Σ+ OH II. Vibrational. J. Chem. Phys. 1978, 68, 5309–5324. [Google Scholar] [CrossRef]
- Levin, D.A.; Laux, C.O.; Kruger, C.H. A general model for the spectral calculation of OH radiation in the ultraviolet. J. Quant. Spectrosc. Radiat. Transfer 1999, 61, 377–392. [Google Scholar] [CrossRef]
- Lucht, R.P.; Sweeney, D.W.; Laurendeau, N.M. Time-resolved fluorescence investigation of rotational transfer in A 2Σ+(v = 0) OH. Appl. Opt. 1986, 25, 4086–4095. [Google Scholar] [CrossRef]
- Raab, F.; Bergeman, T.; Lieberman, D.; Metcalf, H. Precision study of the A 2Σ+ state of the OH radical. Phys. Rev. A 1981, 24, 3120–3135. [Google Scholar] [CrossRef]
- Sechler, T.D.; Dempsey, L.O.; Lester, M.I. State-to-State Vibrational Energy Transfer in OH A2Σ+ with N2. J. Phys. Chem. A 2009, 113, 8845–8851. [Google Scholar] [CrossRef] [PubMed]
- Steffens, K.L.; Crosley, D.R. Vibrational energy transfer in OH A2Σ+ between 195 and 295 K. J. Chem. Phys. 2000, 112, 9427–9432. [Google Scholar] [CrossRef]
- Parigger, C.G.; Helstern, C.M.; Jordan, B.S.; Surmick, D.M.; Splinter, R. Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications. Molecules 2020, 25, 615. [Google Scholar] [CrossRef] [PubMed]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons Ltd.: New York, NY, USA, 2006. [Google Scholar]
- Singh, J.P.; Thakur, S.N. (Eds.) Laser Induced Breakdown Spectroscopy; Elsevier Science: New York, NY, USA, 2007. [Google Scholar]
- Gautam, G.; Helstern, C.M.; Drake, K.A.; Parigger, C.G. Imaging of Laser-induced Plasma Expansion Dynamics in Ambient Air. Int. Rev. At. Mol. Phys. 2016, 7, 45–51. [Google Scholar]
- Brieschenk, S.; O’Byrne, S.; Kleine, H. Visualization of jet development in laser-induced plasmas. Opt. Lett. 2013, 38, 664–666. [Google Scholar] [CrossRef]
- Harilal, S.S.; Miloshevky, G.V.; Diwakar, K.; LaHaye, N.; Hassanein, A. Experimental and computational study of complex shockwave dynamics in laser ablation plumes in argon atmosphere. Phys. Plasmas 2012, 19, 083504. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B. Computer Program for Calculation of Complex Equilibrium Compositions, Rocket Performance, Incident and Reflected Shocks, and Chapman-Jouguet Detonations; Interim Revision, NASA Report SP-273; NASA Lewis Research Center: Cleveland, OH, USA, 1976. [Google Scholar]
- McBride, B.J.; Gordon, S. Computer Program for Calculating and Fitting Thermodynamic Functions, NASA RP-1271, 1992, On-Line 2005 Version. Available online: https://cearun.grc.nasa.gov/ (accessed on 14 December 2019).
- Engineering Toolbox, Water Vapor in Air. Available online: https://www.engineeringtoolbox.com/water-vapor-air-d_854.html (accessed on 15 February 2020).
- Engineering Toolbox, Air Composition. Available online: https://www.engineeringtoolbox.com/air-composition-d_212.html (accessed on 15 February 2020).
- Kogelnik, H.; Li, T. Laser Beams and Resonators. Appl. Opt. 1966, 5, 1550–1567. [Google Scholar] [CrossRef]
- Technical Note: Gaussian Beam Optics. Available online: https://www.newport.com/n/gaussian-beam-optics (accessed on 15 February 2020).
- Parigger, C.G.; Helstern, C.M.; Gautam, G. Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma. Atoms 2019, 7, 74. [Google Scholar] [CrossRef]
- Guan, G. On the Analysis of Emission Spectra and Interference Images. Ph.D. Dissertation, University of Tennessee, Knoxville, TN, USA, 1998. [Google Scholar]
- Hornkohl, J.O.; (University of Tennessee Space Institute, Tullahoma, TN 37388, USA). Personal communication, 1996.
- Parigger, C.G.; Guan, G.; Hornkohl, J.O. Laser-induced breakdown spectroscopy: Analysis of OH spectra. In Laser Induced Plasma Spectroscopy and Applications; Trends in Optics and Photonics Series; Optical Society of America: Washington, DC, USA, 2002; Volume 81, pp. 102–103. [Google Scholar]
| Species | Mole Fraction |
|---|---|
| nitrogen | 0.78 |
| oxygen | 0.21 |
| argon | 0.00934 |
| hydrogen | 0.000005 |
| water | 0.01144 |
| Time Delay (s) | R (mm) for Air [ kg/m3] |
|---|---|
| 20 | 9.21 |
| 25 | 10.1 |
| 50 | 13.3 |
| 75 | 15.7 |
| 100 | 17.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parigger, C.G.; Helstern, C.M.; Jordan, B.S.; Surmick, D.M.; Splinter, R. Laser-Plasma Spectroscopy of Hydroxyl with Applications. Molecules 2020, 25, 988. https://doi.org/10.3390/molecules25040988
Parigger CG, Helstern CM, Jordan BS, Surmick DM, Splinter R. Laser-Plasma Spectroscopy of Hydroxyl with Applications. Molecules. 2020; 25(4):988. https://doi.org/10.3390/molecules25040988
Chicago/Turabian StyleParigger, Christian G., Christopher M. Helstern, Benjamin S. Jordan, David M. Surmick, and Robert Splinter. 2020. "Laser-Plasma Spectroscopy of Hydroxyl with Applications" Molecules 25, no. 4: 988. https://doi.org/10.3390/molecules25040988
APA StyleParigger, C. G., Helstern, C. M., Jordan, B. S., Surmick, D. M., & Splinter, R. (2020). Laser-Plasma Spectroscopy of Hydroxyl with Applications. Molecules, 25(4), 988. https://doi.org/10.3390/molecules25040988