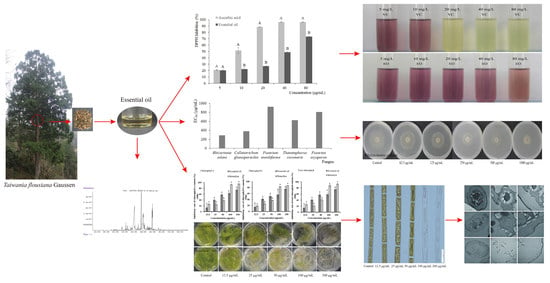
Chemical Composition, Algicidal, Antimicrobial, and Antioxidant Activities of the Essential Oils of Taiwania flousiana Gaussen
Abstract
1. Introduction
2. Results
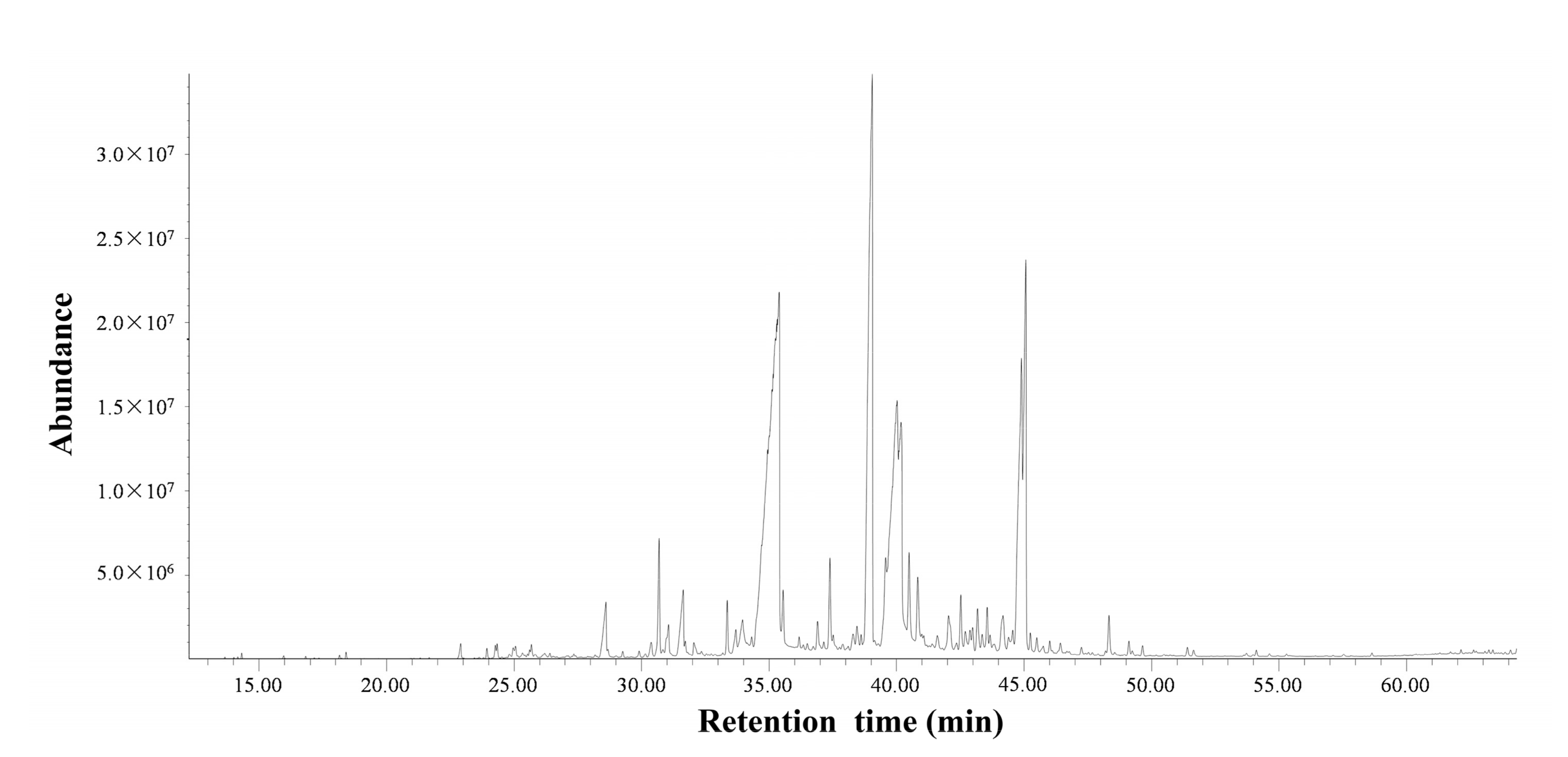
2.1. Chemical Components Identified in the Essential Oil
2.2. Algicidal Activity and Algicidal Mechanism of Action of T. flousiana Essential Oil
2.3. The Effect of Light on the Algicidal Activity of T. flousiana Essential Oil in S. communis
2.4. Antifungal Activities of T. flousiana Essential Oil
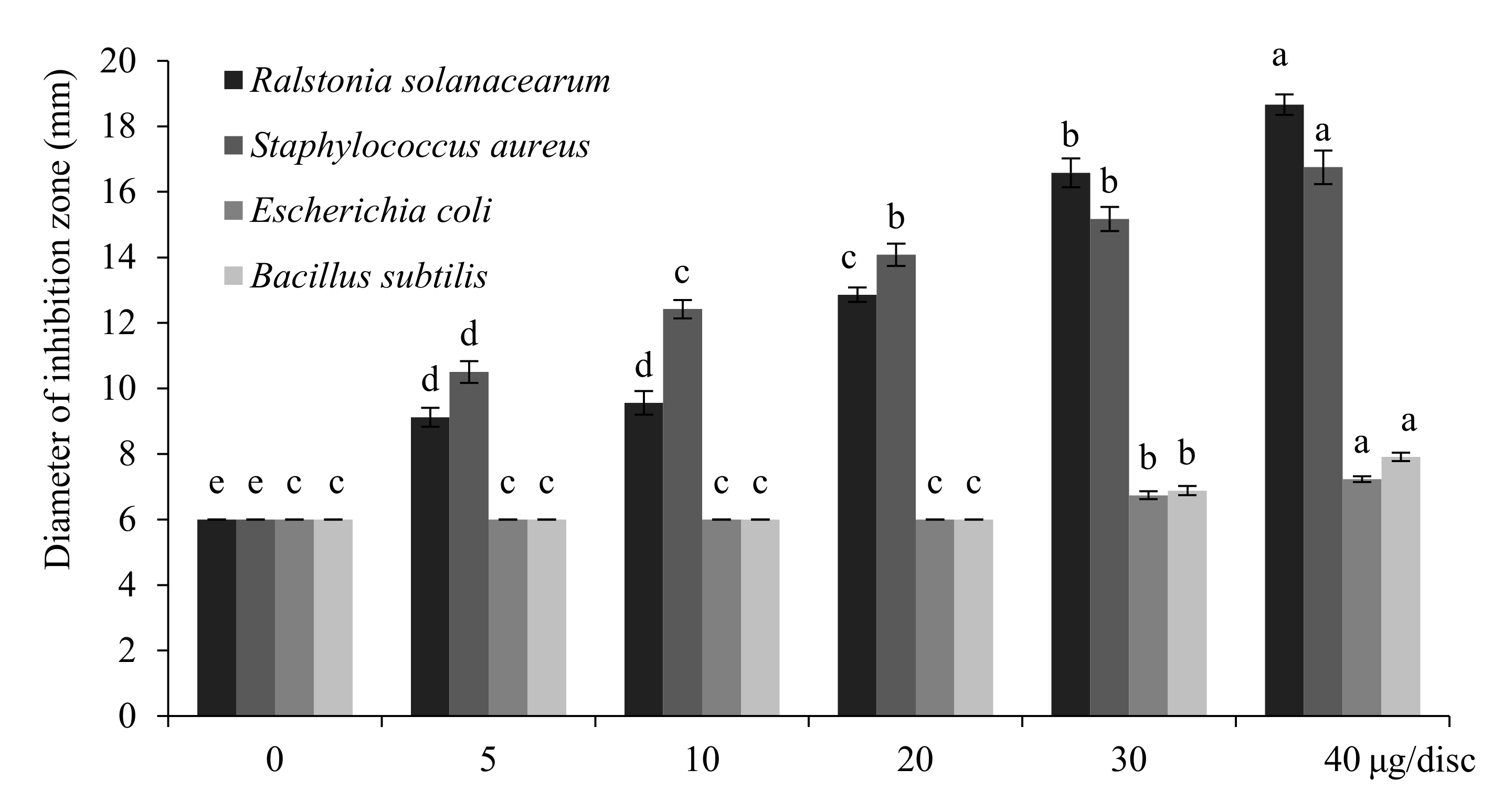
2.5. Antibacterial Activities of T. flousiana Essential Oil
2.6. Antioxidant Activity
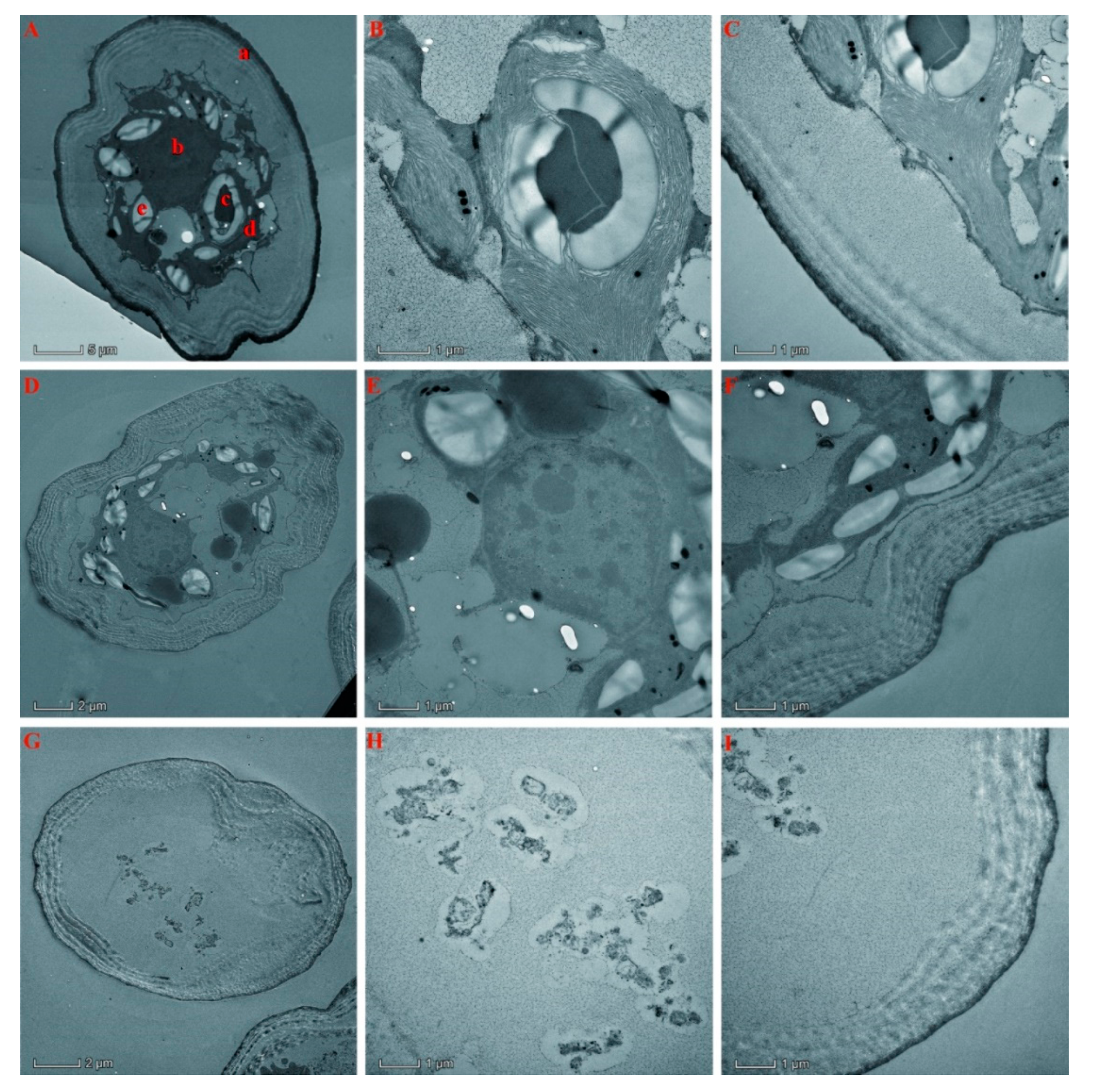
2.7. Effects of Essential Oil on Algal Internal Structure
2.8. Alteration of the Cell Ultrastructure of S. communis by the Essential Oil of T. flousiana
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Isolation of Essential Oil
4.3. Analysis of the Essential Oil
4.4. Identification of the Essential Oil Chemical Constituents
4.5. Evaluation of the Algicidal Activity with Light
4.6. Evaluation of the Algicidal Activity without Light
4.7. Morphological Changes of S. communis Treated with the Essential Oil of T. flousiana
4.8. Determination of Antimicrobial Effects of the Essential Oils on Mycelial Growth
4.9. Determination of Antioxidant Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, J.G.; Zhou, G.; Liu, F.; Yang, Z.; Mo, S.Z.; He, B. Accumulation and distribution pattern of carbon in ecosystems of Taiwania flousiana and successive rotations of Cunninghamia lanceolata plantations. Chin. Subtropical Agric. Res. 2018, 14, 29–33. [Google Scholar]
- Yin, R.P.; Lu, D.W.; Zhang, C.H.; Jiang, Y.; Yang, D.J.; Pu, Y.Q. The biological characteristics of planted Taiwania flousiana. J. West. China For. Sci. 2018, 47, 50–55. [Google Scholar]
- Nie, S. Studies on the pulping paper-making of Taiwania flousiana. Chin. J. Fujian Technol. 1998, 5, 1–5. [Google Scholar]
- Chen, K. Cultivation Method of Taiwania flousiana with High Survival Rate and High Germination Rate. CN Patent CN107969276A, 1 May 2018. [Google Scholar]
- Lian, Y.J.; Lin, X.S.; Chen, Y.P.; Zhou, Z.Z.; Zhang, X.D.; Zheng, Z.W.; Mao, S.P.; Lin, W.F.; You, F.L. Method for Raising Seedlings of Taiwania flousiana. CN Patent CN106613621A, 10 May 2017. [Google Scholar]
- Jiang, F.; Deng, L.; Liu, L.L.; Li, F.S.; Liu, S.L.; Lin, X.H.; Wang, J.S. Non-Woven Lightweight Matrix Seedling Breeding Method of Taiwania flousiana. CN Patent CN104350992A, 18 February 2015. [Google Scholar]
- Huang, C.B.; Cao, J.Z.; Wu, Q.B.; Wei, J.G.; Meng, Y.H.; Li, B.P. Comparative analysis on soil physi-chemical properties and the tree growth in Taiwania flousiana plantations and successive rotation plantations of Cunninghamia lanceolata. Scientia Silvae Sinicae 2010, 46, 1–7. [Google Scholar]
- He, B.; Huang, H.C.; Cao, M.; Huang, C.B.; Meng, Y.H.; Rong, Y.; Chen, Y.P. Concentration, accumulation and distribution of microelements in Taiwania flousiana plantation. Chin. J. Nanjing For. Univ. 2009, 33, 69–73. [Google Scholar]
- Xiang, Y.; Yang, S.P.; Zhan, Z.J.; Yue, J.M. Terpenoids and phenols from Taiwania flousiana. Acta. Bot. Sin. 2004, 46, 1002–1008. [Google Scholar]
- Kagamizono, T.; Kyo, T.; Tanaka, T.; Maejima, A.; Arai, Y.; Sho, S.; Chin, Y.; Tei, K. Lignans as Bone Resorption Inhibitors. JP Patent JP09012592A, 14 January 1997. [Google Scholar]
- Zhou, L.J.; Huang, J.G.; Li, J.L. Herbicidal Plant Extract and Application the Extract of Taiwania flousiana. CN Patent CN106942286B, 19 July 2019. [Google Scholar]
- Lu, C.M.; Wu, G.R.; Tao, M.X.; Zhou, C.F.; Wei, J.C. Effects of osmatic stress and paraquat on SOD in Spirogyra communis(Hassall) Kutzing. J. Plant Resour. Environ. 1999, 8, 13–17. [Google Scholar]
- Takano, T.; Higuchi, S.; Ikegaya, H.; Matsuzaki, R.; Kawachi, M.; Takahashi, F.; Nozaki, H. Identification of 13 Spirogyra species (Zygnemataceae) by traits of sexual reproduction induced under laboratory culture conditions. Sci. Rep. 2019, 9, 7458. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Everhart, S.E.; Brown, P.J.; Tenuta, A.U.; Dorrance, A.E.; Bradley, C.A. Genetic Structure of Rhizoctonia solani AG-2-2IIIB from Soybean in Illinois, Ohio, and Ontario. Phytopathology 2019, 109, 2132–2141. [Google Scholar] [CrossRef]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Phys. 2019, 155, 101–107. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.; Koutsoumanis, K.; Fasseas, K.; Gje, N. Inhibition of oregano essential oil and EDTA on Escherichia coli o157:h7. Ital. J. Food Sci. 2001, 13, 65–75. [Google Scholar]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Schrader, K.K.; Wedge, D.E.; Duke, S.O. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry 2005, 66, 2689–2695. [Google Scholar] [CrossRef]
- Ghosh, S.; Ozek, T.; Tabanca, N.; Ali, A.; Rehman, J.U.; Khan, I.A.; Rangan, L. Chemical composition and bioactivity studies of Alpinia nigra essential oils. Ind. Crops Prod. 2014, 53, 111–119. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Alencar, S.M.D.; Sousa, R.L.M.D.; Moreno, A.M.; Gloria, E.M.D. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Baczek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Weglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Said, Z.B.O.S.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlou, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus, from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Kivcak, B.; Mert, T.; Demirci, B.; Baser, K.H.C. Composition of the essential oil of Arbutus unedo. Chem. Nat. Compd. 2001, 37, 445–446. [Google Scholar] [CrossRef]
- Altintas, A.; Kose, Y.B.; Yucel, E.; Demirci, B.; Baser, K.H.C. Composition of the essential oil of Centaurea dichroa. Chem. Nat. Compd. 2004, 40, 604–605. [Google Scholar] [CrossRef]
- Kheder, F.B.H.; Mahjoub, M.A.; Zaghrouni, F.; Kwaja, S.; Helal, A.N.; Mighri, Z. Chemical composition antioxidant and antimicrobial activities of the essential oils of Matricaria aurea Loefl. growing in Tunisia. J. Essent. Oil Bear. Pl. 2014, 17, 493–505. [Google Scholar] [CrossRef]
- Kushnarenko, S.V.; Karasholakova, L.N.; Ozek, G.; Abidkulova, K.T.; Mukhitdinov, N.M.; Baser, K.H.C.; Ozek, T. Investigation of essential oils from three natural populations of Lonicera iliensis. Chem. Nat. Compd. 2016, 52, 751–753. [Google Scholar] [CrossRef]
- Pino, J.A.; Marquez, E.; Castro, D. Volatile and non-volatile acids of noni (Morinda citrifolia L.) fruit. J. Sci. Food Agric. 2009, 89, 1247–1249. [Google Scholar] [CrossRef]
- Senatore, F.; Formisano, C.; Rigano, D.; Piozzi, F.; Rosselli, S. Chemical composition of the essential oil from aerial parts of Stachys palustris L. (Lamiaceae) growing wild in Southern Italy. Croat. Chem. Acta 2007, 80, 135–139. [Google Scholar]
- Atti-Santos, A.C.; Pansera, M.R.; Paroul, N.; Atti-Serafini, L.; Moyna, P. Seasonal variation of essential oil yield and composition of Thymus vulgaris L. (Lamiaceae) from South Brazil. J. Essent. Oil Res 2004, 16, 294–295. [Google Scholar] [CrossRef]
- Sezik, E.; Kocakulak, E.; Baser, K.H.C.; Ozek, T. Composition of the essential oils of Juniperus oxycedrus SUBSP. macrocarpa from Turkey. Chem. Nat. Compd. 2005, 41, 352–354. [Google Scholar] [CrossRef]
- Chéraif, I.; Jannet, H.B.; Hammami, M.; Khouja, M.L.; Mighri, Z. Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochem. Syst. Ecol. 2007, 35, 813–820. [Google Scholar] [CrossRef]
- Karik, Ü.; Çinar, O.; Tunçtürk, M.; Sekeroglu, N.; Gezici, S. Essential oil composition of some sage (Salvia spp.) species cultivated in İzmir (Turkey) ecological conditions. Indian J. Pharm. Educ. Res. 2018, 52, 102–107. [Google Scholar] [CrossRef]
- Lis, A.; Banaszczak, P. Composition of the essential oil of Phellodendron piriforme. Chem. Nat. Compd. 2011, 47, 641–643. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B. The essential oil of Senecio farfarifolius Boiss. et Kotschy growing in Turkey. J. Essent. Oil Res. 2004, 16, 558–559. [Google Scholar] [CrossRef]
- Linde, J.; Combrinck, S.; Vuuren, S.V.; Rooy, J.V.; Ludwiczuk, A.; Mokgalaka, N. Volatile constituents and antimicrobial activities of nine South African liverwort species. Phytochem. Lett. 2016, 16, 61–69. [Google Scholar] [CrossRef]
- Fkiri, S.; Ghazghazi, H.; Rigane, G.; Ben Salem, R.; Mezni, F.; Khaldi, A.; Khouja, M.L.; Nasr, Z. Chemical compositions and biological activities essential oil from the needles of North African Pinus Pinaster Var. Rev. Roum. Chim. 2019, 64, 511–517. [Google Scholar] [CrossRef]
- Öztürk, B.; Özek, G.; Özek, T.; Baser, K.H.C. Chemical diversity in volatiles of Helichrysum plicatum DC. subspecies in Turkey. Rec. Nat. Prod. 2014, 8, 373–384. [Google Scholar]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M.; Helal, A.N.; Mighri, Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef]
- Clery, R.A.; Cason, J.R.L.; Zelenay, V. Constituents of cypriol oil (Cyperus scariosus R.Br.): N-Containing molecules and key aroma components. J. Agr. Food Chem. 2016, 64, 4566–4573. [Google Scholar] [CrossRef]
- Altintas, A.; Kosar, M.; Kirimer, N.; Baser, K.H.C.; Demirci, B. Composition of the essential oils of Lycium barbarum and L. ruthenicum fruits. Chem. Nat. Compd. 2006, 42, 24–25. [Google Scholar] [CrossRef]
- Boussaada, O.; Saidana, D.; Chriaa, J.; Chraif, I.; Ammar, S.; Mahjoub, M.A.; Mighri, Z.; Daami, M.; Helal, A.N. Chemical composition and antimicrobial activity of volatile components of Scorzonera undulata. J. Essent. Oil Res. 2008, 20, 358–362. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation Carol Stream: Chicago, IL, USA, 2007; pp. 1–804. [Google Scholar]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Melusova, M.; Kozics, K.; Slamenova, D. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. J. Agric. Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Arisoy, K.; Tepe, B.; Cakir, A.; Abali, G.; Mete, E. Studies on the antioxidant activity of essential oil and different solvent extracts of Vitex agnus-castus L. fruits from Turkey. Food Chem. Toxicol. 2009, 47, 2479–2483. [Google Scholar] [CrossRef]
- Chang, S.T.; Cheng, S.S.; Wang, S.Y. Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides). J. Chem. Ecol. 2001, 27, 717–724. [Google Scholar] [CrossRef]
- Chang, S.T.; Chen, P.F.; Wang, S.Y.; Wu, H.H. Antimite activity of essential oils and their constituents from Taiwania Cryptomerioides. J. Med. Entomol. 2001, 38, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Frag. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Gross, E.M.; Hilt, S.; Lombardo, P.; Mulderij, G. Searching for allelopathic effects of submerged macrophytes on phytoplankton-state of the art and open questions. Hydrobiologia 2007, 584, 77–88. [Google Scholar] [CrossRef]
- Katarína, K.; Katarína, K.; Svajlenová, O.; Ján, V. Biological activity of copper (II) N-salicylideneaminoacidato complexes. Reduction of chlorophyll content in freshwater alga Chlorella vulgaris and inhibition of photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 2004, 58, 357–361. [Google Scholar]
- Han, H.; Chen, Y.; Jørgensen, S.E.; Nielsen, S.N.; Hu, W. A system-dynamic model on the competitive growth between Potamogeton malaianus Miq. and Spirogyra sp. Ecol. Model. 2009, 220, 2206–2217. [Google Scholar] [CrossRef]
- Zuo, S.; Zhou, S.; Ye, L.; Ma, S. Synergistic and antagonistic interactions among five allelochemicals with antialgal effects on bloom-forming Microcystis aeruginosa. Ecol. Eng. 2016, 97, 486–492. [Google Scholar] [CrossRef]
- Wang, H.; Liang, F.; Zhang, L. Composition and anti-cyanobacterial activity of essential oils from six different submerged macrophytes. Pol. J. Environ. Stud. 2015, 24, 333–338. [Google Scholar] [CrossRef]
- Wang, H.; Xi, B.; Cheng, S.; Wang, Y.; Zhang, L. Phenolic and fatty acids from pomegranate peel and seeds: Extraction, identification and determination of their anti-algal activity. Fresen. Environ. Bull. 2015, 24, 3921–3925. [Google Scholar]
- Chen, Y.H.; Lin, C.Y.; Yen, P.L.; Yeh, T.F.; Cheng, S.S.; Chang, S.T. Antifungal agents from heartwood extract of Taiwania cryptomerioides, against brown root rot fungus Phellinus noxius. Wood Sci. Technol. 2017, 51, 1–13. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, J.H.; Shyur, L.F.; Kuo, Y.H.; Chang, S.T. Antioxidant activity of abietane-type diterpenes from heartwood of Taiwania cryptomerioides Hayata. Holzforschung 2002, 56, 487–492. [Google Scholar] [CrossRef]
- Ho, C.L.; Yang, S.S.; Chang, T.M.; Su, Y.C. Composition, antioxidant, antimicrobial and anti-wood-decay fungal activities of the twig essential oil of Taiwania cryptomerioides from Taiwan. Nat. Prod. Commun. 2012, 7, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Costantin, M.B.; Sartorelli, P.; Rodrigues, G.V.; Limberger, R.; Henriques, A.T.; Kato, M.J.; Emerenciano, V.P. Computer-aided method for identification of components in essential oils by 13C NMR spectroscopy. Anal. Chim. Acta. 2001, 447, 125–134. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Pacheco, R.; Ferreira, A.F.; Pinto, T.; Nobre, B.P.; Loureiro, D.; Moura, P.; Gouveia, L.; Silva, C.M. The production of pigments & hydrogen through a Spirogyra sp. biorefinery. Energ. Convers. Manage. 2015, 89, 789–797. [Google Scholar]
- Dere, S.; Gunes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Houot, V.; Etienne, P.; Petitot, A.; Barbier, S.; Blein, J.; Suty, L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 2001, 52, 1721–1730. [Google Scholar]
- Boubaker, H.; Karim, H.; Hamdaoui, A.E.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four thymus, species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 9th ed.; CLSI: Wayne, PA, USA, 2006; pp. 2–9. [Google Scholar]
- Verma, S.K.; Goswami, P.; Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R.; Darokar, M.P. Chemical composition and antimicrobial activity of bergamot-mint (Mentha citrata Ehrh.) essential oils isolated from the herbage and aqueous distillate using different methods. Ind. Crops Prod. 2016, 91, 152–160. [Google Scholar] [CrossRef]
- El-Gawad, A.M.A. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
Sample Availability

| No. | Compounds | Molecular Formula | Percentage (%) | RI a | RI b(Reference) | Methods of Identification |
|---|---|---|---|---|---|---|
| Fatty Acids | ||||||
| 1 | Nonanoic acid | C9H18O2 | 0.03 | 1264 | 2192 [24] | a,c,d |
| 2 | Decanoic acid | C10H20O2 | 0.03 | 1361 | 2298 [25] | a,c,d |
| 3 | Dodecanoic acid | C12H24O2 | 0.18 | 1560 | 2503 [24] | a,c,d |
| 4 | Tridecanoic acid | C13H26O2 | 0.02 | 1657 | 2617 [25] | a,c,d |
| 5 | Tetradecanoic acid | C14H28O2 | 1.10 | 1764 | 2670 [25] | a,c,d |
| 6 | 14-Pentadecenoic acid | C15H28O2 | 0.52 | 1848 | 3181 [26] | a,c,d |
| 7 | Pentadecanoic acid | C15H30O2 | 1.37 | 1868 | 2822 [25] | a,c,d |
| 8 | Palmitoleic acid | C16H30O2 | 0.81 | 1946 | 2948 [27] | a,c,d |
| 9 | 9-Hexadecenoic acid | C16H30O2 | 0.09 | 1952 | - | a,c,d |
| 10 | Hexadecanoic acid | C16H32O2 | 27.13 | 1992 | 2931 [25] | a,c,d |
| 11 | cis-10-Heptadecenoic acid | C17H32O2 | 0.08 | 2055 | - | a,c,d |
| 12 | Heptadecanoic acid | C17H34O2 | 0.20 | 2068 | 2305 [28] | a,c,d |
| 13 | Linoleic acid | C18H32O2 | 13.48 | 2155 | 3157 [29] | a,c,d |
| 14 | α-Linolenic acid | C18H30O2 | 5.41 | 2160 | 3193 [29] | a,c,d |
| Monoterpenes | ||||||
| 15 | α-Terpineol | C10H18O | 0.03 | 1194 | 1706 [24] | a,c,d |
| 16 | Carvacrol | C10H14O | 0.02 | 1301 | 2241 [30] | a,c,d |
| Sesquiterpenes | ||||||
| 17 | (-)-Spathulenol | C15H24O | 0.09 | 1599 | 2144 [25] | a,c,d |
| 18 | Widdrol | C15H26O | 0.12 | 1611 | 2179 [31] | a,c,d |
| 19 | Cedrol | C15H26O | 0.13 | 1614 | 2093 [32] | a,c,d |
| 20 | 6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hepta-1,5-dien-4-ol | C15H24O | 0.05 | 1631 | - | a,c,d |
| 21 | 8-Cedren-13-ol | C15H24O | 0.11 | 1637 | 2199 [33] | a,c,d |
| 22 | γ-Eudesmol | C15H26O | 0.12 | 1640 | 2193 [24] | a,c,d |
| 23 | epi-α-Muurolol | C15H26O | 0.06 | 1650 | 1621 [34] | a,c,d |
| 24 | β-Eudesmol | C15H26O | 0.06 | 1660 | 2257 [24] | a,c,d |
| 25 | T-cadinol | C15H26O | 0.13 | 1662 | 2187 [31] | a,c,d |
| 26 | Germacra-4(15),5E,10(14)-trien-1β-ol | C15H24O | 0.05 | 1667 | - | a,c,d |
| 27 | Humulenol-II | C15H24O | 0.09 | 1681 | - | a,c,d |
| 28 | α-Bisabolol | C15H26O | 0.05 | 1688 | 2232 [35] | a,c,d |
| 29 | Longifolaldehyde | C15H24O | 0.02 | 1692 | - | a,c,d |
| 30 | β-Acoradienol | C15H24O | 0.05 | 1787 | - | a,c,d |
| 31 | Drimenol | C15H26O | 0.04 | 1817 | 1772 [36] | a,c,d |
| Diterpenes | ||||||
| 32 | Ambrial | C16H26O | 0.07 | 1809 | - | a,c,d |
| 33 | Biformene | C20H32 | 0.32 | 1937 | 1907 [37] | a,c,d |
| 34 | Cembrene | C20H32 | 0.16 | 1958 | - | a,c,d |
| 35 | Manoyl oxide | C20H34O | 0.64 | 2000 | 2376 [31] | a,c,d |
| 36 | 13-epi-Manoyl oxide | C20H34O | 0.11 | 2021 | 2335 [38] | a,c,d |
| 37 | Manool | C20H34O | 0.03 | 2027 | 2180 [39] | a,c,d |
| 38 | Geranyl linalool | C20H34O | 0.06 | 2033 | 1912 [37] | a,c,d |
| 39 | Abietatriene | C20H30 | 1.04 | 2063 | 2065 [37] | a,c,d |
| 40 | Thunbergol | C20H34O | 0.02 | 2076 | - | a,c,d |
| 41 | Abieta- 7, 13- diene | C20H32 | 0.04 | 2088 | - | a,c,d |
| 42 | 2-Penten-1-ol, 3-methyl-5-[octahydro-4,5-dimethyl-7a-(1-methylethenyl)-1H-inden-4-yl- | C20H34O | 16.16 | 2121 | - | a,c,d |
| 43 | Sandaracopimarinal | C20H30O | 0.17 | 2192 | - | a,c,d |
| 44 | Larixol | C20H34O2 | 0.28 | 2211 | - | a,c,d |
| 45 | 4-epi-Dehydroabietol | C20H30O | 0.77 | 2227 | - | a,c,d |
| 46 | Nimbiol | C18H24O2 | 0.26 | 2258 | - | a,c,d |
| 47 | Isopimara-7,15-dien-3-one | C20H30O | 0.26 | 2261 | - | a,c,d |
| 48 | trans-Totarol | C20H30O | 0.19 | 2286 | 2280 [32] | a,c,d |
| 49 | Dehydroabietal | C20H28O | 0.78 | 2305 | - | a,c,d |
| 50 | Podocarpa-6,8,11,13-tetraen-12-ol, 13-isopropyl-, acetate | C22H30O2 | 7.56 | 2332 | - | a,c,d |
| 51 | Ferruginol | C20H30O | 6.52 | 2339 | 2327 [37] | a,c,d |
| 52 | Hinokione | C20H28O2 | 0.51 | 2463 | - | a,c,d |
| 53 | Dronabinol | C21H30O2 | 0.11 | 2515 | - | a,c,d |
| Esters | ||||||
| 54 | Methyl pentadecanoate | C15H30O2 | 0.22 | 1825 | 2099 [29] | a,c,d |
| 55 | Diisobutyl phthalate | C16H22O4 | 0.14 | 1870 | - | a,c,d |
| 56 | Cyperolactone | C15H22O2 | 0.02 | 1873 | 2480 [40] | a,c,d |
| 57 | Methyl hexadecanoate | C17H34O2 | 0.46 | 1926 | 2226 [41] | a,c,d |
| 58 | Methyl linoleate | C19H34O2 | 0.35 | 2099 | 2490 [42] | a,c,d |
| 59 | Methyl linolenate | C19H32O2 | 0.17 | 2105 | 2478 [42] | a,c,d |
| Phenols | ||||||
| 60 | 2-Allyl-4-methylphenol | C10H12O | 0.05 | 1373 | - | a,c,d |
| 61 | 2,2-Methylene-bis(4-methyl-6-tert-butylphenol) | C23H32O2 | 0.10 | 2421 | - | a,c,d |
| Alkanes | ||||||
| 62 | Docosane | C22H46 | 0.07 | 2200 | 2196 [34] | a,c,d |
| 63 | Pentacosane | C25H52 | 0.05 | 2499 | 2500 [25] | a,c,d |
| 64 | Heptacosane | C27H56 | 0.06 | 2698 | 2700 [25] | a,c,d |
| 65 | Nonacosane | C29H60 | 0.03 | 2895 | 2900 [25] | a,c,d |
| Others | ||||||
| 66 | 2,5,5,8a-Tetramethyl-4-methylene-6,7,8,8a-tetrahydro-4H,5H-chromen-4a-yl hydroperoxide | C14H22O3 | 0.02 | 1740 | - | a,c,d |
| 67 | cis-9-Hexadecenal | C16H30O | 0.03 | 1750 | - | a,c,d |
| 68 | 1-Hexadecanol | C16H34O | 0.17 | 1881 | 2384 [27] | a,c,d |
| 69 | 13-Heptadecyn-1-ol | C17H32O | 0.03 | 2041 | - | a,c,d |
| Total | 89.70 | |||||
| Pigment | Treatment | Time (h) | Regression Equation | r | IC50 (μg/mL) | 95%CL (μg/mL) |
|---|---|---|---|---|---|---|
| Chlorophyll a | Essential oil | 24 | y = 1.2251 + 1.9312x | 0.9327 | 90.10 | 74.01–109.68 |
| 48 | y = −0.0821 + 3.0372x | 0.9896 | 47.13 | 42.09–52.76 | ||
| 72 | y = 1.6596 + 2.0762x | 0.9437 | 40.64 | 34.98–47.21 | ||
| 96 | y = 2.6742 + 1.3534x | 0.9536 | 52.29 | 42.40–64.50 | ||
| Butachlor | 24 | y = 0.7963 + 2.4091x | 0.9459 | 55.58 | 48.57–63.60 | |
| 48 | y = 0.9345 + 2.5763x | 0.9794 | 37.85 | 33.08–43.30 | ||
| 72 | y = 1.5056 + 2.2331x | 0.9946 | 36.71 | 31.66–42.58 | ||
| 96 | y = 1.4714 + 2.2568x | 0.9174 | 36.60 | 31.49–42.54 | ||
| Chlorophyll b | Essential oil | 24 | y = 0.7342 + 2.1024x | 0.9438 | 106.91 | 86.86–131.58 |
| 48 | y = −1.2225 + 3.7115x | 0.9759 | 47.49 | 42.73–52.77 | ||
| 72 | y = 1.9149 + 1.7859x | 0.9560 | 53.39 | 45.22–63.05 | ||
| 96 | y = 2.7763 + 1.2659x | 0.9584 | 57.09 | 45.60–71.47 | ||
| Butachlor | 24 | y = 0.6332 + 2.3004x | 0.9186 | 79.12 | 67.10–93.00 | |
| 48 | y = −0.0687 + 2.8175x | 0.9467 | 62.95 | 55.74–71.10 | ||
| 72 | y = 1.5328 + 2.0981x | 0.9424 | 44.93 | 38.67–52.20 | ||
| 96 | y = 1.8578 + 1.8762x | 0.9530 | 47.29 | 40.24–55.57 | ||
| Total chlorophyll | Essential oil | 24 | y = 1.2976 + 1.9193x | 0.9309 | 84.92 | 70.19–102.75 |
| 48 | y = −0.0821 + 3.0372x | 0.9623 | 46.62 | 41.67–52.15 | ||
| 72 | y = 1.6945 + 2.2008x | 0.9200 | 31.77 | 27.16–37.17 | ||
| 96 | y = 2.7412 + 1.3035x | 0.9482 | 54.05 | 43.48–67.18 | ||
| Butachlor | 24 | y = 0.6609 + 2.4596x | 0.9436 | 58.09 | 50.76–66.49 | |
| 48 | y = 0.7453 + 2.6025x | 0.9719 | 43.14 | 37.87–49.14 | ||
| 72 | y = 1.7028 + 2.0456x | 0.9566 | 40.91 | 35.07–47.74 | ||
| 96 | y = 1.7113 + 2.0495x | 0.9256 | 40.24 | 34.43–47.04 |
| Treatment | Regression Equation | r | IC50 (μg/mL) | 95%CL (μg/mL) | |
|---|---|---|---|---|---|
| With Light | chlorophyll a | y = −1.9294 + 3.7360x | 0.9062 | 71.58 | 57.66–88.84 |
| chlorophyll b | y = −1.5076 + 3.3476x | 0.9310 | 87.89 | 67.42–114.59 | |
| total chlorophyll | y = −2.0191 + 3.6402x | 0.9473 | 84.77 | 65.94–108.97 | |
| Without Light | chlorophyll a | y = 2.1333 + 0.9279x | 0.9814 | 1229.24 | 121.24–12463.23 |
| chlorophyll b | y = 1.6774 + 1.0713x | 0.9071 | 1156.28 | 156.28–10205.66 | |
| total chlorophyll | y = 2.1286 + 0.9302x | 0.9328 | 1221.58 | 120.76–12357.62 | |
| Fungus | Time (h) | Regression Equation | r | EC50 (μg/mL) | 95%CL(μg/mL) |
|---|---|---|---|---|---|
| Rhizoctonia solani | 24 | y = 1.8819 + 1.2679x | 0.9876 | 287.94 | 195.23–424.68 |
| Colletotrichum gloeosporioiles | 48 | y = 2.8088 + 0.8498x | 0.9261 | 378.90 | 205.68–698.17 |
| Fusarium moniliforme | 72 | y = 2.4418 + 0.8627x | 0.9865 | 923.03 | 529.24–1609.80 |
| Thanatephorus cucumeris | 72 | y = 1.4164 + 1.2822x | 0.9716 | 623.36 | 453.38–857.06 |
| Fusariun oxysporun | 72 | y = 1.5112 + 1.1997x | 0.9754 | 809.07 | 545.56–1199.86 |
| Didymella bryoniae | 72 | y = 1.5316 + 0.9910x | 0.9122 | 3162.34 | 1187.37–8422.33 |
| Treatment | Regression Eq | IC50 (μg/mL) | r | 95% CL (μg/mL) |
|---|---|---|---|---|
| Essential oil | y = 1.6712 + 2.1903x | 33.51 | 0.9766 | 26.15–42.95 |
| Ascorbic acid | y = 3.5608 + 1.6001x | 7.98 | 0.9808 | 5.76–11.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Huang, J.; Yang, S.; Li, J.; Zhou, L. Chemical Composition, Algicidal, Antimicrobial, and Antioxidant Activities of the Essential Oils of Taiwania flousiana Gaussen. Molecules 2020, 25, 967. https://doi.org/10.3390/molecules25040967
Liu H, Huang J, Yang S, Li J, Zhou L. Chemical Composition, Algicidal, Antimicrobial, and Antioxidant Activities of the Essential Oils of Taiwania flousiana Gaussen. Molecules. 2020; 25(4):967. https://doi.org/10.3390/molecules25040967
Chicago/Turabian StyleLiu, Hongmei, Jiguang Huang, Sifan Yang, Jialin Li, and Lijuan Zhou. 2020. "Chemical Composition, Algicidal, Antimicrobial, and Antioxidant Activities of the Essential Oils of Taiwania flousiana Gaussen" Molecules 25, no. 4: 967. https://doi.org/10.3390/molecules25040967
APA StyleLiu, H., Huang, J., Yang, S., Li, J., & Zhou, L. (2020). Chemical Composition, Algicidal, Antimicrobial, and Antioxidant Activities of the Essential Oils of Taiwania flousiana Gaussen. Molecules, 25(4), 967. https://doi.org/10.3390/molecules25040967