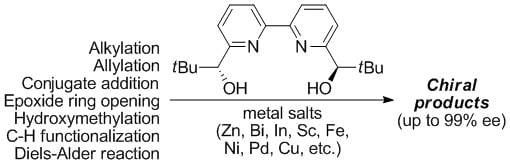
Applications of Bolm’s Ligand in Enantioselective Synthesis
Abstract
1. Introduction
2. Bolm’s Ligand
2.1. Bolm’s Ligand Properties and Synthesis
2.2. Alkylation and Allylation of Aldehydes
2.3. Conjugate Addition
2.4. Opening of Meso-Epoxides
2.5. Mukaiyama Aldol Reaction
2.6. Hydroxymethylation
2.7. α-Amination of β-Ketocarbonyl Compounds
2.8. C-H Functionalization of Indole
2.9. Diels–Alder Reaction
2.10. Other Applications
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Note
- Liu, B.; Yu, W.-L.; Pei, J.; Liu, S.-Y.; Lai, Y.-H.; Huang, W. Design and Synthesis of Bipyridyl-Containing Conjugated Polymers: Effects of Polymer Rigidity on Metal Ion Sensing. Macromolecules 2001, 34, 7932–7940. [Google Scholar] [CrossRef]
- Mathieu, J.; Fraisse, B.; Lacour, D.; Ghermani, N.; Montaigne, F.; Marsura, A. An Original Supramolecular Helicate from a Bipyridine–Bipyrazine Ligand Strand and NiII by Self-Assembly. Eur. J. Inorg. Chem. 2006, 133–136. [Google Scholar] [CrossRef]
- Schubert, U.S.; Kersten, J.L.; Pemp, A.E.; Eisenbach, C.D.; Newkome, G.R. A New Generation of 6,6′-Disubstituted 2,2′-Bipyridines: Towards Novel Oligo (Bipyridine) Building Blocks for Potential Applications in Materials Science and Supramolecular Chemistry. Eur. J. Org. Chem. 1998, 2573–2581. [Google Scholar] [CrossRef]
- Balzani, V.; Juris, A. Photochemistry and Photophysics of Ru (II)-Polypyridine Complexes in the Bologna Group. From Early Studies to Recent Developments. Coord. Chem. Rev. 2001, 211, 97–115. [Google Scholar] [CrossRef]
- Margel, S.; Smith, W.; Anson, F.C. Electrochemistry of 2,2′-Bipyridine Complexes of Cobalt in the Presence of Acrylonitrile. J. Electrochem. Soc. 1978, 125, 241–246. [Google Scholar] [CrossRef]
- Smith, A.P.; Fraser, C.L. 1.1-Bipyridine Ligands. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: Oxford, UK, 2003. [Google Scholar]
- Fletcher, N.C. Chiral 2,2′-bipyridines: ligands for asymmetric induction. J. Chem. Soc. Perkin 1 2002, 1831–1842. [Google Scholar] [CrossRef]
- Malkov, A.V.; Kocovsky, P. Chiral Bipyridine Derivatives in Asymmetric Catalysis. Curr. Org. Chem. 2003, 17, 1737–1757. [Google Scholar] [CrossRef]
- Bolm, C.; Zehnder, M.; Bur, D. Optically Active Bipyridines in Asymmetric Catalysis. Angew. Chem. Int. Ed. Engl. 1990, 29, 205–207. [Google Scholar] [CrossRef]
- Bolm, C.; Ewald, M.; Felder, M.; Schlingloff, G. Enantioselective Synthesis of Optically Active Pyridine Derivatives and C2-Symmetric 2,2′-Bipyridines. Chem. Ber. 1992, 125, 1169–1190. [Google Scholar] [CrossRef]
- Ishikawa, S.; Hamada, T.; Manabe, K.; Kobayashi, S. New Efficient Method for the Synthesis of Chiral 2,2′-Bipyridyl Ligands. Synthesis 2005, 2176–2182. [Google Scholar]
- Kobayashi, S.; Endo, T.; Yoshino, T.; Schneider, U.; Ueno, M. Allylation Reactions of Aldehydes with Allylboronates in Aqueous Media: Unique Reactivity and Selectivity That Are Only Observed in the Presence of Water. Chem. Asian J. 2013, 8, 2033–2045. [Google Scholar] [CrossRef]
- Kokubo, M.; Naito, T.; Kobayashi, S. Chiral Zinc (II) and Copper (II)-Catalyzed Asymmetric Ring-Opening Reactions of Meso-Epoxides with Aniline and Indole Derivatives. Tetrahedron 2010, 66, 1111–1118. [Google Scholar] [CrossRef]
- Plancq, B.; Lafantaisie, M.; Companys, S.; Maroun, C.; Ollevier, T. Highly Enantioselective Iron (II)-Catalyzed Opening Reaction of Aromatic Meso -Epoxides with Indoles. Org. Biomol. Chem. 2013, 11, 7463–7466. [Google Scholar] [CrossRef]
- Ollevier, T.; Plancq, B. Highly Enantioselective Mukaiyama Aldol Reaction in Aqueous Conditions Using a Chiral Iron (II) Bipyridine Catalyst. Chem. Commun. 2012, 48, 2289–2291. [Google Scholar] [CrossRef]
- Plancq, B.; Ollevier, T. Iron (II)-Catalyzed Enantioselective Meso-Epoxide-Opening with Anilines. Chem. Commun. 2012, 48, 3806–3808. [Google Scholar] [CrossRef][Green Version]
- Kokubo, M.; Naito, T.; Kobayashi, S. Metal-Controlled Reversal of Enantioselectivity in Catalyzed Asymmetric Ring-Opening Reactions of Meso-Epoxides in Water. Chem. Lett. 2009, 38, 904–905. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ogino, T.; Shimizu, H.; Ishikawa, S.; Hamada, T.; Manabe, K. Bismuth Triflate−Chiral Bipyridine Complexes as Water-Compatible Chiral Lewis Acids. Org. Lett. 2005, 7, 4729–4731. [Google Scholar] [CrossRef]
- Nandakumar, M.V.; Tschöp, A.; Krautscheid, H.; Schneider, C. Indium–Bipyridine-Catalyzed, Enantioselective Thiolysis of Meso-Epoxides. Chem. Commun. 2007, 2756–2758. [Google Scholar] [CrossRef]
- Bolm, C.; Schlingloff, G.; Harms, K. Catalyzed Enantioselective Alkylation of Aldehydes. Chem. Ber. 1992, 125, 1191–1203. [Google Scholar] [CrossRef]
- Kobayashi, S.; Endo, T.; Schneider, U.; Ueno, M. Aldehyde Allylation with Allylboronates Providing α-Addition Products. Chem. Commun. 2010, 46, 1260–1262. [Google Scholar] [CrossRef]
- Kobayashi, S.; Endo, T.; Ueno, M. Chiral Zinc-Catalyzed Asymmetric α-Alkylallylation and α-Chloroallylation of Aldehydes. Angew. Chem. Int. Ed. 2011, 50, 12262–12265. [Google Scholar] [CrossRef]
- Bolm, C.; Ewald, M. Optically Active Bipyridines in Nickel Catalyzed Enantioselective Conjugate Addition to Enones. Tetrahedron Lett. 1990, 31, 5011–5012. [Google Scholar] [CrossRef]
- Bolm, C.; Ewald, M.; Felder, M. Catalytic Enantioselective Conjugate Addition of Dialkylzinc Compounds to Chalcones. Chem. Ber. 1992, 125, 1205–1215. [Google Scholar] [CrossRef]
- Ogawa, C.; Kizu, K.; Shimizu, H.; Takeuchi, M.; Kobayashi, S. Chiral Scandium Catalysts for Enantioselective Michael Reactions of β-Ketoesters. Chem. Asian J. 2006, 1, 121–124. [Google Scholar] [CrossRef]
- Bonollo, S.; Lanari, D.; Pizzo, F.; Vaccaro, L. Sc (III)-Catalyzed Enantioselective Addition of Thiols to α,β-Unsaturated Ketones in Neutral Water. Org. Lett. 2011, 13, 2150–2152. [Google Scholar] [CrossRef]
- Ueno, M.; Kitanosono, T.; Sakai, M.; Kobayashi, S. Chiral Sc-Catalyzed Asymmetric Michael Reactions of Thiols with Enones in Water. Org. Biomol. Chem. 2011, 9, 3619–3621. [Google Scholar] [CrossRef]
- Lauzon, S.; Keipour, H.; Gandon, V.; Ollevier, T. Asymmetric FeII-Catalyzed Thia-Michael Addition Reaction to α, β-Unsaturated Oxazolidin-2-One Derivatives. Org. Lett. 2017, 19, 6324–6327. [Google Scholar] [CrossRef]
- Kobayashi, S.; Xu, P.; Endo, T.; Ueno, M.; Kitanosono, T. Chiral Copper (II)-Catalyzed Enantioselective Boron Conjugate Additions to α, β-Unsaturated Carbonyl Compounds in Water. Angew. Chem. Int. Ed. 2012, 5, 12763–12766. [Google Scholar] [CrossRef]
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S. A Cu (II)-Based Strategy for Catalytic Enantioselective β-Borylation of α, β-Unsaturated Acceptors. Chem. Commun. 2015, 51, 11685–11688. [Google Scholar] [CrossRef]
- Kitanosono, T.; Kobayashi, S. Asymmetric Boron Conjugate Additions to Enones in Water Catalyzed by Copper (0). Asian J. Org. Chem. 2013, 2, 961–966. [Google Scholar] [CrossRef]
- Kitanosono, T.; Xu, P.; Isshiki, S.; Zhu, L.; Kobayashi, S. Cu (II)-Catalyzed Asymmetric Boron Conjugate Addition to α,β-Unsaturated Imines in Water. Chem. Commun. 2014, 50, 9336–9339. [Google Scholar] [CrossRef]
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S. Chiral Cu (II)-Catalyzed Enantioselective β-Borylation of α,β-Unsaturated Nitriles in Water. Beilstein J. Org. Chem. 2015, 11, 2007–2011. [Google Scholar] [CrossRef]
- Kitanosono, T.; Xu, P.; Kobayashi, S. Heterogeneous and Homogeneous Chiral Cu (II) Catalysis in Water: Enantioselective Boron Conjugate Additions to Dienones and Dienoesters. Chem. Commun. 2013, 49, 8184–8186. [Google Scholar] [CrossRef]
- Bergmeier, S.C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Schneider, C.; Sreekanth, A.R.; Mai, E. Scandium–Bipyridine-Catalyzed Enantioselective Addition of Alcohols and Amines to Meso-Epoxides. Angew. Chem. Int. Ed. 2004, 43, 5691–5694. [Google Scholar] [CrossRef]
- Mai, E.; Schneider, C. Scandium–Bipyridine-Catalyzed Enantioselective Aminolysis of Meso-Epoxides. Chem. Eur. J. 2007, 13, 2729–2741. [Google Scholar] [CrossRef]
- Azoulay, S.; Manabe, K.; Kobayashi, S. Catalytic Asymmetric Ring Opening of Meso-Epoxides with Aromatic Amines in Water. Org. Lett. 2005, 7, 4593–4595. [Google Scholar] [CrossRef]
- Bonollo, S.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. Zn (II)-Catalyzed Desymmetrization of Meso-Epoxides by Aromatic Amines in Water. Synlett 2008, 1574–1578. [Google Scholar]
- Bonollo, S.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. Zr (DS)4 as an Efficient Catalyst for the Aminolysis of Epoxides in Water. Synlett 2007, 2683–2686. [Google Scholar]
- Mai, E.; Schneider, C. Indium-Bipyridine Catalyzed, Enantioselective Aminolysis of Meso-Epoxides. Synlett 2007, 2136–2138. [Google Scholar] [CrossRef]
- Tschöp, A.; Marx, A.; Sreekanth, A.R.; Schneider, C. Scandium-Bipyridine-Catalyzed, Enantioselective Alcoholysis of Meso-Epoxides. Eur. J. Org. Chem. 2007, 2318–2327. [Google Scholar] [CrossRef]
- Boudou, M.; Ogawa, C.; Kobayashi, S. Chiral Scandium-Catalysed Enantioselective Ring-Opening of Meso-Epoxides with N-Heterocycle, Alcohol and Thiol Derivatives in Water. Adv. Synth. Catal. 2006, 348, 2585–2589. [Google Scholar] [CrossRef]
- Ogawa, C.; Wang, N.; Kobayashi, S. Chiral Scandium-Catalyzed Highly Stereoselective Ring-Opening of Meso-Epoxides with Thiols. Chem. Lett. 2007, 36, 34–35. [Google Scholar] [CrossRef]
- Tschöp, A.; Nandakumar, M.V.; Pavlyuk, O.; Schneider, C. Scandium–Bipyridine-Catalyzed, Enantioselective Selenol Addition to Aromatic Meso-Epoxides. Tetrahedron Lett. 2008, 49, 1030–1033. [Google Scholar] [CrossRef]
- It may seem that the results are in contrast with non-linear effect experiments on conjugate addition with Sc(III) triflate in water where the non-linearity was observed. On the other hand, Kobayashi et al. observed that the presence of additive can influence non-linear effect in Fe(II) catalyzed Mukaiyama aldol reaction [49]. Perhaps the use of pyridine as an additive during conjugate addition could clear up discrepancy in results.
- Mai, E.; Schneider, C. Enantioselective Synthesis of Boc-Protected 1,2-Amino Alcohols through Aminolysis of Meso-Epoxides with Benzophenone Imine. ARKIVOC 2008, xvi, 216–222. [Google Scholar]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef]
- Kitanosono, T.; Ollevier, T.; Kobayashi, S. Iron- and Bismuth-Catalyzed Asymmetric Mukaiyama Aldol Reactions in Aqueous Media. Chem. Asian J. 2013, 8, 3051–3062. [Google Scholar] [CrossRef]
- Lafantaisie, M.; Mirabaud, A.; Plancq, B.; Ollevier, T. Iron (II)-Derived Lewis Acid/Surfactant Combined Catalysis for the Enantioselective Mukaiyama Aldol Reaction in Pure Water. ChemCatChem 2014, 6, 2244–2247. [Google Scholar] [CrossRef]
- Sameera, W.M.C.; Hatanaka, M.; Kitanosono, T.; Kobayashi, S.; Morokuma, K. The Mechanism of Iron (II)-Catalyzed Asymmetric Mukaiyama Aldol Reaction in Aqueous Media: Density Functional Theory and Artificial Force-Induced Reaction Study. J. Am. Chem. Soc. 2015, 137, 11085–11094. [Google Scholar] [CrossRef]
- Ishikawa, S.; Hamada, T.; Manabe, K.; Kobayashi, S. Catalytic Asymmetric Hydroxymethylation of Silicon Enolates Using an Aqueous Solution of Formaldehyde with a Chiral Scandium Complex. J. Am. Chem. Soc. 2004, 126, 12236–12237. [Google Scholar] [CrossRef]
- Kokubo, M.; Ogawa, C.; Kobayashi, S. Lewis Acid Catalysis in Water with a Hydrophilic Substrate: Scandium-Catalyzed Hydroxymethylation with Aqueous Formaldehyde in Water. Angew. Chem. Int. Ed. 2008, 47, 6909–6911. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.R.W.; Sanderson, J.M. The Synthesis of Peptides and Proteins Containing Non-Natural Amino Acids. Chem. Soc. Rev. 2004, 33, 422–430. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, A.J.; Kooijman, M.; Hennink, W.E.; Mastrobattista, E. Nonnatural Amino Acids for Site-Specific Protein Conjugation. Bioconjug. Chem. 2009, 20, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nandakumar, M.V.; Krautscheid, H.; Schneider, C. Copper–Bipyridine-Catalyzed Enantioselective α-Amination of β-Keto Esters. Tetrahedron Lett. 2010, 51, 1860–1862. [Google Scholar] [CrossRef]
- Kitanosono, T.; Miyo, M.; Kobayashi, S. Surfactant-Aided Chiral Palladium (II) Catalysis Exerted Exclusively in Water for the C–H Functionalization of Indoles. ACS Sustain. Chem. Eng. 2016, 4, 6101–6106. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, L.-W.; Li, L.; Yang, L.; Xia, C.-G. Enantioselective Michael-Type Friedel–Crafts Reactions of Indoles to Enones Catalyzed by a Chiral Camphor-Based Brønsted Acid. Eur. J. Org. Chem. 2006, 5225–5227. [Google Scholar] [CrossRef]
- Li, M.; Carreras, V.; Jalba, A.; Ollevier, T. Asymmetric Diels–Alder Reaction of α, β-Unsaturated Oxazolidin-2-One Derivatives Catalyzed by a Chiral Fe (III)-Bipyridine Diol Complex. Org. Lett. 2018, 20, 995–998. [Google Scholar] [CrossRef]
- Lee, W.-S.; Yeung, C.-T.; Sham, K.-C.; Wong, W.-T.; Kwong, H.-L. Chiral Copper–Bipyridine Complexes: Synthesis, Characterization and Mechanistic Studies on Asymmetric Cyclopropanation. Polyhedron 2011, 30, 178–186. [Google Scholar] [CrossRef]
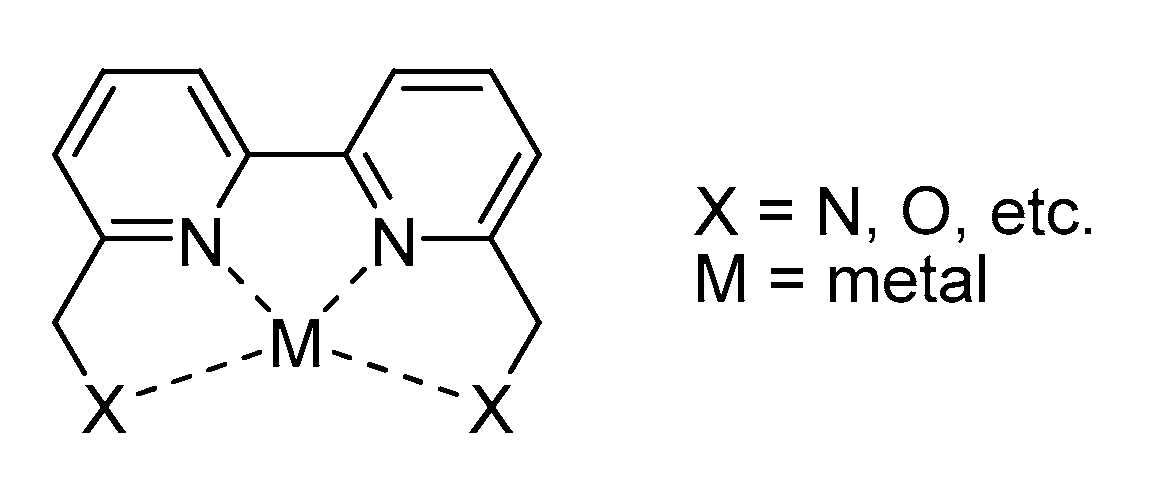

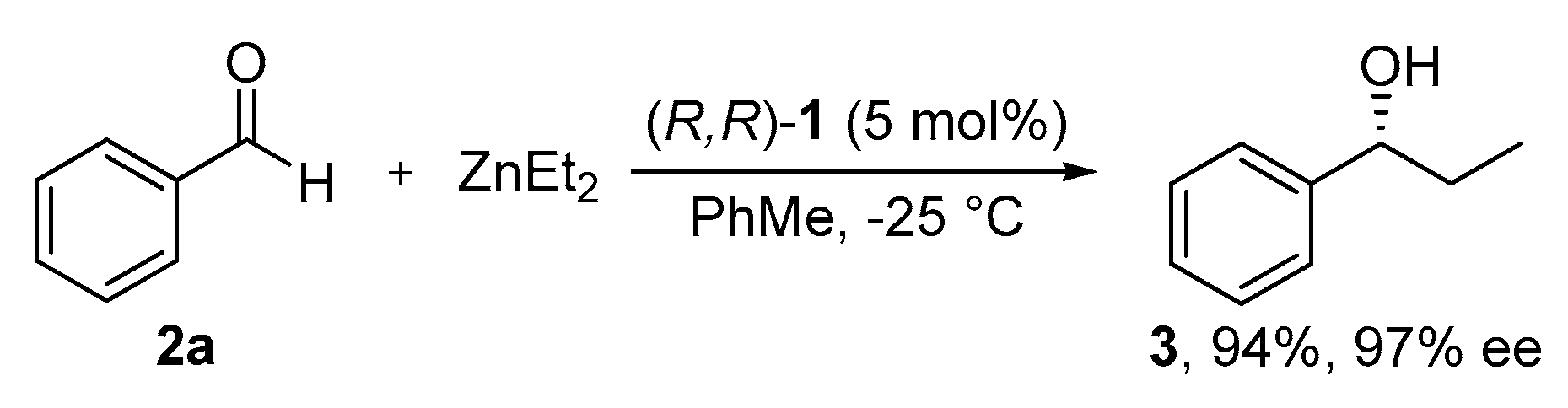
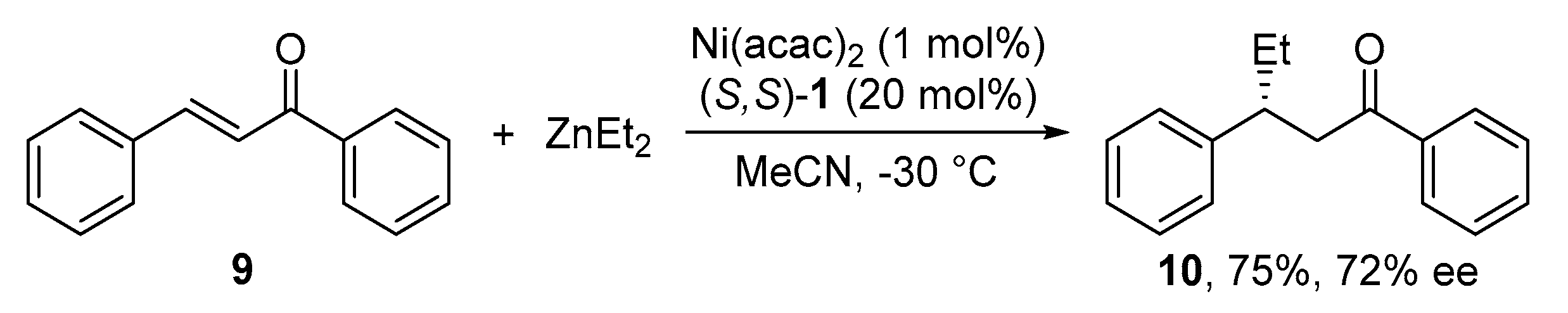
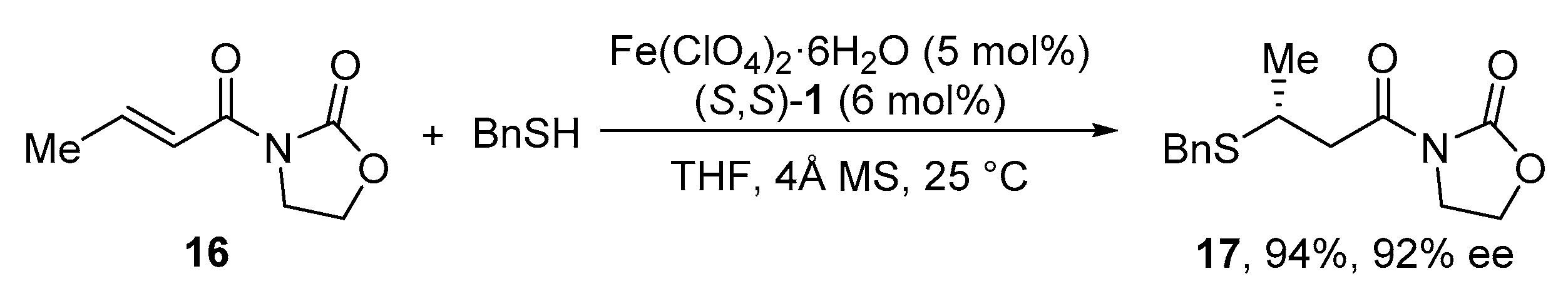
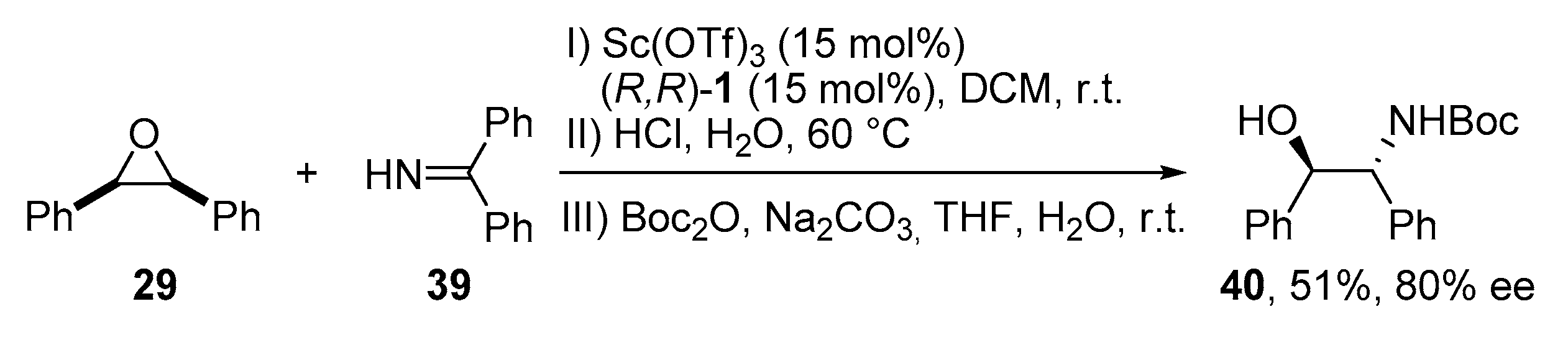
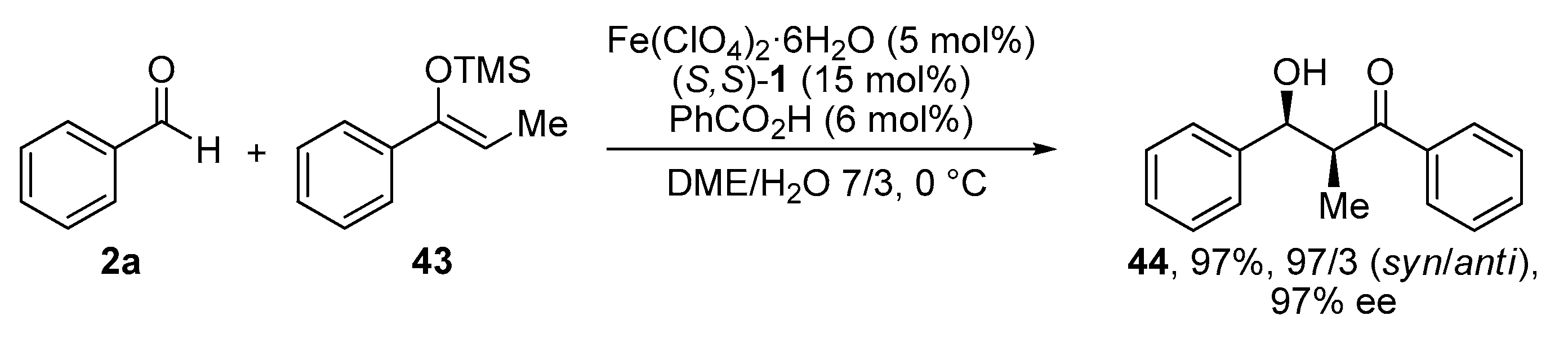
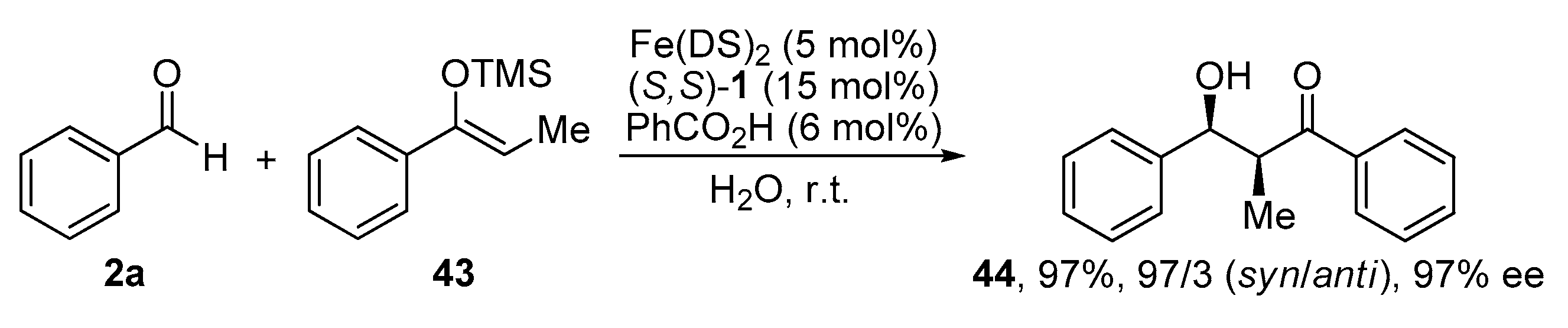
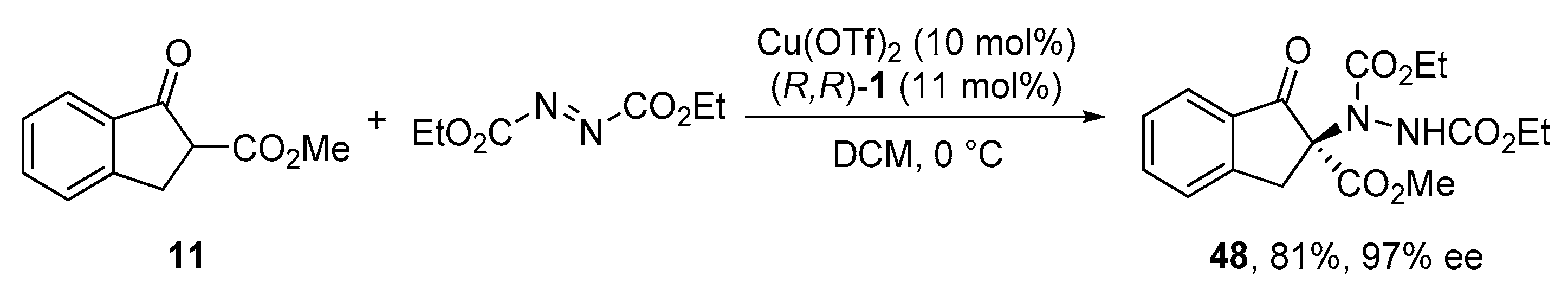
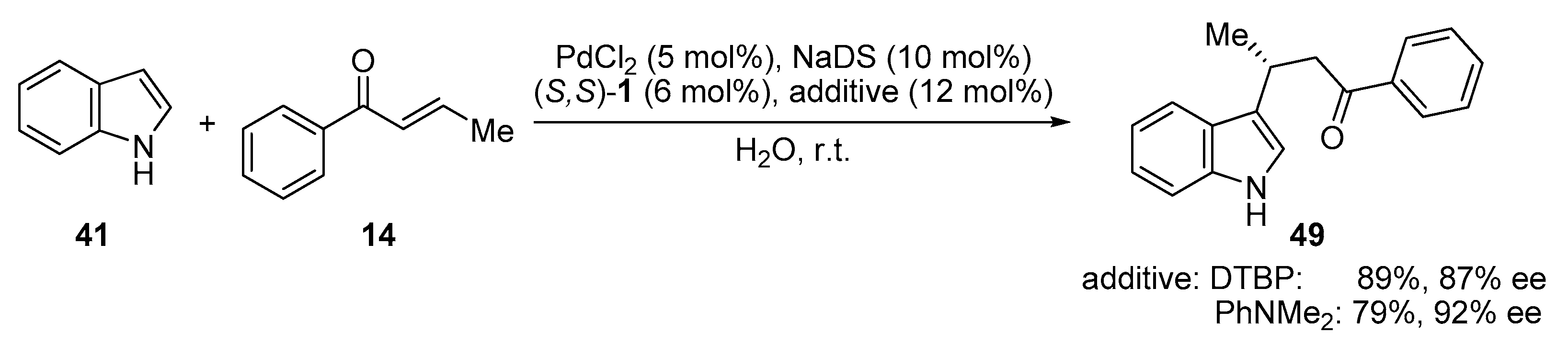
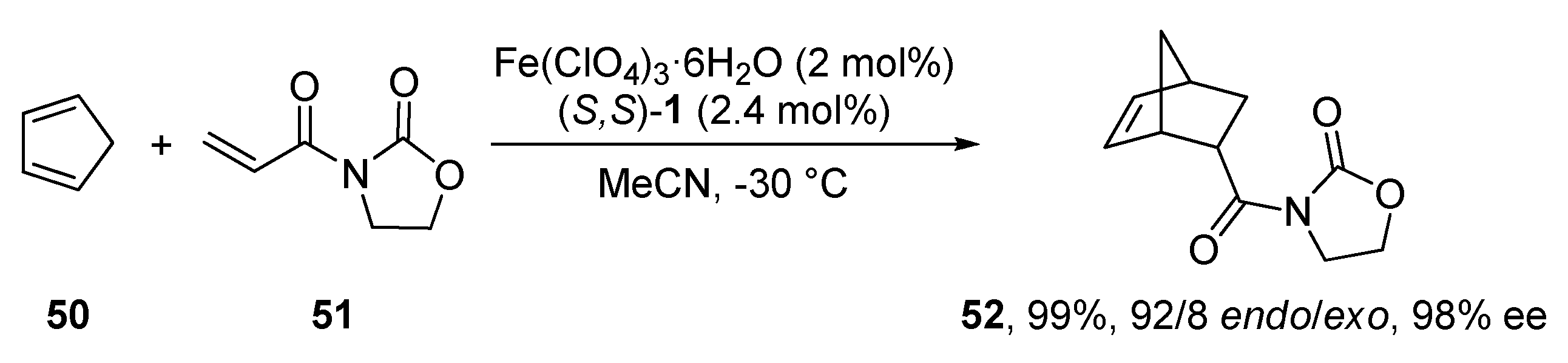
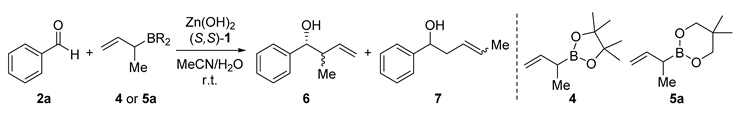
| Entry | 4 or 5a | Zn(OH)2 (mol%) | (S,S)-1 (mol%) | MeCN/H2O | Yield (%) | 6/7 | dr (syn/anti) | ee (syn) (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | - | - | 4/1 | 77 | 5/95 | - | - |
| 2 | 5a | 10 | - | 4/1 | 86 | 45/55 | 70/30 | - |
| 3 | 5a | 10 | 12 | 7/3 | 92 | >99/1 | 91/9 | 71 |
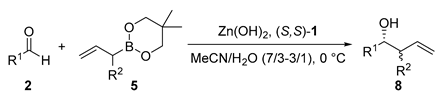
| Entry | R1 | R2 | Zn(OH)2 (mol%) | (S,S)-1 (mol%) | Yield (%) | dr (syn/anti) | ee (syn) (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | PhCH2CH2 | 2b | Me | 5a | 5 | 6 | 94 | 83/17 | 85 |
| 2 | PhCH2CH2 | 2b | Et | 5b | 5 | 6 | 96 | 75/25 | 91 |
| 3 | PhCH2CH2 | 2b | iBu | 5c | 5 | 6 | 95 | 75/25 | 91 |
| 4 | PhCH2CH2 | 2b | Cl | 5d | 3 | 3.6 | 92 | 93/7 | 95 |
| 5 | Ph | 2a | Cl | 5d | 5 | 6 | 92 | 96/4 | 88 |
| 6 | 4-MeC6H4 | 2c | Cl | 5d | 3 | 3.6 | 91 | 96/4 | 87 |
| 7 | 4-BrC6H4 | 2d | Cl | 5d | 7.5 | 9 | quant | 95/5 | 85 |
| 8 | 1-naphthyl | 2e | Cl | 5d | 5 | 6 | 94 | 93/7 | 85 |
| 9 | CH3(CH2)10 | 2f | Cl | 5d | 2 | 2.4 | 92 | 93/7 | 93 |
| Entry | Solvent 1 | T (°C) | c (M) | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 1 | DCM | 10 | 0.08 | 22 | 53 |
| 2 | DCM | 20 | 0.08 | 61 | 80 |
| 3 | DCM | 30 | 0.08 | 98 | 81 |
| 4 | PhMe | 10 | 0.08 | quant. | 5 |
| 5 | MeCN/DCM | 30 | 0.08 | 76 | 8 |
| 6 | DCE | 30 | 0.08 | 94 | 84 |
| 7 | DCE | 30 | 0.04 | 96 | 89 |
| 8 | DCE | 40 | 0.02 | 94 | 92 |

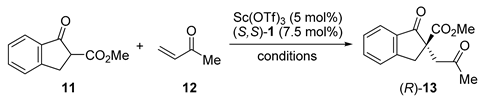
| Entry | Sc(OTf)3 (mol%) | 1 (mol%) | Base (mol%) | T (°C) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | 2 | 5 (R,R) | - | 30 | 46 | 57 (R) |
| 2 | 1 | 2 (R,R) | NaOH (3) | 30 | 95 | 91 (R) |
| 3 | 1 | 1.2 (S,S) | Pyridine (10) | r.t. | 92 | 93 (S) |
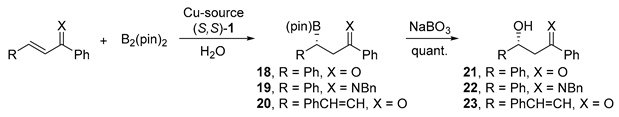
| Entry | R | X | Cu-Source | (mol%) | (S,S)-1 (mol%) | Additive (mol%) | T (°C) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ph | O | Cu(OH)2 | 5 | 6 | AcOH (6) | 5 | 95 | 99 |
| 2 | Ph | O | Cu(OAc)2 | 5 | 6 | MeOH (100) | r.t. | 92 | 92 |
| 3 | Ph | O | Cu powder | 10 | 12 | - | 30 | 92 | 83 |
| 4 | Ph | NBn | Cu(OAc)2 | 5 | 6 | - | r.t. | 91 | >99 |
| 5 | PhCH=CH | O | Cu(OAc)2 | 5 | 6 | - | 5 | 91 | 91 |
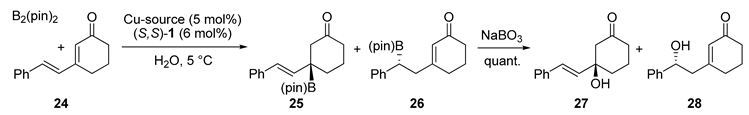
| Entry | Cu-Source | Additive (mol%) | Yield (%) | 27/28 | ee (%) |
|---|---|---|---|---|---|
| 1 | Cu(OH)2 | - | 81 | <1/99 | 76 |
| 2 | Cu(OH)2 | AcOH (6 mol%) | 92 | >99/1 | 87 |
| 3 | Cu(OAc)2 | - | 94 | >99/1 | 91 |
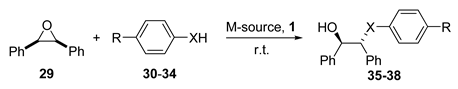
| Entry | X | R | 30–34 | (eq.) | M-source 1 | (mol%) | 1 (mol%) | Additive (mol%) | Solvent | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NH | H | 30 | 1 | Sc(OTf)3 | 10 | 10 (R,R) | - | DCM | 95 | 93 (R,R) |
| 2 | NH | H | 30 | 1 | Sc(DS)3 | 1 | 1.2 (S,S) | - | H2O | 89 | 91 (S,S) |
| 3 | NH | H | 30 | 1.5 | Sc(OTf)3 | 10 | 12 (S,S) | - | DCM | 85 | 93 (S,S) |
| 4 | NH | H | 30 | 1.5 | Sc(UDST)3 | 10 | 12 (S,S) | - | H2O | 87 | 95 (S,S) |
| 5 | NH | H | 30 | 1.5 | Cu(OTf)2 | 10 | 12 (S,S) | - | DCM | 18 | 80 (R,R) |
| 6 | NH | H | 30 | 1.5 | Cu(UDST)2 | 10 | 12 (S,S) | - | H2O | 82 | 80 (R,R) |
| 7 | NH | H | 30 | 1.5 | Zn(OTf)2 | 10 | 12 (S,S) | - | DCM | 60 | 90 (R,R) |
| 8 | NH | H | 30 | 1.5 | Zn(UDST)2 | 10 | 12 (S,S) | - | H2O | 97 | 92 (R,R) |
| 9 | NH | H | 30 | 1 | Zn(OTf)2 | 5 | 5 (R,R) | NaDS (5 mol%) | H2O | 97 | 90 (S,S) |
| 10 | NH | H | 30 | 1 | ZrCl4 | 5 | 10 (R,R) | NaDS (20 mol%) | H2O | 55 | 22 (R,R) |
| 11 | NH | H | 30 | 2 | In(OTf)3 | 10 | 10 (R,R) | - | DCM | 69 | 89 (R,R) |
| 12 | NH | H | 30 | 1 | Fe(ClO4)2·6H2O | 5 | 6 (S,S) | - | DCM | 90 | 95 (S,S) |
| 13 | CH2O | MeO | 31 | 2 | Sc(OTf)3 | 10 | 10 (R,R) | - | DCM | 82 | 97 (R,R) |
| 14 | CH2O | Br | 32 | 1 | Sc(DS)3 | 10 | 12 (S,S) | - | H2O | 34 | 86 (S,S) |
| 15 | S | H | 33 | 3 | Sc(OTf)3 | 10 | 12 (S,S) | - | DCM | 84 | 94 (S,S) |
| 16 | S | H | 33 | 3 | Sc(DS)3 | 10 | 12 (S,S) | - | H2O | 73 | 89 (S,S) |
| 17 | S | H | 33 | 1.5 | InBr3 | 10 | 11 (R,R) | - | DCM | 81 | 96 (R,R) |
| 18 | Se | H | 34 | 3 | Sc(OTf)3 | 10 | 10 (R,R) | - | DCM | 77 | 93 (R,R) |
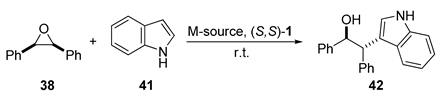
| Entry | 42 (eq.) | M-source (mol%) | (S,S)-1 (mol%) | Solvent | Yield (%) | ee (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 1.2 | Sc(OTf)3 | 10 | 12 | DCM | Traces | - |
| 2 | 1.1 | Sc(DS)3 | 5 | 6 | H2O | 85 | 93 (R,R) |
| 3 | 1.2 | Sc(UDST)3 | 10 | 12 | H2O | 69 | 92 (R,R) |
| 4 | 1.2 | Cu(OTf)2 | 10 | 12 | DCM | 60 | 86 (S,S) |
| 5 | 1.2 | Cu(UDST)2 | 10 | 12 | H2O | 80 | 96 (S,S) |
| 6 | 1.2 | Zn(OTf)2 | 10 | 12 | DCM | Traces | - |
| 7 | 1.2 | Zn(UDST)2 | 10 | 12 | H2O | 8 | 80 (S,S) |
| 8 1 | 1.2 | Fe(ClO4)2·6H2O | 5 | 6 | DCM | 90 | >99 (R,R) |

| Silyl Enol Ether | Aldehyde | ||
|---|---|---|---|
| R2 = Bulky | R2 = Non-Bulky | ||
| R1 | Electron Rich | Electron Poor | |
| Neutral | A | B | C |
| EDG | A | C | C |
| EWG | A | B | C |

| Entry | R | M-source 1 | (mol%) | (S,S)-1 (mol%) | Additive | Solvent | T (°C) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H | Sc(OTf)3 | 10 | 12 | - | DCM | −20 | 80 | 90 |
| 2 | H | Bi(OTf)3 | 1 | 3 | 2,2′-bipyridine (5 mol%) | H2O | 0 | 93 | 91 |
| 3 | Me | Sc(DS)3 | 10 | 12 | Triton® X-705 | H2O | r.t. | 73 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednářová, E.; Malatinec, Š.; Kotora, M. Applications of Bolm’s Ligand in Enantioselective Synthesis. Molecules 2020, 25, 958. https://doi.org/10.3390/molecules25040958
Bednářová E, Malatinec Š, Kotora M. Applications of Bolm’s Ligand in Enantioselective Synthesis. Molecules. 2020; 25(4):958. https://doi.org/10.3390/molecules25040958
Chicago/Turabian StyleBednářová, Eva, Štefan Malatinec, and Martin Kotora. 2020. "Applications of Bolm’s Ligand in Enantioselective Synthesis" Molecules 25, no. 4: 958. https://doi.org/10.3390/molecules25040958
APA StyleBednářová, E., Malatinec, Š., & Kotora, M. (2020). Applications of Bolm’s Ligand in Enantioselective Synthesis. Molecules, 25(4), 958. https://doi.org/10.3390/molecules25040958