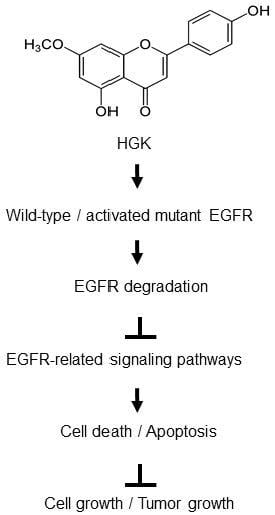
Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Flavonoids from Genkwa Flos
2.2. The Cytotoxic Effect of HGK
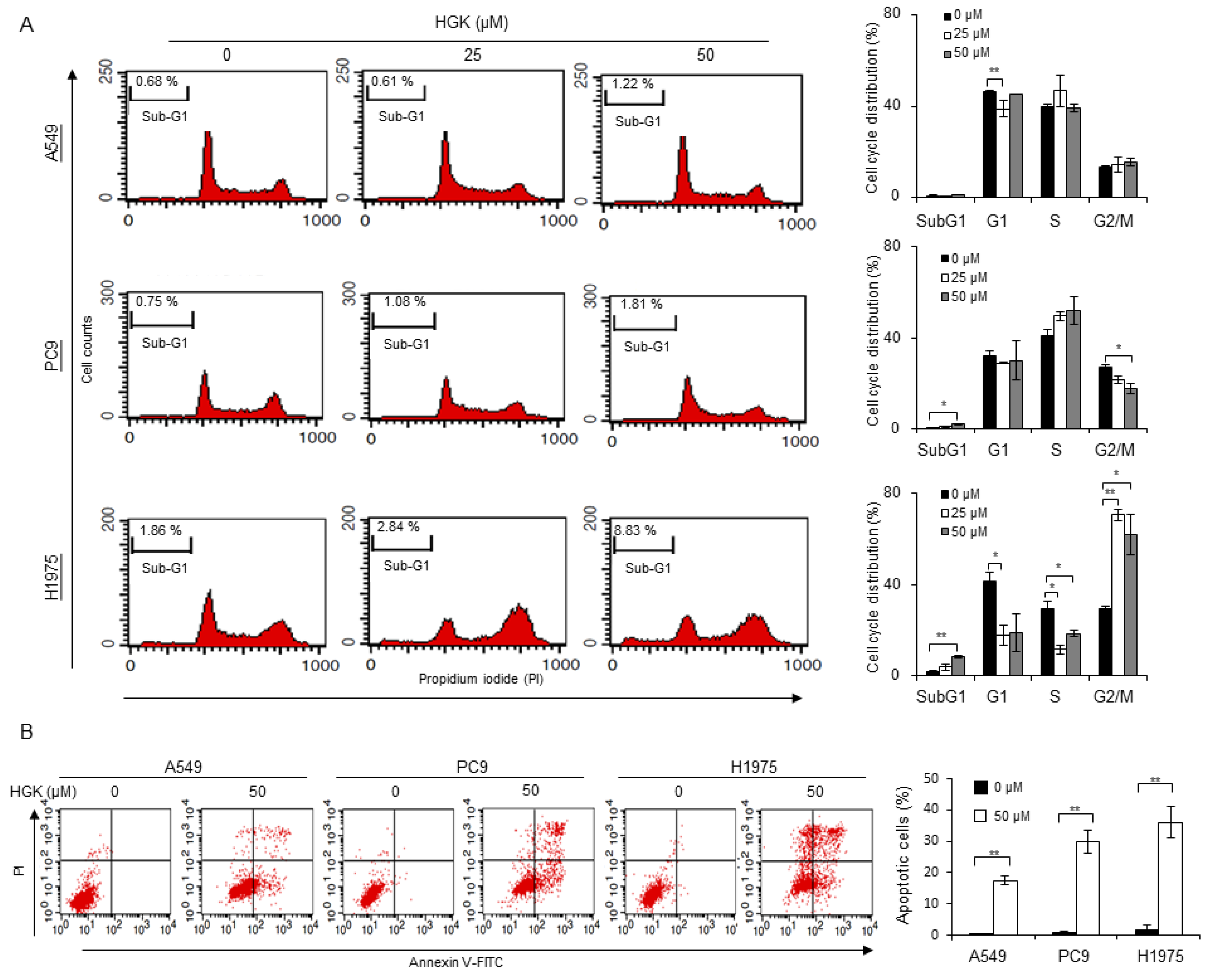
2.3. Effects of HGK on the Cell Cycle Progression and Apoptosis
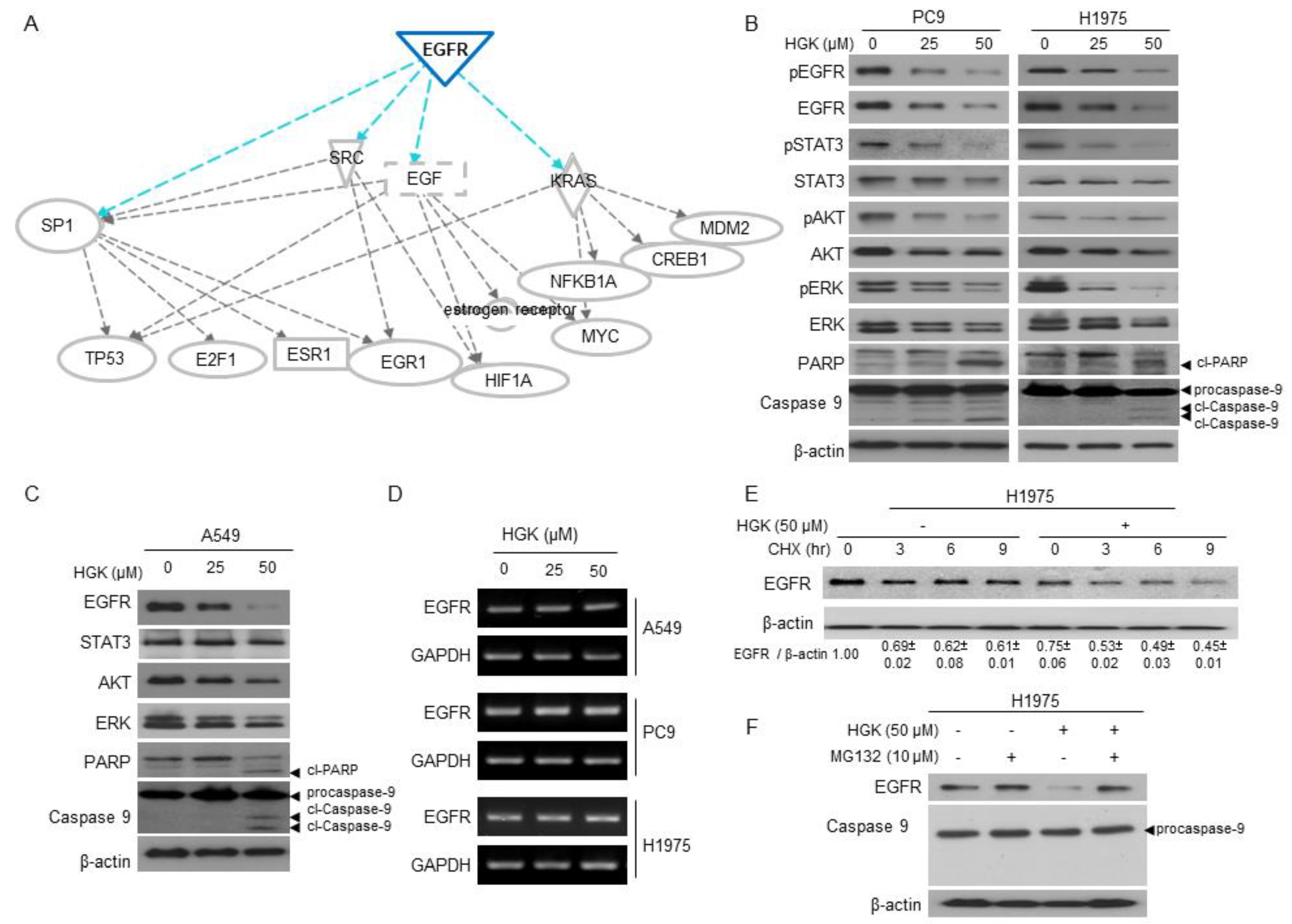
2.4. The Molecular Mechanism of Antitumor Activity by HGK in H1975 Cells
2.5. The Effects of HGK on EGFR Expression and Downstream Pathways
2.6. Antitumor Activity of HGK in a Xenograft Mouse Model
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Isolation and Identification of Flavonoids
4.3. Cell Lines and Culture
4.4. Antibodies, Oligonucleotides, and Reagents
4.5. Assays for Viability, Cell Proliferation Capacity, Clonogenic Ability, and Apoptosis
4.6. Cell-Cycle Analysis
4.7. RT-PCR, Western Blotting, and Immunohistochemistry (IHC)
4.8. Whole-Transcriptome Sequencing
4.9. Functional Enrichment Analysis
4.10. In Vivo Tumor Xenograft Study
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Linardou, H.; Dahabreh, I.J.; Bafaloukos, D.; Kosmidis, P.; Murray, S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat. Rev. Clin. Oncol. 2009, 6, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Chmielecki, J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat. Rev. Cancer 2010, 10, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Jänne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef]
- Kai, H.; Koine, T.; Baba, M.; Okuyama, T. Pharmacological Effects of Daphne genkwa and Chinese Medical Prescription, “Jyu-So-To”. Yakugaku Zasshi 2004, 124, 349–354. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Fan, C.Q.; Ding, J.; Yue, J.M. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorg. Med. Chem. 2005, 13, 645–655. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Park, B.-Y.; Kwon, O.-K.; Yuk, J.-E.; Oh, S.-R.; Kim, H.-S.; Lee, H.-K.; Ahn, K.-S. Anti-inflammatory activity of (−)-aptosimon isolated from Daphne genkwa in RAW264.7 cells. Int. Immunopharmacol. 2009, 9, 878–885. [Google Scholar] [CrossRef]
- Park, B.-Y.; Min, B.S.; Ahn, K.-S.; Kwon, O.-K.; Joung, H.; Bae, K.-H.; Lee, H.-K.; Oh, S.-R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model. J. Ethnopharmacol. 2007, 111, 496–503. [Google Scholar] [CrossRef]
- Park, B.-Y.; Min, B.-S.; Oh, S.-R.; Kim, J.-H.; Bae, K.-H.; Lee, H.-K. Isolation of flavonoids, a biscoumarin and an amide from the flower buds ofDaphne genkwa and the evaluation of their anti-complement activity. Phytotherapy Res. 2006, 20, 610–613. [Google Scholar] [CrossRef]
- Wang, C.-F.; Li, R.-R.; Huang, L.-L.; Zhong, L.-Q.; Yuan, S.-T. [Studies on chemical constituents of Daphne genkwa]. J. Chin. Med. Mater. 2009, 32, 508–511. [Google Scholar]
- Hong, J.-Y.; Chung, H.-J.; Lee, H.-J.; Park, H.J.; Lee, S.K. Growth Inhibition of Human Lung Cancer Cells via Down-regulation of Epidermal Growth Factor Receptor Signaling by Yuanhuadine, a Daphnane Diterpene fromDaphne genkwa. J. Nat. Prod. 2011, 74, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Hong, J.Y.; Lee, H.J.; Bae, S.Y.; Jung, C.; Park, H.J.; Lee, S.K. Anti-Tumor Activity of Yuanhuacine by Regulating AMPK/mTOR Signaling Pathway and Actin Cytoskeleton Organization in Non-Small Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0144368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Gao, X.; Chen, C.; Tan, R. Total flavonoids of Daphne genkwa root significantly inhibit the growth and metastasis of Lewis lung carcinoma in C57BL6 mice. Int. Immunopharmacol. 2007, 7, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-J.; Yang, X.-L.; Song, Z.-J.; Wang, J.-Y.; Zhang, W.-J.; He, X.; Zhang, R.-Q.; Zhang, C.-F.; Li, F.; Yu, C.; et al. Antitumor Activity of Total Flavonoids from Daphne genkwa in Colorectal Cancer. Phytotherapy Res. 2015, 30, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Leu, Y.-L.; Horng, J.-T. Daphne Genkwa Sieb. et Zucc. Water-Soluble Extracts Act on Enterovirus 71 by Inhibiting Viral Entry. Viruses 2012, 4, 539–556. [Google Scholar] [CrossRef]
- Ulubelen, A.; Bücker, R.; Mabry, T.J. Flavone 5-O-glucosides from Daphne sericea. Phytochemistry 1982, 21, 801–803. [Google Scholar] [CrossRef]
- Das, K.C.; Farmer, W.J.; Weinstein, B. Phytochemical studies. IX. A new flavone, velutin. J. Org. Chem. 1970, 35, 3989–3990. [Google Scholar] [CrossRef]
- Pang, Y.-X.; Wang, D.; Fan, Z.; Chen, X.L.; Yu, F.-L.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera—A Phytochemical and Pharmacological Review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.S.; Yin, L.H.; Na Xu, L.; Jinyong, P.; Zhou, H.; Kang, W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chem. Interact. 2013, 206, 346–355. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lee, P.-C.; Wang, J.J.; Hsu, Y.-C. Anticancer Effect and Mechanism of Hydroxygenkwanin in Oral Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.-F.; Chen, C.-Y.; Yang, W.-H.; Chen, C.-C.; Chang, J.-L.; Leu, Y.-L.; Liou, M.-J.; Wang, T.H. Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression. Biomology 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Kao, S.-H.; Wang, W.-L.; Hong, T.-M.; Chen, C.-Y.; Hsiao, T.-H.; Salunke, S.B.; Chen, J.J.W.; Su, K.-Y.; Yang, S.-C.; et al. Small Molecule T315 Promotes Casitas B-Lineage Lymphoma–Dependent Degradation of Epidermal Growth Factor Receptor via Y1045 Autophosphorylation. Am. J. Respir. Crit. Care Med. 2016, 193, 753–766. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yu, Z.-Y.; Chuang, Y.-S.; Huang, R.-M.; Wang, T.-C. Sulforaphane attenuates EGFR signaling in NSCLC cells. J. Biomed. Sci. 2015, 22, 38. [Google Scholar] [CrossRef]
- Sigismund, S.; Algisi, V.; Nappo, G.; Conte, A.; Pascolutti, R.; Cuomo, A.; Bonaldi, T.; Argenzio, E.; Verhoef, L.G.G.C.; Maspero, E.; et al. Threshold-controlled ubiquitination of the EGFR directs receptor fate. EMBO J. 2013, 32, 2140–2157. [Google Scholar] [CrossRef]
- Vucic, D.; Dixit, V.M.; Wertz, I.E. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Boil. 2011, 12, 439–452. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-Y.; Ueng, S.-H.; Hsueh, C.; Yeh, C.-T.; Ho, J.-Y.; Chou, L.-F.; Wang, T.H. Corylin increases the sensitivity of hepatocellular carcinoma cells to chemotherapy through long noncoding RNA RAD51-AS1-mediated inhibition of DNA repair. Cell Death Dis. 2018, 9, 543. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Chen, C.-Y.; Huang, P.-R.; Hsu, C.-W.; Wu, C.-C.; Wang, T.-C. Proteomic analyses of genes regulated by heterogeneous nuclear ribonucleoproteins A/B in Jurkat cells. Proteomics 2014, 14, 1357–1366. [Google Scholar] [CrossRef]
- Wang, T.-H.; Chan, C.-W.; Fang, J.-Y.; Shih, Y.-M.; Liu, Y.-W.; Wang, T.-C.; Chen, C.-Y. 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Cell Death Dis. 2017, 8, e2638. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Jan, C.-I.; Lo, J.-F.; Yang, S.-C.; Chang, Y.-L.; Pan, S.-H.; Wang, W.-L.; Hong, T.-M.; Yang, P.-C. Tid1-L Inhibits EGFR Signaling in Lung Adenocarcinoma by Enhancing EGFR Ubiquitinylation and Degradation. Cancer Res. 2013, 73, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Lin, Y.-H.; Yang, S.-C.; Chang, P.-C.; Wang, T.-C.; Chen, C.-Y. Tid1-S regulates the mitochondrial localization of EGFR in non-small cell lung carcinoma. Oncogensis 2017, 6, e361. [Google Scholar] [CrossRef] [PubMed]
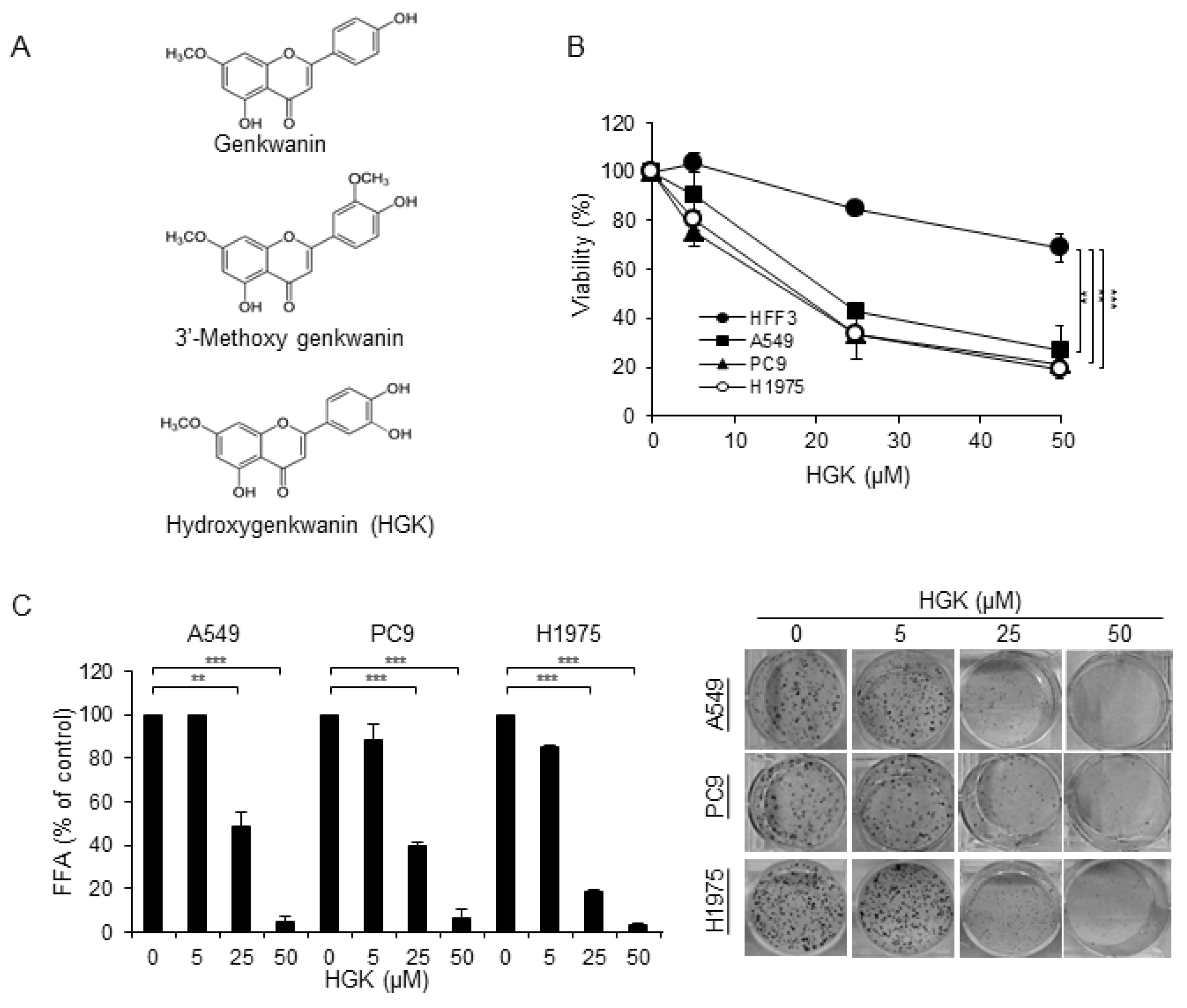

| Name of Compounds (100 μM) | Cell Viability (%) | |
|---|---|---|
| HFF3 | A549 | |
| Genkwanin | 86.46 ± 2.4 | 91.64 ± 11.0 |
| 3′-Methoxy genkwanin | 55.15 ± 6.9 | 67.31 ± 5.0 |
| Hydroxygenkwanin (HGK) | 74.87 ± 3.4 | 22.03 ± 2.9 |
| Ingenuity Canonical Pathways | p-Value |
|---|---|
| Mitochondrial Dysfunction | 5.55 × 10−22 |
| Sirtuin Signaling Pathway | 1.29 × 10−20 |
| Oxidative Phosphorylation | 4.41 × 10−18 |
| Protein Ubiquitination Pathway | 5.23 × 10−16 |
| Estrogen Receptor Signaling | 4.34 × 10−12 |
| Molecular and Cellular Functions | p-Value |
|---|---|
| Cell Death and Survival | 2.32 × 10−6 to 2.37 × 10−42 |
| Cellular Development | 2.05 × 10−6 to 2.40 × 10−39 |
| Cellular Growth and Proliferation | 2.05 × 10−6 to 2.40 × 10−39 |
| RNA Post-Transcriptional Modification | 8.49 × 10−7 to 2.98 × 10−38 |
| Cell Cycle | 2.26 × 10−6 to 1.61 × 10−33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, Y.-L.; Wang, T.-H.; Wu, C.-C.; Huang, K.-Y.; Jiang, Y.-W.; Hsu, Y.-C.; Chen, C.-Y. Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation. Molecules 2020, 25, 941. https://doi.org/10.3390/molecules25040941
Leu Y-L, Wang T-H, Wu C-C, Huang K-Y, Jiang Y-W, Hsu Y-C, Chen C-Y. Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation. Molecules. 2020; 25(4):941. https://doi.org/10.3390/molecules25040941
Chicago/Turabian StyleLeu, Yann-Lii, Tong-Hong Wang, Chih-Ching Wu, Kuo-Yen Huang, Yu-Wen Jiang, Yi-Chiung Hsu, and Chi-Yuan Chen. 2020. "Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation" Molecules 25, no. 4: 941. https://doi.org/10.3390/molecules25040941
APA StyleLeu, Y.-L., Wang, T.-H., Wu, C.-C., Huang, K.-Y., Jiang, Y.-W., Hsu, Y.-C., & Chen, C.-Y. (2020). Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation. Molecules, 25(4), 941. https://doi.org/10.3390/molecules25040941