Electrochemical Properties and Structure of Multi-Ferrocenyl Phosphorus Thioesters
Abstract
1. Introduction
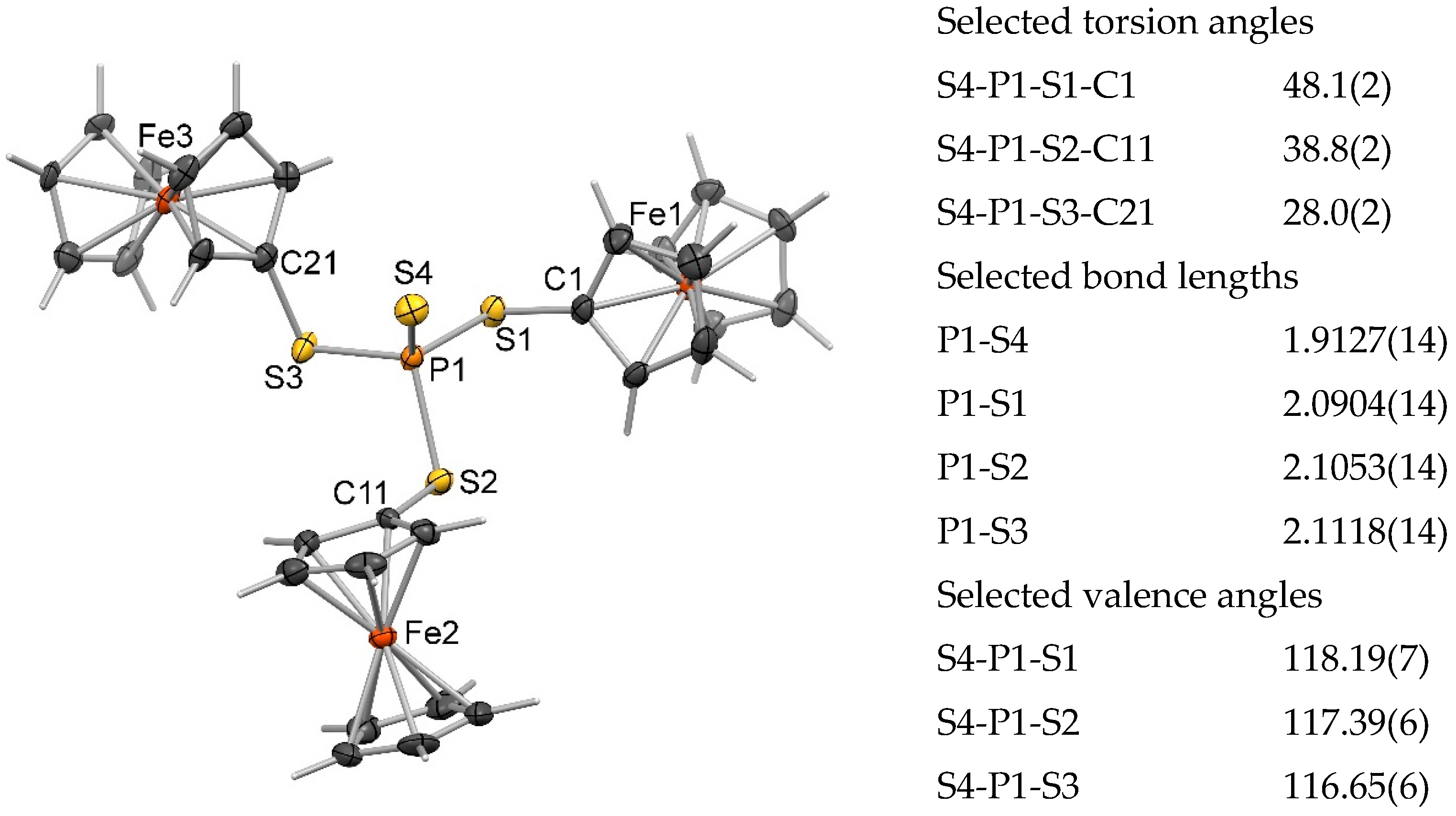
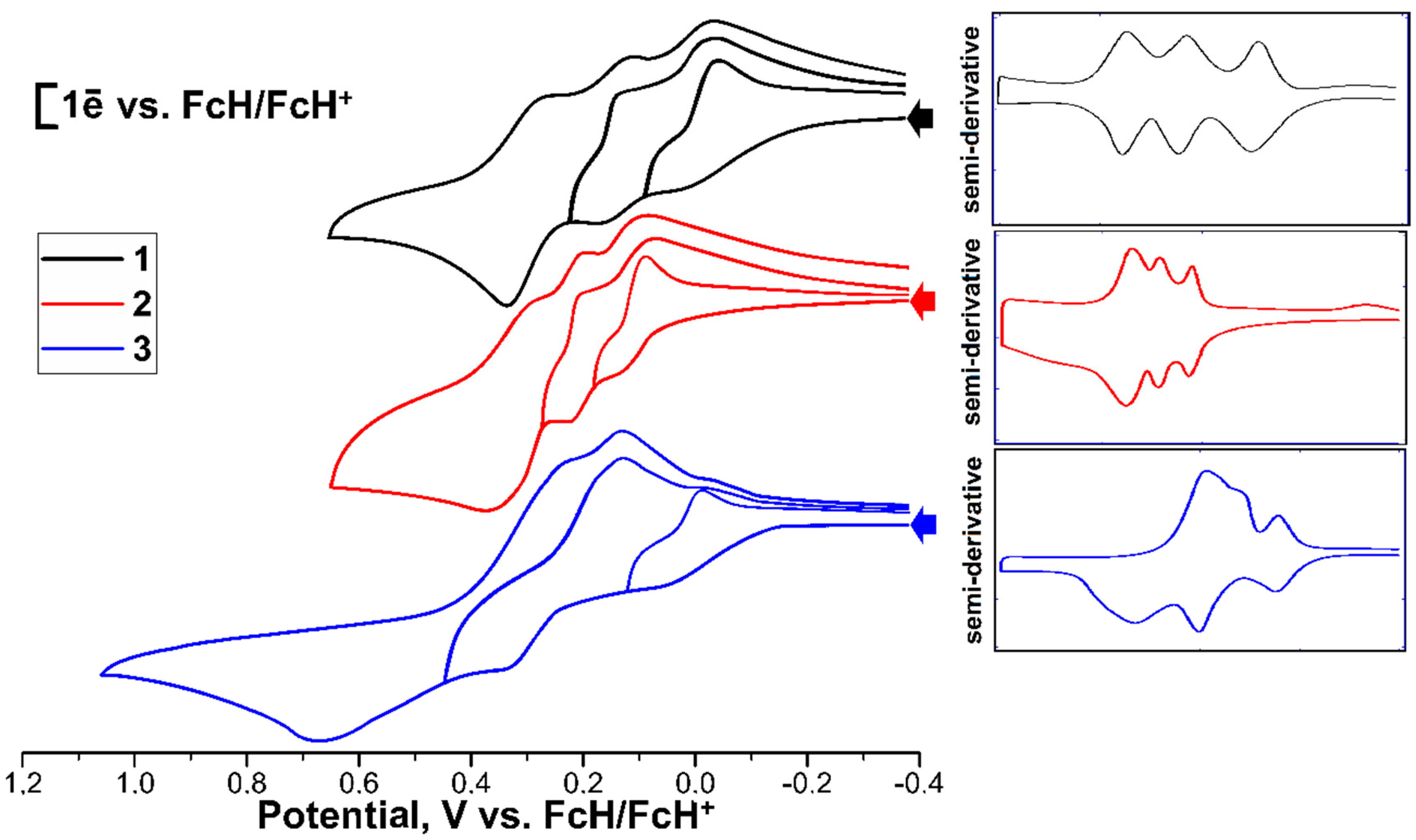
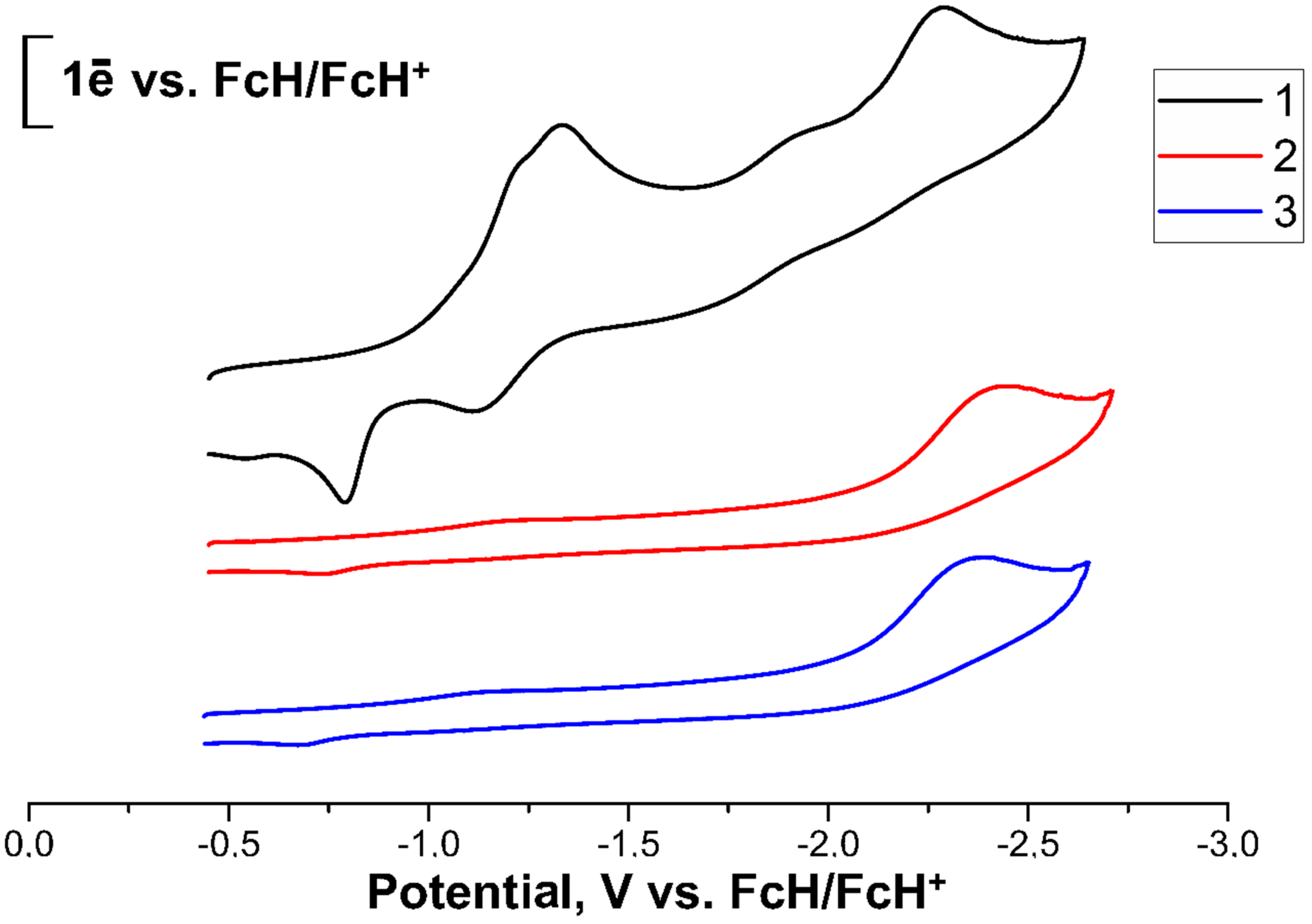
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Research Subjects
3.2. Synthesis of Triferrocenyltetrathiophosphate (3)
3.3. X-ray Crystallography
3.4. NMR Experiments
3.5. UV/Vis Experiments
3.6. Calculations
3.7. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Djeda, R.; Rapakousiou, A.; Liang, L.; Guidolin, N.; Ruiz, J.; Astruc, D. Click Syntheses of 1,2,3-Triazolylbiferrocenyl Dendrimers and the Selective Roles of the Inner and Outer Ferrocenyl Groups in the Redox Recognition of ATP2− and Pd2+. Angew. Chem. Int. Ed. 2010, 49, 8152–8156. [Google Scholar] [CrossRef]
- Pfaff, U.; Filipczyk, G.; Hildebrandt, A.; Korb, M.; Lang, H. 1, 3, 5-Triferrocenyl-2, 4, 6-tris (ethynylferrocenyl)-benzene–a new member of the family of multiferrocenyl-functionalized cyclic systems. Dalton Trans. 2014, 43, 16310–16321. [Google Scholar] [CrossRef] [PubMed]
- Lohan, M.; Justaud, F.; Lang, H.; Lapinte, C. Synthesis, Spectroelectrochemical, and EPR Spectroscopic Studies of Mixed Bis (alkynyl) biferrocenes of the Type (L n MC ≡C)(L n M′ C ≡C) bfc. Organometallics 2012, 31, 3565–3574. [Google Scholar] [CrossRef]
- Diallo, A.K.; Absalon, C.; Ruiz, J.; Astruc, D. Ferrocenyl-terminated redox stars: Synthesis and electrostatic effects in mixed-valence stabilization. J. Am. Chem. Soc. 2010, 133, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bond, A.D.; Leonard, P.W.; Lorenz, U.J.; Timofeeva, T.V.; Vollhardt, K.P.C.; Yakovenko, A.A. Hexaferrocenylbenzene. Chem. Comm. 2006, 24, 2572–2574. [Google Scholar] [CrossRef]
- Nlate, S.; Ruiz, J.; Blais, J.C.; Astruc, D. Ferrocenylsilylation of dendrons: A fast convergent route to redox-stable ferrocene dendrimers. Chem. Comm. 2000, 5, 417–418. [Google Scholar] [CrossRef]
- Sakamoto, R.; Murata, M.; Nishihara, H. Visible-Light Photochromism of Bis (ferrocenylethynyl) ethenes Switches Electronic Communication between Ferrocene Sites. Angew. Chem. Int. Ed. 2006, 45, 4793–4795. [Google Scholar] [CrossRef]
- Kishore, P.V.; Liao, J.H.; Hou, H.N.; Lin, Y.R.; Liu, C.W. Ferrocene-functionalized Cu (I)/Ag (I) dithiocarbamate clusters. Inorg. Chem. 2016, 55, 3663–3673. [Google Scholar] [CrossRef]
- Shah, H.H.; Changez, M.; Singh, V.; Luqman, M.; Ismail, Y.; Raithby, P.R.; Marken, F. Estimation of energy levels of self-assembled ferrocenyls and investigation of charge-driven electro-crystallization of ferricenyl materials. Energy Procedia 2016, 100, 149–154. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.J.; Ma, J.Q.; Wang, C.H.; Tan, H.; Huang, J.; Xu, L. Construction of multiferrocenes end-capped metallodendrimers via coordination-driven self-assembly and their electrochemical behavior. J. Organomet. Chem. 2016, 823, 1–7. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Li, Q.J.; Chen, L.J.; Tan, H.; Wang, C.H.; Lehman, D.A.; Yang, H.B. Facile self-assembly of supramolecular hexakisferrocenyl triangles via coordination-driven self-assembly and their electrochemical behavior. Organometallics 2011, 30, 3637–3642. [Google Scholar] [CrossRef]
- Muraoka, H.; Ozawa, K.; Ogawa, S. Electrochemical studies of the multi-step multi-electron redox process of tetraferrocenyloligothiophenes with electron donor and acceptor abilities. Heteroat. Chem. 2018, 29, e21455. [Google Scholar] [CrossRef]
- Miesel, D.; Hildebrandt, A.; Lang, H. Molecular electrochemistry of multi-redox functionalized 5-membered heterocycles. Curr. Opin. Electrochem. 2018, 8, 39–44. [Google Scholar] [CrossRef]
- Gao, R.X.; Gao, Y.Y.; Xie, R.J.; Han, L.M. How many ferrocene units of multi-ferrocenyl complexes can react with the electrode? Chem. Pap. 2020, 74, 895–901. [Google Scholar] [CrossRef]
- Ajayi, T.J.; Ollengo, M.; le Roux, L.; Pillay, M.N.; Staples, R.J.; Biros, S.M.; Wenderich, K.; Mei, B.; van Zyl, W.E. Heterodimetallic Ferrocenyl Dithiophosphonate Complexes of Nickel (II), Zinc (II) and Cadmium (II) as Sensitizers for TiO2-Based Dye-Sensitized Solar Cells. ChemistrySelect 2019, 4, 7416–7424. [Google Scholar] [CrossRef]
- Herberhold, M.; Hofmann, A.; Milius, W.; de Biani, F.F.; Zanello, P. Oligonuclear ferrocenolato and 1,1′-ferrocenediolato derivatives of phosphorus: Synthesis, structure and electrochemical behaviour. Inorg. Chim. Acta 1998, 273, 24–30. [Google Scholar] [CrossRef]
- Herberhold, M.; Hofmann, A.; Milius, W. Phosphor (III)-und Phosphor (V)-Verbindungen mit Ferrocenolato-und 1,1′-Ferrocendiolato-Substituenten. J. Organomet. Chem. 1998, 555, 187–200. [Google Scholar] [CrossRef]
- Korb, M.; Schaarschmidt, D.; Lang, H. Anionic phospho-Fries rearrangement at ferrocene: One-pot approach to P, O-substituted ferrocenes. Organometallics 2014, 33, 2099–2108. [Google Scholar] [CrossRef]
- Korb, M.; Lehrich, S.W.; Lang, H. Reactivity of Ferrocenyl Phosphates Bearing (Hetero-) Aromatics and [3] Ferrocenophanes toward Anionic Phospho-Fries Rearrangements. J. Org. Chem. 2017, 82, 3102–3124. [Google Scholar] [CrossRef]
- Milyukov, V.A.; Zverev, A.V.; Podlesnov, S.M.; Krivolapov, D.B.; Litvinov, I.A.; Gubaidullin, A.T.; Kataeva, O.N.; Ginzburg, A.; Sinyashin, O.G. Novel Organometallic Derivatives of Thioesters of the Trivalent Phosphorus Acids: Synthesis and Structure. Eur. J. Inorg. Chem. 2000, 2000, 225–228. [Google Scholar] [CrossRef]
- Seiler, P.T.; Dunitz, J.D. The structure of triclinic ferrocene at 101, 123 and 148 K. Acta Crystallogr. Sect. B 1979, 35, 2020–2032. [Google Scholar] [CrossRef]
- Seiler, P.; Dunitz, J.D. Low-temperature crystallization of orthorhombic ferrocene: Structure analysis at 98 K. Acta Crystallogr. Sect. B 1982, 38, 1741–1745. [Google Scholar] [CrossRef]
- Brock, C.P.; Fu, Y. Rigid-body disorder models for the high-temperature phase of ferrocene. Acta Crystallogr. Sect. B 1997, 53, 928–938. [Google Scholar] [CrossRef]
- Takusagawa, F.; Koetzle, T.F. A neutron diffraction study of the crystal structure of ferrocene. Acta Crystallogr. Sect. B 1979, 35, 1074–1081. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. Cambridge structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Gerasimova, T.; Shekurov, R.; Gilmanova, L.; Laskin, A.; Katsyuba, S.; Kovalenko, V.; Khrizanforov, M.; Milyukov, V.; Sinyashin, O. IR and UV study of reversible water-induced structural transformations of poly (manganese 1, 1′-ferrocenediyl-bis (H-phosphinate)) and poly (cobalt 1,1′-ferrocenediyl-bis (H-phosphinate)). J. Mol. Struc. 2018, 1166, 237–242. [Google Scholar] [CrossRef]
- Bruker. APEX3 Crystallography Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker. SAINT. Crystallography Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D.J. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Johnson, E.R. A Density-Functional Model of the Dispersion Interaction. J. Chem. Phys. 2005, 123, 154101–154109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Becke, A.D. A Post-Hartree-Fock Model of Intermolecular Interactions: Inclusion of Higher-Order Corrections. J. Chem. Phys. 2006, 124, 174104–174109. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
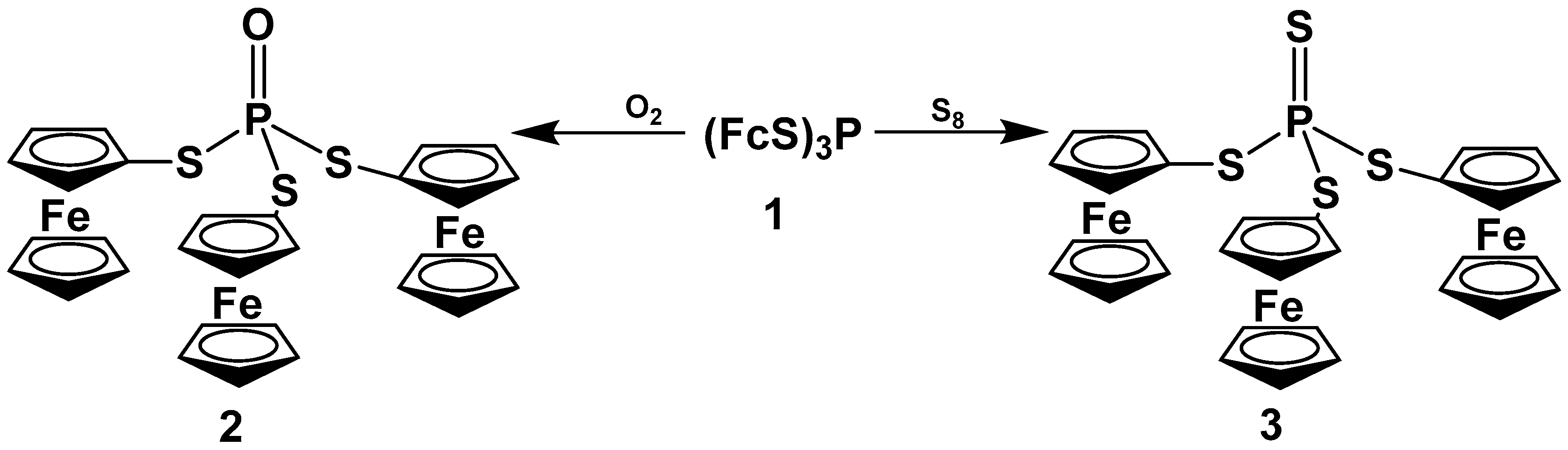
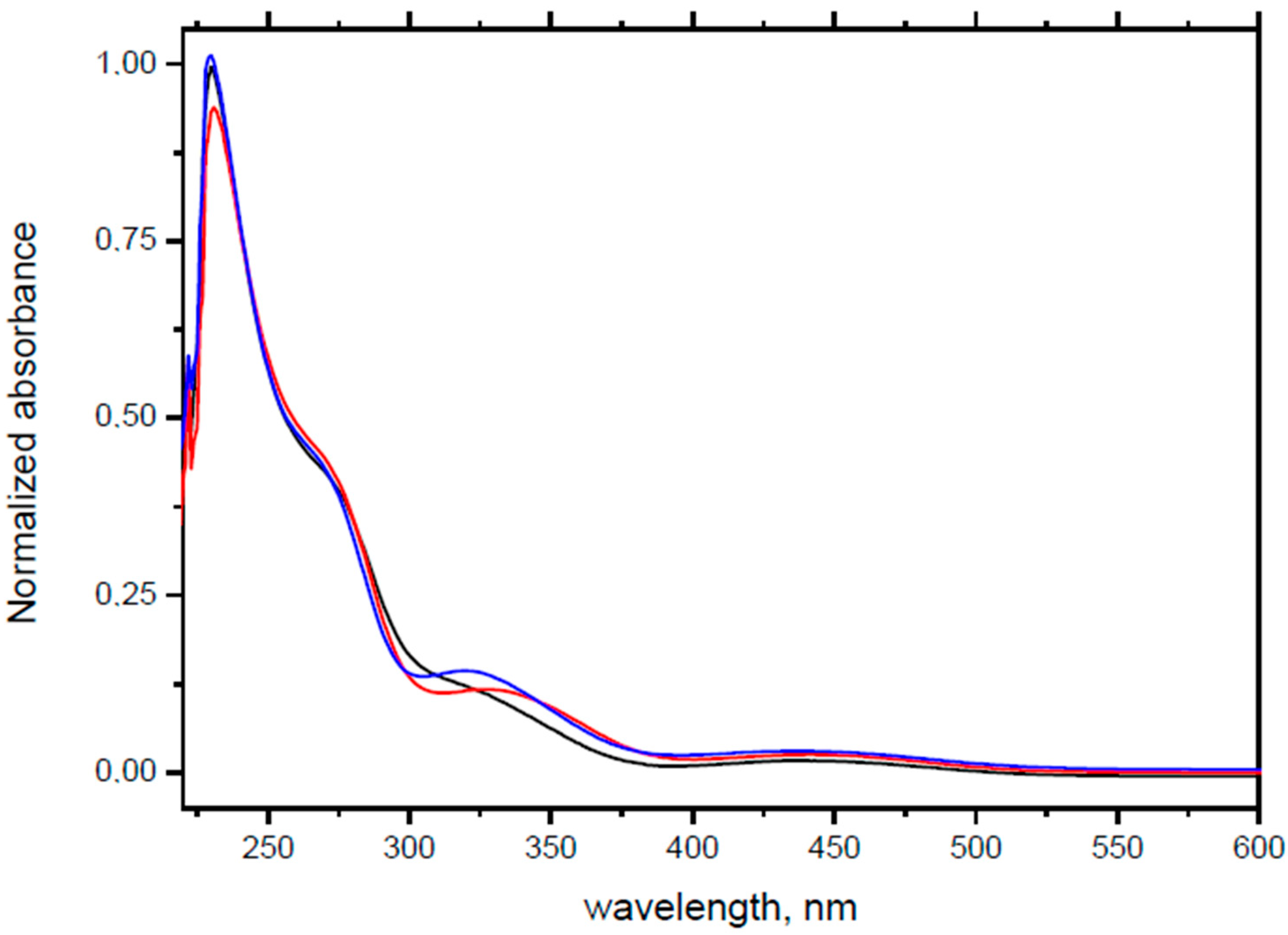
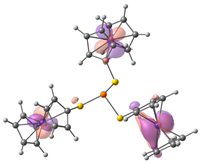
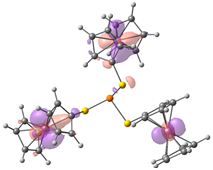
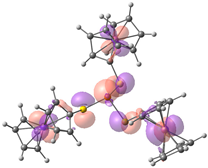
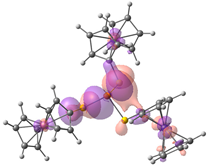
| HOMO-2. | HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|---|
| Compound 1 (ΔEHOMO − LUMO = 5.24 eV) | |||||
| −5.92 | −5.92 | −5.89 | −0.54 | −0.48 | −0.48 |
 |  |  | 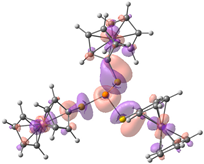 | 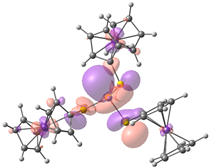 |  |
| Compound 2 (ΔEHOMO − LUMO = 5.03 eV) | |||||
| −5.98 | −5.95 | −5.93 | −0.90 | −0.15 | −0.14 |
 |  | 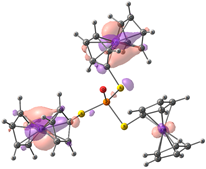 | 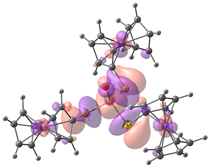 | 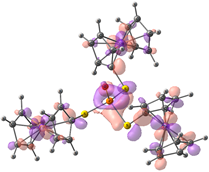 | 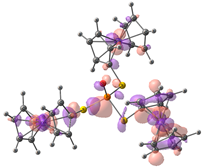 |
| Compound 3 (ΔEHOMO − LUMO = 4.86 eV) | |||||
| −5.99 | −5.95 | −5.95 | −1.09 | −0.35 | −0.34 |
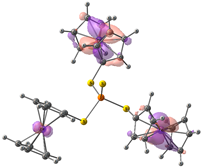 | 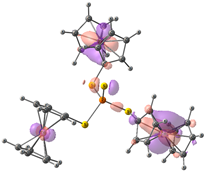 |  | 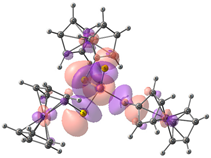 |  | 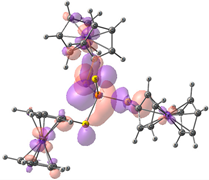 |
| Oxidation | 1Epf/Epb, V | ΔEpf–b, V | 2Epf/Epb, V | Δ Epf-b, V | 3Epf/Epb, V | Δ Epf–b, V |
|---|---|---|---|---|---|---|
| Fc3S3P (1) | 0.00/−0.06 | 0.06 | 0.16/0.10 | 0.06 | 0.32/0.26 | 0.06 |
| Fc3S3P=O (2) | 0.14/0.08 | 0.06 | 0.23/0.17 | 0.06 | 0.35/0.29 | 0.06 |
| Fc3S3P=S (3) | 0.04/−0.02 | 0.06 | 0.33/0.12 | 0.21 | 0.64/0.25 | 0.39 |
| Fc3O3P=O [19] | 0.03/−0.03 | 0.06 | 0.24/0.17 | 0.07 | 0.44/0.37 | 0.07 |
| Reduction | ||||||
| Fc3S3P (1) | −1.23/−0.79 | 0.44 | −1.33/−1.11 | 0.22 | −2.28/irrev | - |
| Fc3S3P=O (2) | −2.44/irrev | - | ||||
| Fc3S3P=S (3) | −2.37/irrev | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekurov, R.; Khrizanforov, M.; Gerasimova, T.; Yamaleeva, Z.; Ivshin, K.; Lakomkina, A.; Bezkishko, I.; Kononov, A.; Sinyashin, O.; Budnikova, Y.; et al. Electrochemical Properties and Structure of Multi-Ferrocenyl Phosphorus Thioesters. Molecules 2020, 25, 939. https://doi.org/10.3390/molecules25040939
Shekurov R, Khrizanforov M, Gerasimova T, Yamaleeva Z, Ivshin K, Lakomkina A, Bezkishko I, Kononov A, Sinyashin O, Budnikova Y, et al. Electrochemical Properties and Structure of Multi-Ferrocenyl Phosphorus Thioesters. Molecules. 2020; 25(4):939. https://doi.org/10.3390/molecules25040939
Chicago/Turabian StyleShekurov, Ruslan, Mikhail Khrizanforov, Tatiana Gerasimova, Zilya Yamaleeva, Kamil Ivshin, Alyona Lakomkina, Ilya Bezkishko, Aleksandr Kononov, Oleg Sinyashin, Yulia Budnikova, and et al. 2020. "Electrochemical Properties and Structure of Multi-Ferrocenyl Phosphorus Thioesters" Molecules 25, no. 4: 939. https://doi.org/10.3390/molecules25040939
APA StyleShekurov, R., Khrizanforov, M., Gerasimova, T., Yamaleeva, Z., Ivshin, K., Lakomkina, A., Bezkishko, I., Kononov, A., Sinyashin, O., Budnikova, Y., Kataeva, O., & Miluykov, V. (2020). Electrochemical Properties and Structure of Multi-Ferrocenyl Phosphorus Thioesters. Molecules, 25(4), 939. https://doi.org/10.3390/molecules25040939









