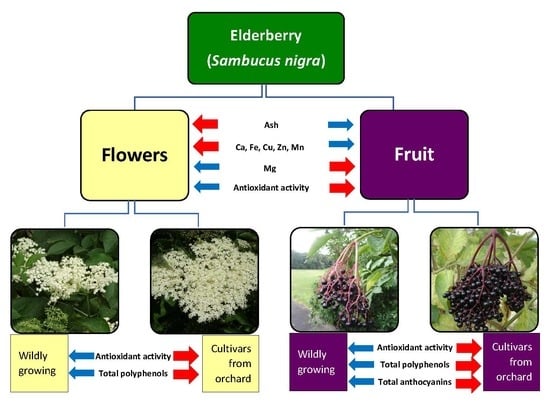
The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Content of Ash and Selected Minerals in Elderberry Flowers and Fruit
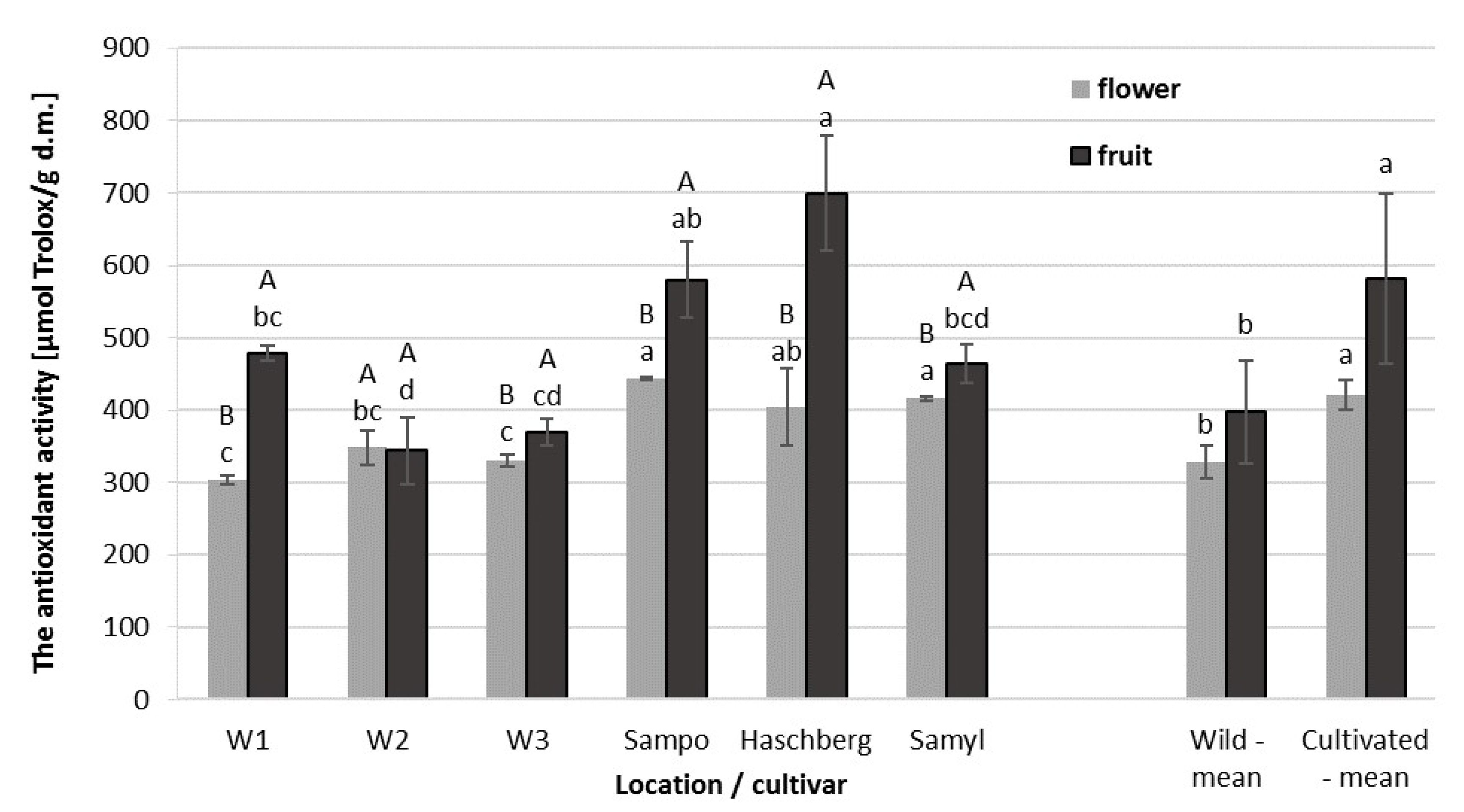
2.2. Antioxidant Capacity (AC) of Elderberry Flower and Fruit Extracts
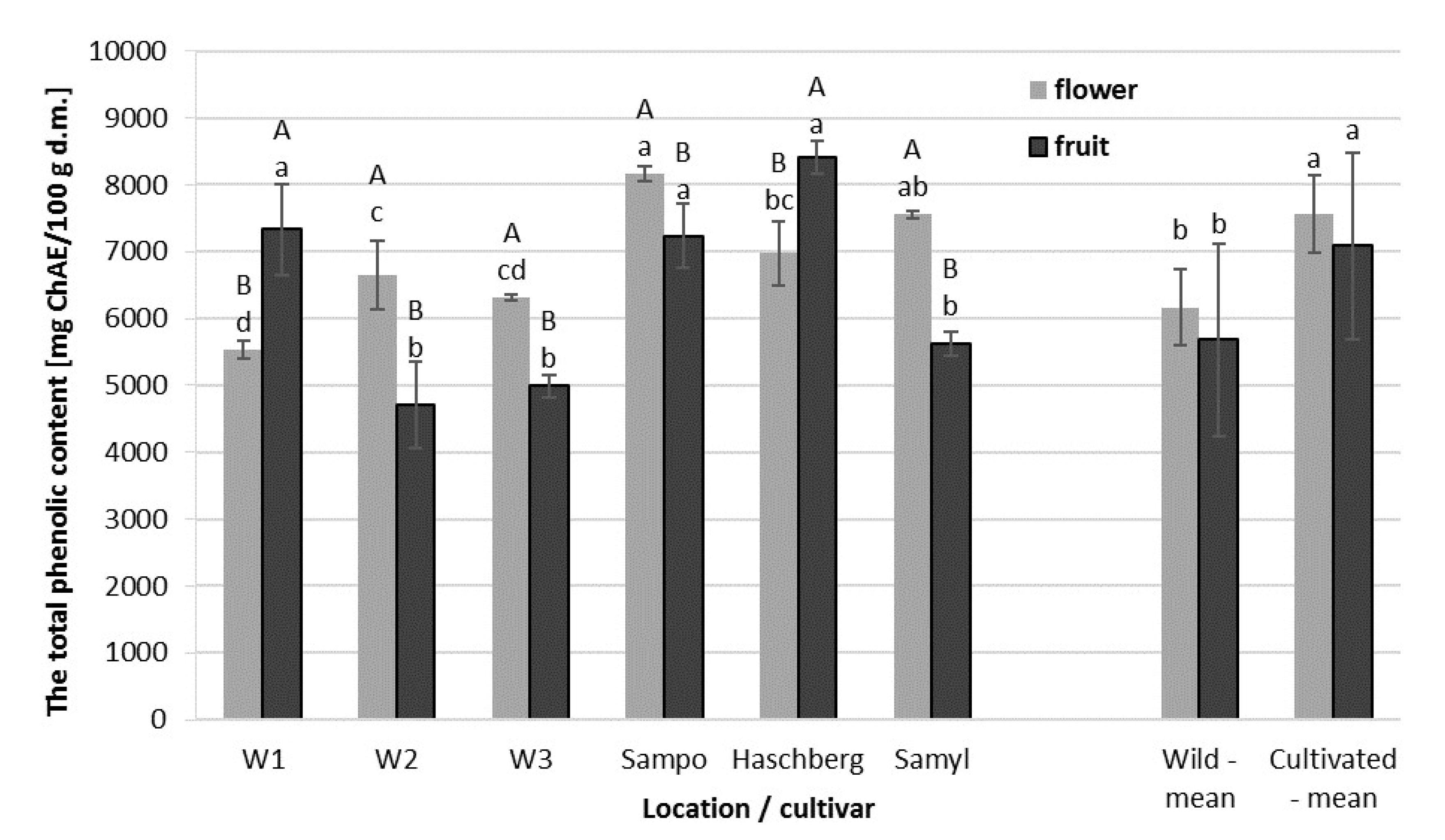
2.3. Total Phenolic Content (TPC) of Elderberry Flower and Fruit Extracts
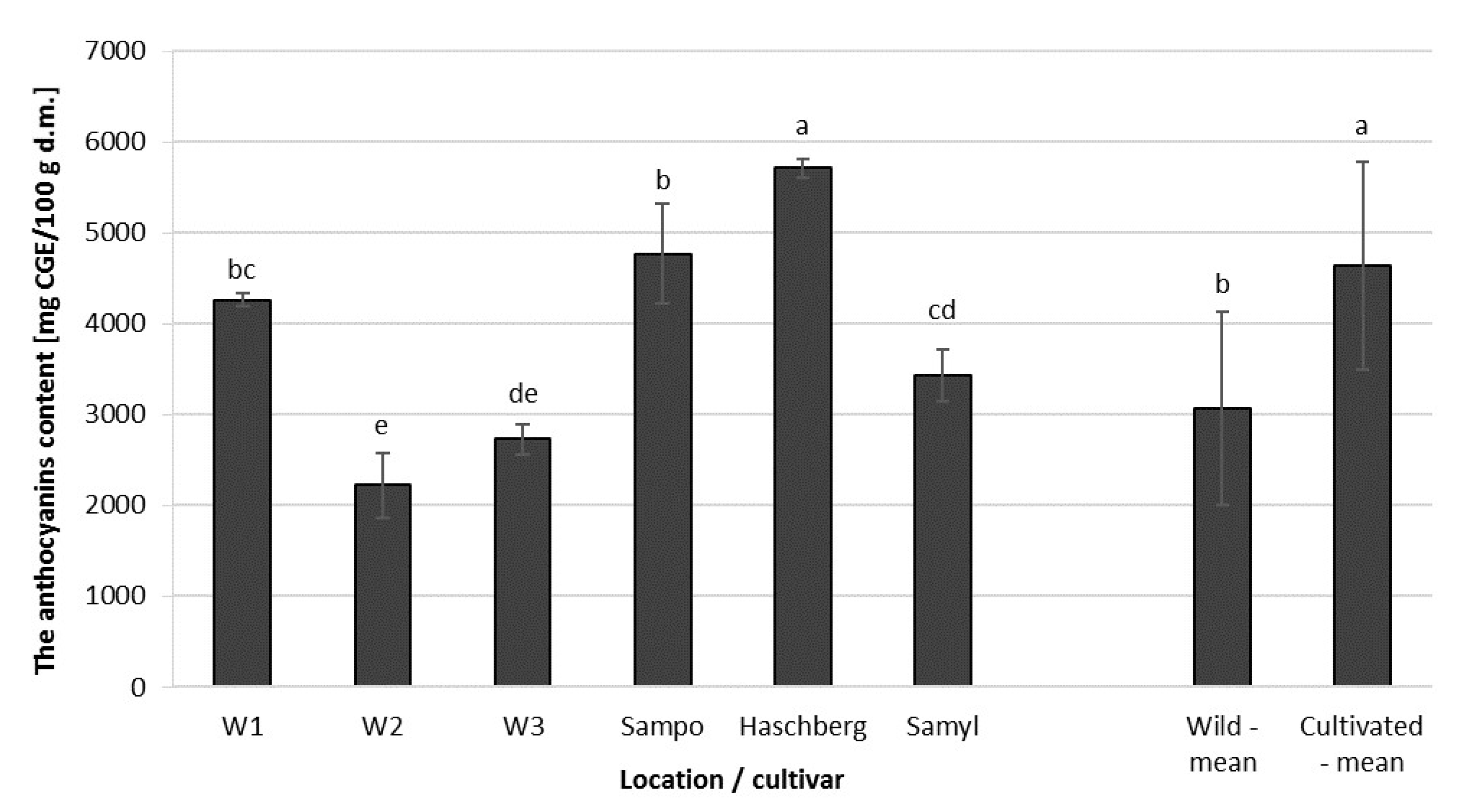
2.4. Total Anthocyanin Content (TAC) in Elderberry Fruit Extracts
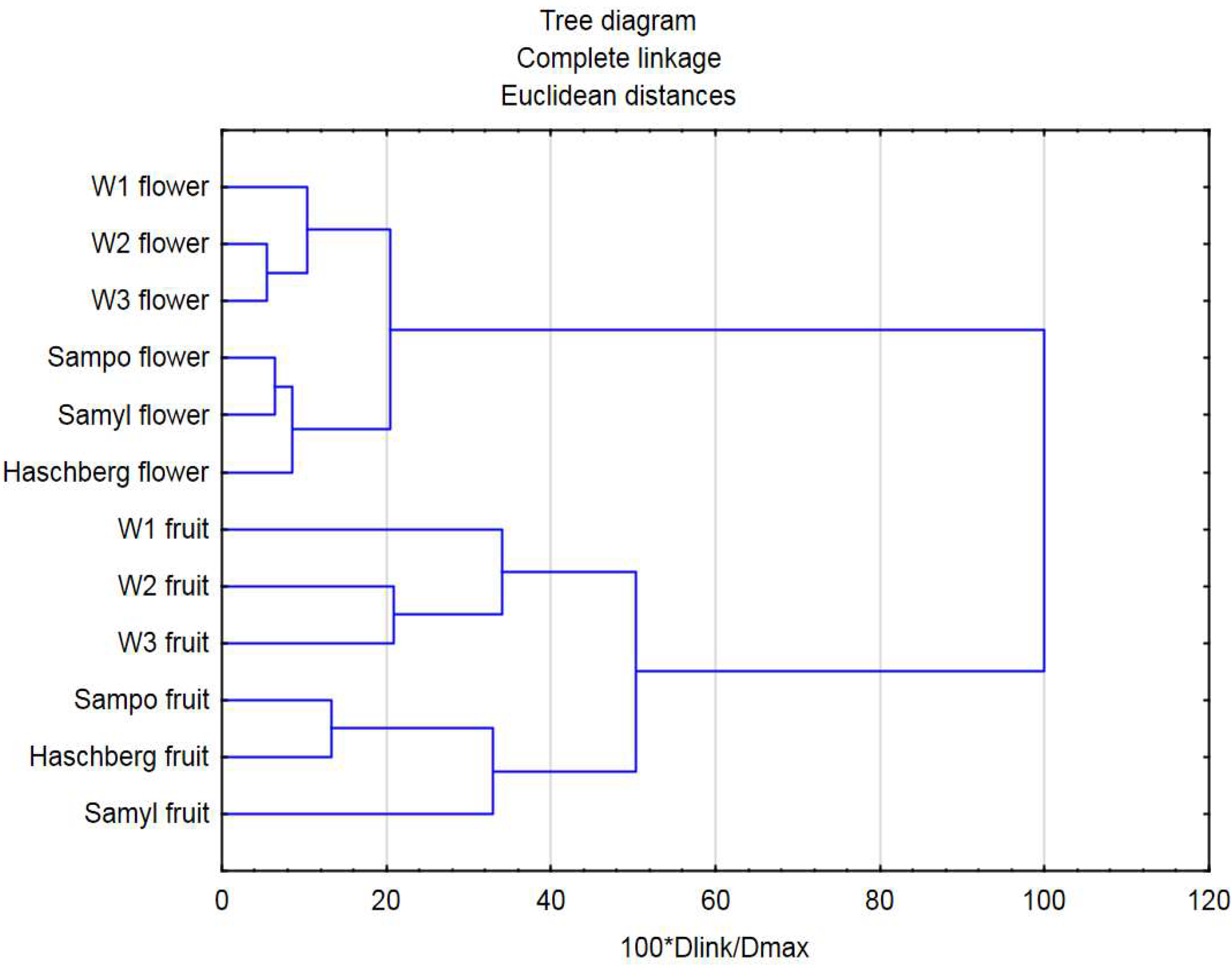
2.5. Assessment of Similarities and Differences between Genotypes Using a Cluster Analysis
3. Materials and Methods
3.1. Plant Material
3.1.1. Flowers
3.1.2. Fruit
3.2. Preparation of Samples
3.3. Determination of Mineral Content by Atomic Absorption Spectrometry Method (AAS)
3.4. Preparation of Extracts
3.5. Measuring Antioxidant Capacity (AC) Using ABTS Radical Cation
3.6. Measurement of Total Phenolic Content (TPC)
3.7. Measurement of Total Anthocyanin Content (TAC)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waźbińska, J. Sambucus for growers. Szkółkarstwo 2002, 6, 29–30. (In Polish) [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT–Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food–A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Sambucus nigra L., Fructus; European Medicine Agency: London, UK, 2013. [Google Scholar]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef]
- Gold, M.; Cernusca, M.M.; Godsey, L.D. A Competitive Market Analysis of the US Elderberry Industry. In HortScience: A publication of the American Society for Horticultural Science, Proceedings of the 2010 ASHS Annual Conference, Palm Desert, CA, USA, 31 July–05 August 2010; American Society for Horticultural Science: Palm Desert, CA, USA, 2010; Volume 45, p. 8. [Google Scholar]
- Vulić, J.J.; Vračar, L.O.; Šumić, Z.M. Chemical characteristics of cultivated elderberry fruit. Acta Period. Technol. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Mratinić, E.; Fotirić, M. Selection of black elderberry (Sambucus nigra L.) and evaluation of its fruits usability as biologically valuable food. Genetika 2007, 39, 305–314. [Google Scholar] [CrossRef]
- Kołodziej, B.; Maksymiec, N.; Drożdżal, K.; Antonkiewicz, J. Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.). J. Elem. 2012, 17, 67–78. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Vespalcová, M.; Matějíček, A.; Kaplan, J. Elemental composition of fruits from different Black elder (Sambucus nigra L.) cultivars grown in the Czech Republik. J. Elem. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries and lingoberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Wilker, M.; Stoyanova, A.; Krastanov, A.; Stanchev, V. Antioxidant activity of extract from elder flower (Sambucus nigra L.). Herba Pol. 2007, 53, 45–54. [Google Scholar]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alkoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Kołodziej, B.; Drożdżal, K. Antioxidant properties of black elder flowers and berries harvested from the wild. Żywn. Nauka Technol. Jakość 2011, 4, 36–44. [Google Scholar]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crop. Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Căta, A.; Ştefănuţ, M.N.; Pop, R.; Tănasie, C.; Moşoarcă, C.; Zamfir, A.D. Evaluation of antioxidant activities of some small fruits containing anthocyanins using electrochemical and chemical methods. Croat. Chem. Acta 2016, 89, 37–48. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, B.M.; de Ancos, B.; Sanchez-Moreno, C.; Fernandez-Ruiz, V.; de Cortes Sanchez-Mata, M.; Camara, M.; Tardio, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The phenolic contents and antioxidant activities of infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J.; García-Viguera, C. Anthocyanin-based natural colorants: A new source of antiradical activity for foodstuff. J. Agric. Food Chem. 2000, 48, 1588–1592. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Kaack, K. ‘Sampo’ and ‘Samdal’, elderberry cultivars for juice concentrates. Fruit Var. J. 1997, 51, 28–31. [Google Scholar]
- Pliszka, B. Polyphenolic content, antiradical activity, stability and microbiological quality of elderberry (Sambucus nigra L.) extracts. Acta Sci. Pol. Technol. Aliment. 2017, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Łysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowiak-Tomczak, D. Apple skin colour changes during harvest as an indicator of maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- De la Guardia, M.; Garrigues, S. Handbook of Mineral Elements in Food; Wiley Blackwell: Pondicherry, India, 2015. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How to improve bayberry (Myrica rubra Sieb. Et Zucc.) juice color quality: Effect of juice processing on bayberry anthocyanins and polyphenolics. J. Agric. Food Chem. 2006, 54, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley: New York, NY, USA, 2001; pp. F1.2.1.–F1.2.13. [Google Scholar]
Sample Availability: The samples can be requested from the authors. |
| Raw Material | Location/Cultivar | Ash | Ca | Mg | Fe | Cu | Zn | Mn |
|---|---|---|---|---|---|---|---|---|
| Flowers | W1 | 1.5 d | 2673.9 b | 493.7 c | 103.0 b | 13.4 a | 39.0 ab | 19.7 e |
| W2 | 2.1 a | 3333.6 a | 1097.6 b | 87.4 b | 6.9 b | 35.2 ab | 60.0 a | |
| W3 | 1.9 bc | 2925.2 ab | 567.2 c | 92.1 b | 13.8 a | 41.1 a | 27.9 d | |
| Sampo | 1.7 cd | 2868.7 ab | 1525.7 a | 54.5 a | 6.5 b | 31.8 b | 37.2 c | |
| Haschberg | 1.9 b | 2724.7 ab | 1555.6 a | 46.8 a | 8.0 b | 38.1 ab | 49.7 b | |
| Samyl | 1.9 bc | 3252.6 ab | 1000.0 b | 52.2 a | 13.1 a | 38.2 ab | 23.1 de | |
| Fruit | W1 | 0.8 d | 2503.2 b | 13555.1 a | 41.3 a | 5.2 b | 12.4 ab | 8.8 de |
| W2 | 0.8 cd | 2563.5 b | 12565.1 a | 35.3 ab | 4.4 b | 10.8 bc | 26.0 a | |
| W3 | 0.6 e | 3033.0 a | 9774.3 b | 28.7 bc | 5.3 b | 14.0 a | 11.9 c | |
| Sampo | 1.0 ab | 662.1 d | 9058.1 b | 26.2 c | 4.9 b | 11.6 abc | 9.8 d | |
| Haschberg | 1.1 a | 1508.2 c | 9731.4 b | 28.1 c | 4.6 b | 9.5 c | 17.1 b | |
| Samyl | 0.9 bc | 1513.9 c | 6909.2 c | 25.7 c | 6.7 a | 9.8 bc | 8.5 e |
| Raw Material | Origin | Ash | Ca | Mg | Fe | Cu | Zn | Mn |
|---|---|---|---|---|---|---|---|---|
| Flowers | Wild growing | 1.8 a | 2977.6 a | 719.5 b | 94.2 a | 11.3 a | 38.4 a | 35.9 a |
| Orchard | 1.8 a | 2948.7 a | 1360.4 a | 51.1 b | 9.2 a | 36.1 a | 36.7 a | |
| Fruit | Wild growing | 0.7 b | 2699.9 a | 11964.8 a | 35.1 a | 5.0 a | 12.4 a | 15.6 a |
| Orchard | 1.0 a | 1228.1 b | 8566.2 b | 26.6 b | 5.4 a | 10.3 b | 11.8 a |
| Raw Material | Origin | Antioxidant Activity [µmol Trolox/g d.m.] | Total Phenolic Content [mg ChAE/100g d.m.] | Total Anthocyanin Content [mg CGE/100 g d.m.] |
|---|---|---|---|---|
| Flowers | Wild growing | 327.7 b | 6164.4 b | - |
| Orchard | 421.5 a | 7561.8 a | - | |
| Fruit | Wild growing | 397.5 b | 5678.8 b | 3071.0 b |
| Orchard | 581.3 a | 7087.3 a | 4638.2 a |
| Raw Material | AC/TPC | AC/TAC | TPC/TAC |
|---|---|---|---|
| Flowers | 0.97 | - | - |
| Fruit | 0.93 | 0.98 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. https://doi.org/10.3390/molecules25040876
Młynarczyk K, Walkowiak-Tomczak D, Staniek H, Kidoń M, Łysiak GP. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules. 2020; 25(4):876. https://doi.org/10.3390/molecules25040876
Chicago/Turabian StyleMłynarczyk, Karolina, Dorota Walkowiak-Tomczak, Halina Staniek, Marcin Kidoń, and Grzegorz P. Łysiak. 2020. "The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L." Molecules 25, no. 4: 876. https://doi.org/10.3390/molecules25040876
APA StyleMłynarczyk, K., Walkowiak-Tomczak, D., Staniek, H., Kidoń, M., & Łysiak, G. P. (2020). The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules, 25(4), 876. https://doi.org/10.3390/molecules25040876