Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage
Abstract
1. Introduction
2. Results and Discussions
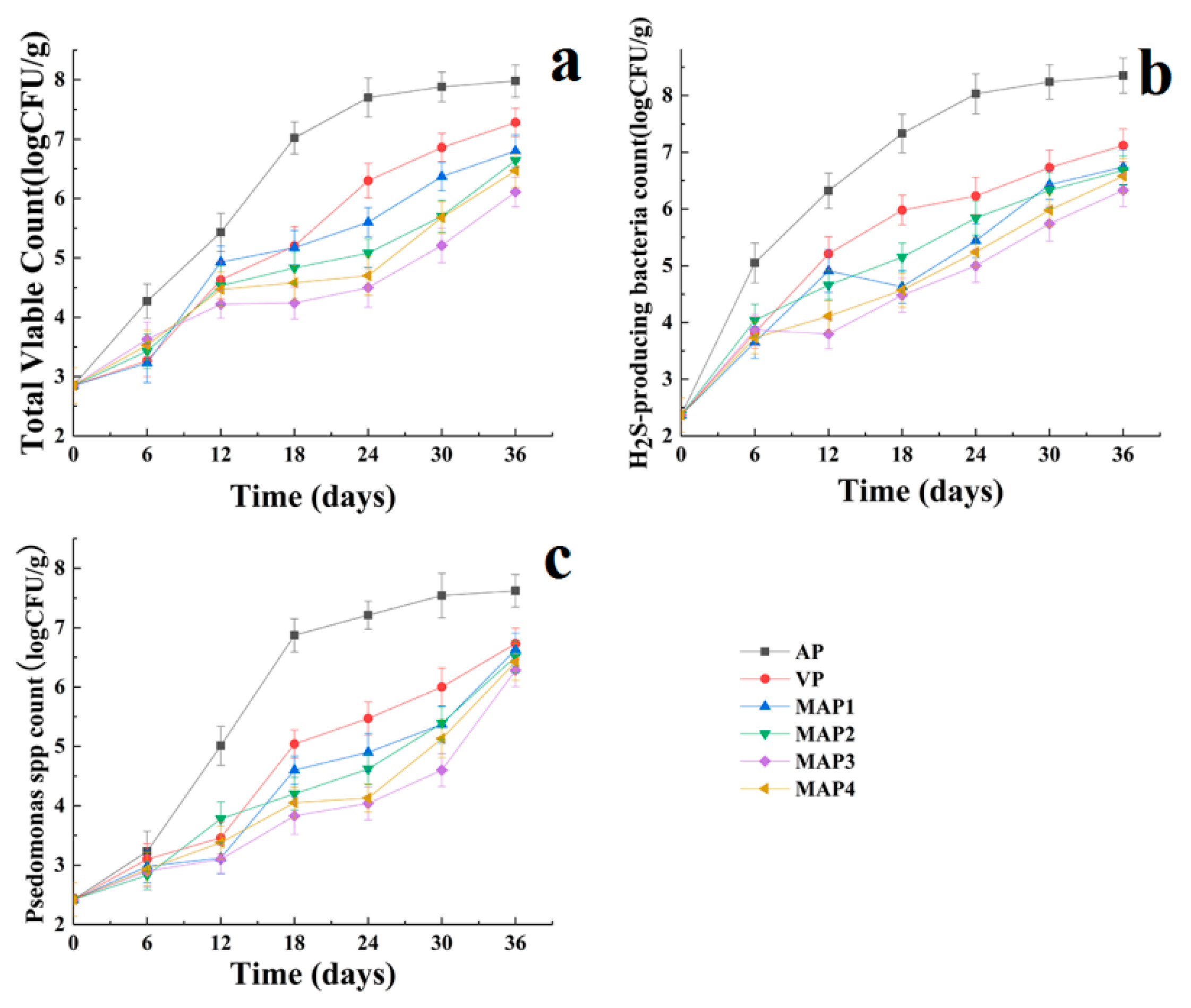
2.1. Microbiological Results
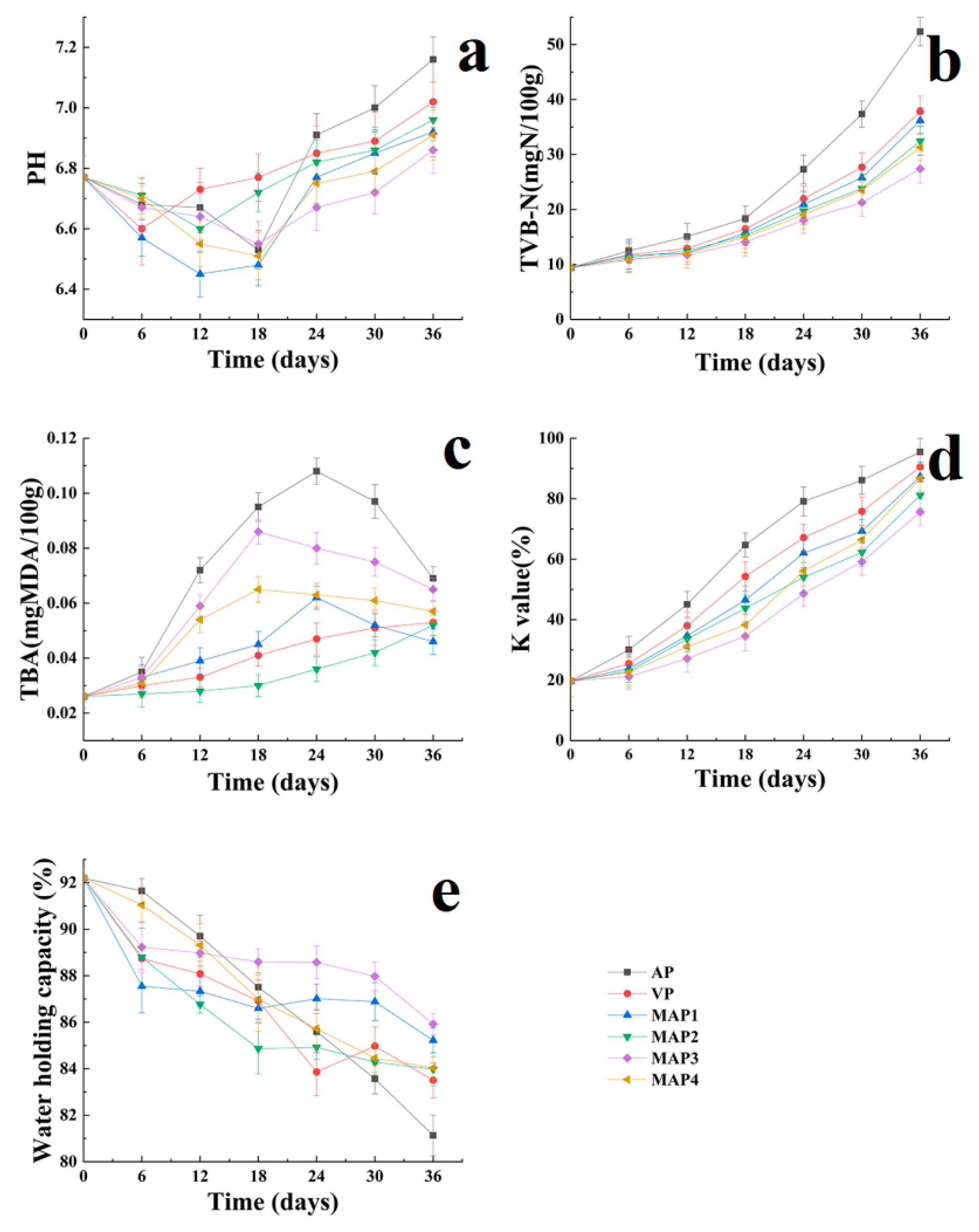
2.2. pH Values
2.3. Total Volatile Basic Nitrogen (TVB-N) Values
2.4. Thiobarbituric Acid Reactive Substances (TBARS) Values
2.5. Free Amino Acids (FAAs)Values
2.6. K Values
2.7. Water Holding Capacity (WHC) Values
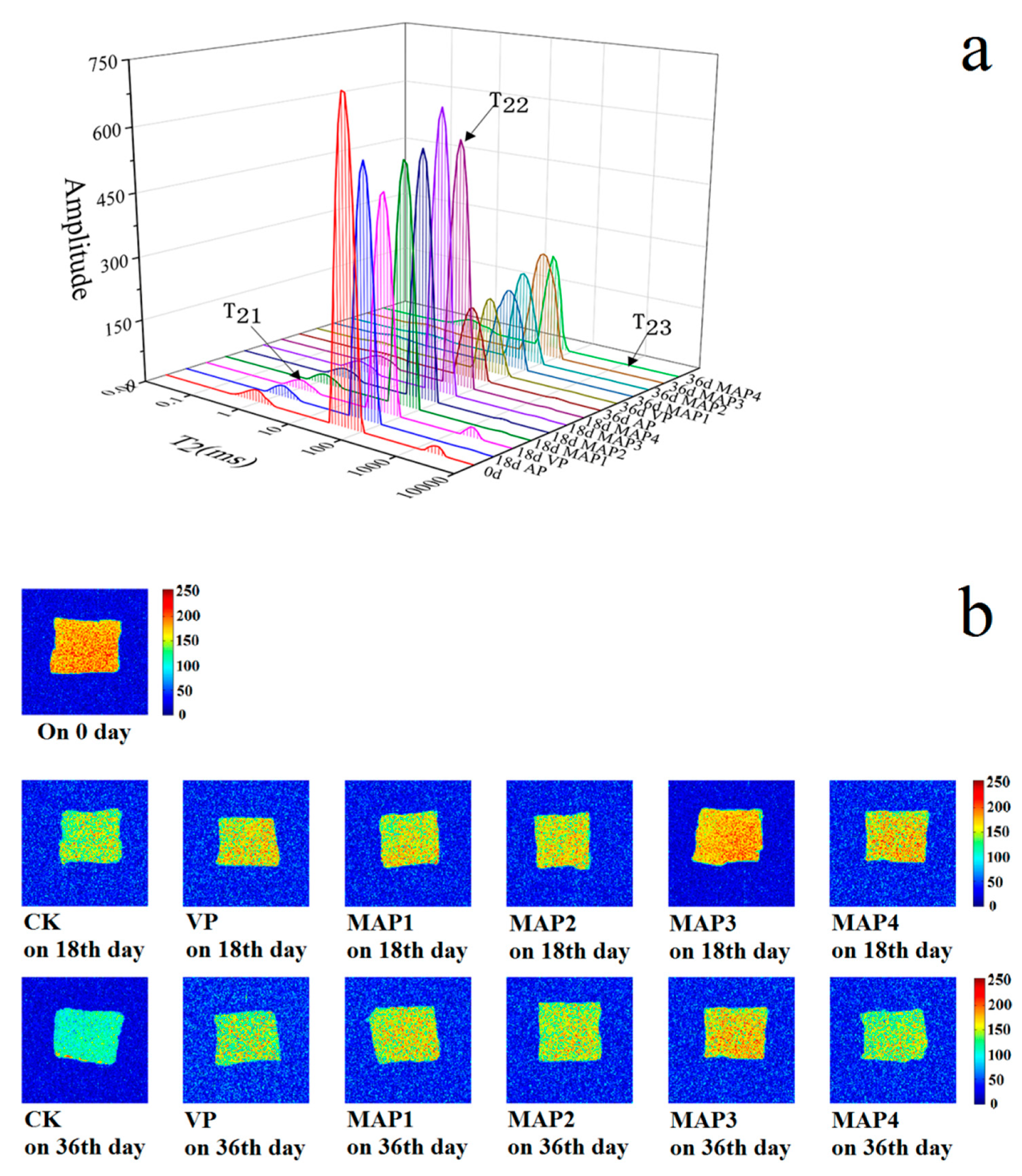
2.8. Water Distribution by Low Field Nuclear Magnetic Resonance (LF-NMR) Analysis
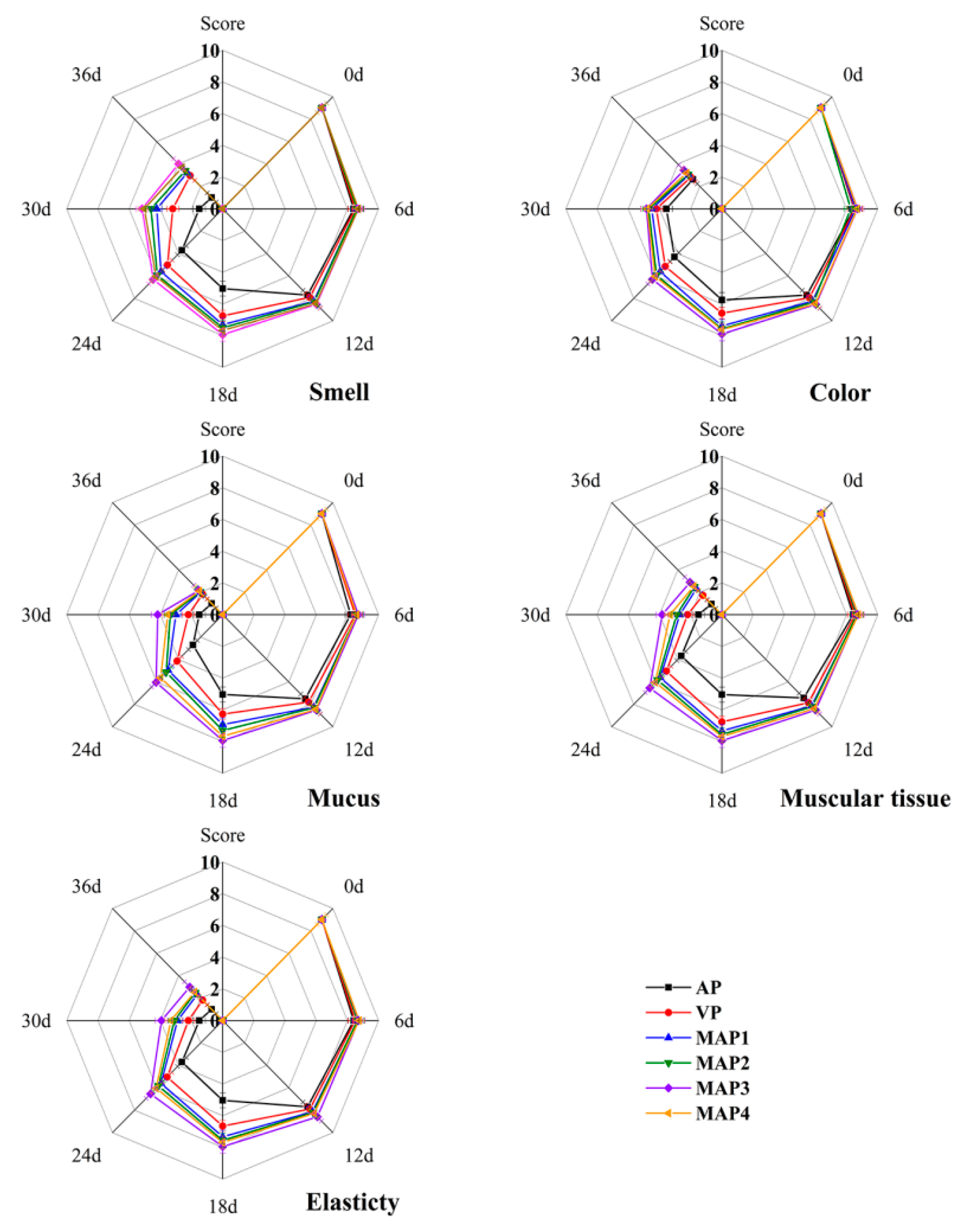
2.9. Organoleptic Properties
3. Materials and Methods
3.1. Preparation of Gelatin Active Coatings Containing Eugenol Emulsions
3.2. Preparation of Sea Bass and Sample Treatments
3.3. Microbiological Analysis
3.4. pH Value Determination
3.5. Water Distribution and Migration
3.6. WHC Determination
3.7. TVB-N Values Determination
3.8. TBARS Value Determination
3.9. Determination of Adenosine Triphosphate (ATP) Related Compounds
3.10. FAA Analysis
3.11. Organoleptic Properties
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; He, Y.; Bi, Y.; Chen, J.; Zhao, Z.; Li, J.; Chen, X. Transcriptome analysis of gill from Lateolabrax maculatus and aqp3 gene expression. Aquaculture and Fisheries 2019, 4, 247–254. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Sun, Y.; Cao, M.; Duan, Y.; Li, H.; Liu, Q.; Gu, Q.; Zhang, J. The efficacy of eugenol and tricaine methanesulphonate as anesthetics for juvenile Chinese sea bass (Lateolabrax maculatus) during simulated transport. J. Appl. Ichthyol. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cao, A.; Bai, F.; Li, J. Effect of ε-polylysine in combination with alginate coating treatment on physicochemical and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) during refrigerated storage. LWT-Food Sci. Technol. 2015, 62, 1053–1059. [Google Scholar] [CrossRef]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. 2019, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Stevik, A.M.; Claussen, I.C. Industrial superchilling, a practical approach. Procedia Food Sci. 2011, 1, 1265–1271. [Google Scholar] [CrossRef][Green Version]
- Magnussen, O.M.; Haugland, A.; Hemmingsen, A.K.T.; Johansen, S.; Nordtvedt, T.S. Advances in superchilling of food-Process characteristics and product quality. Trends Food Sci.T. 2008, 19, 418–424. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Grøvlen, M.S.; Rustad, T. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets–changes in texture, drip loss, protein solubility and oxidation. Int G Food Sci Tech. 2019, 54, 2228–2235. [Google Scholar] [CrossRef]
- Luan, L.; Fu, S.; Yuan, C.; Ishimura, G.; Chen, S.; Chen, J.; Hu, Y. Combined effect of superchilling and tea polyphenols on the preservation quality of hairtail (Trichiurus haumela). Int. J. Food Prop. 2017, 20, S992–S1001. [Google Scholar] [CrossRef]
- Xu, Y.; Li, T.; Zhang, C.; Li, X.; Yi, S.; Li, J.; Sun, X. Protein degradation of olive flounder (Paralichthys olivaceus) muscle after postmortem superchilled and refrigerated storage. Int. J. Food Prop. 2018, 21, 1911–1922. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Taoukis, P.S. Effect of storage temperature and osmotic pre-treatment with alternative solutes on the shelf-life of gilthead seabream (Sparus aurata) fillets. Acquaculture and Fisheries 2017, 2, 39–47. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ianniello, R.G. Dynamics of bacterial communities and interaction networks in thawed fish fillets during chilled storage in air. Int. J. Food Microbiol. 2019, 293, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Granda, X.; Fernández-Pan, I.; Rovira, J.; Maté, J. I Effect of antimicrobial edible coatings and modified atmosphere packaging on the microbiological quality of cold stored hake (Merluccius merluccius) fillets. J. Food Quality 2018, 2018, 6194906. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Yang, H.; Xiao, Y.; Xiong, Y.L. Super-chilling (-0.7 oC) with high-CO2 packaging inhibits biochemical changes of microbial origin in catfish (Clarias gariepinus) muscle during storage. Food Chem. 2016, 206, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.-J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef] [PubMed]
- De Witt, C.; Oliveira, A. Modified atmosphere systems and shelf life extension of fish and fishery products. Foods 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, H.; Li, L.; Yang, X.; Hao, S.; Chen, S.; Deng, J. The effects of modified atmosphere packaging and enzyme inhibitors on protein oxidation of tilapia muscle during iced storage. LWT-Food Sci. Technol. 2018, 87, 186–193. [Google Scholar] [CrossRef]
- Messina, C.M.; Bono, G.; Renda, G.; La Barbera, L.; Santulli, A. Effect of natural antioxidants and modified atmosphere packaging in preventing lipid oxidation and increasing the shelf-life of common dolphin fish (Coryphaena hippurus) fillets. LWT-Food Sci. and Technol. 2015, 62, 271–277. [Google Scholar] [CrossRef]
- Yew, C.C.; Bakar, F.A.; Rahman, R.A.; Bakar, J.; Zaman, M.Z.; Velu, S.; Shariat, M. Effects of modified atmosphere packaging with various carbon dioxide composition on biogenic amines formation in Indian mackerel (Rastrelliger kanagurta) stored at 5 ± 1 °C. Packag. Technol. Sci. 2014, 27, 249–254. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Azevedo, A.L.A.; Pereira, A.M.G.; Rocha, R.R.; Cavalcante, R.M.B.; Matos, M.N.C.; Lopes, P.H.R.; Gomes, G.A.; Rodrigues, T.H.S.; dos Santos, H.S.; et al. Chemical composition and antimicrobial effectiveness of Ocimum gratissimum L. essential oil against multidrug-resistant isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. [Google Scholar] [CrossRef] [PubMed]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Sharifi-Rad, M.; Sharifi-Rad, J.; Quek, S.Y. Evaluation of Allium paradoxum (M.B.) G. Don. and Eryngium caucasicum trauve. Extracts on the shelf-life and quality of silver carp (Hypophthalmichthys molitrix) fillets during refrigerated storage. J. Food Safety 2017, 37, e12321. [Google Scholar] [CrossRef]
- Kuuliala, L.; Al Hage, Y.; Ioannidis, A.G.; Sader, M.; Kerckhof, F.M.; Vanderroost, M.; Boon, N.; De Bates, B.; De Meulenaer, B.; Ragaert, P.; et al. Microbiological, chemical and sensory spoilage analysis of raw Atlantic cod (Gadus morhua) stored under modified atmospheres. Food Microbiol. 2018, 70, 232–244. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag. Technol Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Grković, N.; Bošković, M.; Baltić, M.Ž.; Dojčinović, S.; Karabasil, N.; Vasilev, D.; Teodorović, V. Inhibition of Listeria monocytogenes growth on vacuum packaged rainbow trout (Oncorhynchus mykiss) with carvacrol and eugenol. J. Food Safety 2019, 39, e12553. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Bono, G.; Okpala, C.O.R.; Vitale, S.; Ferrantelli, V.; Di Noto, A.; Costa, A.; Di Bella, C.; Lo Monaco, D. Effects of different ozonized slurry-ice treatments and superchilling storage (−1 °C) on microbial spoilage of two important pelagic fish species. Food Sci. Nutr. 2017, 5, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The dynamics of Pseudomonas and volatilome during the spoilage of gutted sea bream stored at 2 °C. Food Control 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Otero, L.; Pérez-Mateos, M.; Holgado, F.; Márquez-Ruiz, G.; López-Caballero, M.E.; Technologies, E. Hyperbaric cold storage: Pressure as an effective tool for extending the shelf-life of refrigerated mackerel (Scomber scombrus, L.). Innov Food Sci Emerg. 2019, 51, 41–50. [Google Scholar] [CrossRef]
- Patel, S. Plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products: A review. Food Addi. Contam. A 2015, 32, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Ravishankar, C. Active and intelligent packaging systems-Application in seafood. World J. Aquac. Res. Dev. 2019, 1, 1003. [Google Scholar]
- Provincial, L.; Gil, M.; Guillén, E.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Effect of modified atmosphere packaging using different CO2 and N2 combinations on physical, chemical, microbiological and sensory changes of fresh sea bass (Dicentrarchus labrax) fillets. J. Food Sci. Ttech. Mys. 2010, 45, 1828–1836. [Google Scholar] [CrossRef]
- Jia, S.; Liu, X.; Huang, Z.; Li, Y.; Zhang, L.; Luo, Y. Effects of chitosan oligosaccharides on microbiota composition of silver carp (Hypophthalmichthys molitrix) determined by culture-dependent and independent methods during chilled storage. Int. J. Food Microbiol. 2018, 268, 81–91. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Tech. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT-Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchus mykiss) fillets. LWT-Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, L.; Gao, Q.; Yang, S.; Li, Z.; Xu, J.; Xue, C. Oxidation evaluation of free astaxanthin and astaxanthin esters in Pacific white shrimp during iced storage and frozen storage. J. Sci. Food Agr. 2019, 99, 2226–2235. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Fuentes, A.; Masot, R.; Alcañiz, M.; Fernández-Segovia, I.; Barat, J.M. Use of the voltammetric tongue in fresh cod (Gadus morhua) quality assessment. Innov. Food Sci. Emerg. 2013, 18, 256–263. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Durmuş, M.; Balikçi, E.; Gökdoğan, S.; Uçar, Y.; Aksun, E.T. Effects of oil-in-water nanoemulsion based on sunflower oil on the quality of farmed sea bass and gilthead sea bream stored at chilled temperature (2 ± 2 °C). J. Aquat. Food Prod. T. 2017, 26, 979–992. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Kazemi, S.M.; Rezaei, M. Antimicrobial effectiveness of gelatin–alginate film containing oregano essential oil for fish preservation. J. Food Safety 2015, 35, 482–490. [Google Scholar] [CrossRef]
- De Lima Veeck, A.P.; Daniel, A.P.; Klein, B.; Quatrin, A.; de Souza Rezer, A.P.; Milani, L.G.; Zeppenfeld, C.C.; da Cunha, M.A.; Heldwein, C.G.; Heinzmann, B.M.; et al. Chemical, microbiological, and sensory parameters during the refrigerated storage of silver catfish (Rhamdia quelen) exposed in vivo to the essential oil of Lippia alba. J. Food Sci. Ttech. Mys. 2018, 55, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157: H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Özpolat, E.; Patir, B. Determination of shelf life for sausages produced from some freshwater fish using two different smoking methods. J. Food Saf. 2016, 36, 69–76. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yang, H.; Huang, L.; Zeng, M. Optimal modified atmosphere for packaging and its effects on quality and shelf-life of pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at -0.8oC. Food Sci. Technol. R. 2016, 22, 173–183. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery-In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gao, H.; Gao, T.; Sun, D.-W. Quality comparison of grass carp and salmon fillets packaged in modified atmosphere with different composite films. J. Food Process Eng. 2018, 41, e12803. [Google Scholar] [CrossRef]
- De Abreu, D.P.; Losada, P.P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int. 2010, 43, 1277–1282. [Google Scholar] [CrossRef]
- Hassoun, A.; Çoban, Ö.E. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Tech. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Li, N.; Mei, J.; Shen, Y.; Xie, J. Quality improvement of half-smooth tongue sole (Cynoglossus Semilaevis) fillets by chitosan coatings containing rosmarinic acid during storage. CyTA – J. Food 2018, 16, 1018–1029. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirose, N.; Ohno, S.; Arakaki, M.; Wada, K. Flavor characteristics and antioxidant capacities of hihatsumodoki (Piper retrofractum Vahl) fresh fruit at three edible maturity stages. J. Food Sci. Ttech. Mys. 2018, 55, 1295. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Tan, X.; Qi, L.; Fan, F.; Guo, Z.; Wang, Z.; Song, W.; Du, M. Analysis of volatile compounds and nutritional properties of enzymatic hydrolysate of protein from cod bone. Food Chem. 2018, 264, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preservation of bream (Megalobrama amblycephala) during superchilling storage. Food Control 2016, 69, 339–345. [Google Scholar] [CrossRef]
- Laly, S.J.; Ashok Kumar, K.N.; Sankar, T.V.; Lalitha, K.V.; Ninan, G. Quality of monosex tilapia under ice storage: Gutting effects on the formation of biogenic amines, biochemical, and microbiological characteristics. Int. J. Food Prop. 2017, 20, 1368–1377. [Google Scholar] [CrossRef]
- Ghalati, L.N.; Khodanazary, A.; Hosseini, S.M.; Matroodi, S. combination effect of phosphate and vacuum packaging on quality parameters of refrigerated Aurigequula fasciata fillets. J. Packag. Technol. Res. 2017, 1, 101–112. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Junior, E.E.R.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.B.; Ofstad, R.; Lødemel, J.B.; Olsen, R.L. Changes in water-holding capacity of halibut muscle during cold storage. LWT-Food Sci. Technol. 2003, 36, 771–778. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, W.; Fan, H.; Xie, J.; Qian, Y.-F. Study on the mobility of water and its correlation with the spoilage process of salmon (Salmo solar) stored at 0 and 4 °C by low-field nuclear magnetic resonance (LF NMR 1 H). J. Food Sci. Ttech. Mys. 2018, 55, 173–182. [Google Scholar] [CrossRef]
- Patel, K.K.; Khan, M.A.; Kar, A. Recent developments in applications of MRI techniques for foods and agricultural produce-an overview. J. Food Sci. Ttech. Mys. 2015, 52, 1–26. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Shen, Y.; Mei, J.; Xie, J. Coating effects of ε-polylysine and rosmarinic acid combined with chitosan on the storage quality of fresh half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets. Coatings 2019, 9, 273. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, S.; Song, Y.; Xia, K.; Xu, X.; Zhu, B.-W.; Tan, M. Non-destructive analysis of caviar compositions using low-field nuclear magnetic resonance technique. J. Food Meas. Charact. 2017, 11, 621–628. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, L.; Zhang, J.; Song, S.; Wang, Z.; Regenstein, J.M.; Luo, Y. Influence of lightly salting and sugaring on the quality and water distribution of grass carp (Ctenopharyngodon idellus) during super-chilled storage. J. Food Eng. 2017, 215, 104–112. [Google Scholar] [CrossRef]
- Reza, M.S.; Bapary, M.A.; Ahasan, C.T.; Islam, M.N.; Kamal, M. Shelf life of several marine fish species of Bangladesh during ice storage. Int. J. Food Sci. Tech. 2009, 44, 1485–1494. [Google Scholar] [CrossRef]
- Li, D.-Y.; Huang, Y.; Wang, K.-X.; Dong, X.-P.; Yu, D.; Ge, L.-H.; Zhou, D.-Y.; Yu, C.-X. Microstructural characteristics of turbot (Scophthalmus maximus) muscle: Effect of salting and processing. Int. J. Food Prop. 2018, 21, 1291–1302. [Google Scholar] [CrossRef]
- Li, N.; Shen, Y.; Liu, W.; Mei, J.; Xie, J. Low-field NMR and MRI to analyze the effect of edible coating incorporated with map on qualities of half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets during refrigerated storage. Appl. Sci. 2018, 8, 1391. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.; Cullen, P.J.; Tiwari, B.; Ojha, K.S.; Bourke, P.; Rico, D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. 2019, 53, 85–91. [Google Scholar] [CrossRef]
- Sun, X.H.; Xiao, L.; Lan, W.Q.; Liu, S.C.; Wang, Q.; Yang, X.H.; Zhang, W.J.; Xie, J. Effects of temperature fluctuation on quality changes of large yellow croaker (Pseudosciaena crocea) with ice storage during logistics process. J. Food Process. Pres. 2018, 42, e13505. [Google Scholar] [CrossRef]
- Wang, Z.; Herremans, E.; Janssen, S.; Cantre, D.; Verboven, P.; Nicolaï, B. Visualizing 3D food microstructure using tomographic methods: Advantages and disadvantages. Annu. Rev. Food Sci. T. 2018, 9, 323–343. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Fan, Y.-F.; Ling, J.-G.; Hu, Y.-Q.; Liu, D.-H.; Chen, S.-G.; Ye, X.-Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Lather, P.; Mohanty, A.K.; Jha, P.; Garsa, A.K.; Sood, S.K. Changes associated with cell membrane composition of Staphylococcus aureus on acquisition of resistance against class IIa bacteriocin and its in vitro substantiation. Eur. Food Res. Technol. 2015, 240, 101–107. [Google Scholar] [CrossRef]
- Li, P.; Peng, Y.; Mei, J.; Xie, J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT-Food Sci. Technol. 2020, 118, 108831. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Jiang, Q. The impact of desmin on texture and water-holding capacity of ice-stored grass carp (Ctenopharyngodon idella) fillet. Int. J. Food Sci. Tech. 2017, 52, 464–471. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.e.; Chen, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT-Food Sci. Technol. 2020, 117, 108633. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Study on the volatile organic compounds and its correlation with water dynamics of bigeye tuna (Thunnus obesus) during cold storage. Molecules 2019, 24, 3119. [Google Scholar] [CrossRef]
- Liu, W.; Shen, Y.; Li, N.; Mei, J.; Xie, J. Application of gelatin incorporated with red pitaya peel methanol extract as edible coating for quality enhancement of crayfish (Procambarus clarkii) during refrigerated storage. J Food Quality 2019, 1715946. [Google Scholar] [CrossRef]
- Lanzarin, M.; Ritter, D.; Novaes, S.; Monteiro, M.; Almeida Filho, E.; Marsico, E.; Franco, R.; Conte, C., Jr.; Freitas, M.Q. Quality Index Method (QIM) for ice stored gutted Amazonian Pintado (Pseudoplatystoma fasciatum× Leiarius marmoratus) and estimation of shelf life. LWT-Food Sci. Technol. 2016, 65, 363–370. [Google Scholar] [CrossRef]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. Freshness assessment and shelf-life prediction for Seriola dumerili from aquaculture based on the quality index method. Molecules 2019, 24, 3530. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds eugenol are available from the authors. |
| Time | Samples | Free Amino Acids | |||||
|---|---|---|---|---|---|---|---|
| Aspartic Acid | Threonine | Serine | Glutamic Acid | Glycine | Alanine | ||
| On day 0 | 3.71 ± 0.36 | 12.36 ± 0.75 | 25.32 ± 0.88 | 11.79 ± 0.43 | 126.58 ± 0.99 | 81.80 ± 0.62 | |
| On 18th day | AP | 4.33 ± 0.26b | 29.45 ± 0.96ab | 27.4 ± 0.82b | 17.86 ± 0.53e | 72.45 ± 0.67e | 87.62 ± 1.77c |
| VP | 1.88 ± 0.22d | 26.04 ± 0.81bc | 24.28 ± 0.76c | 19.82 ± 0.65d | 78.58 ± 0.78d | 90.14 ± 0.83c | |
| MAP1 | 3.20 ± 0.41c | 33.10 ± 0.87a | 28.10 ± 0.73b | 23.34 ± 0.59c | 82.24 ± 0.64c | 94.01 ± 1.85b | |
| MAP2 | 3.46 ± 0.19c | 30.71 ± 0.83a | 30.14 ± 0.81a | 23.94 ± 0.38c | 88.32 ± 0.98b | 95.54 ± 0.83b | |
| MAP3 | 6.54 ± 0.38a | 30.87 ± 0.58a | 22.12 ± 0.72d | 27.34 ± 0.43a | 99.30 ± 0.49a | 103.14 ± 0.88a | |
| MAP4 | 4.37 ± 0.25b | 22.47 ± 0.61c | 25.49 ± 0.73c | 25.65 ± 0.65b | 89.64 ± 1.26b | 97.75 ± 2.93b | |
| On 36th day | AP | 3.81 ± 0.14d | 16.89 ± 0.43d | 11.47 ± 2.2e | 20.87 ± 2.7c | 58.32 ± 1.07e | 59.10 ± 3.43f |
| VP | 4.07 ± 0.55d | 20.06 ± 0.58c | 18.40 ± 1.7d | 27.70 ± 4.8ac | 71.77 ± 3.75d | 70.81 ± 1.52e | |
| MAP1 | 5.04 ± 0.35d | 33.90 ± 0.55a | 70.29 ± 5.3a | 38.06 ± 4.5b | 97.23 ± 2.6c | 87.85 ± 2.08d | |
| MAP2 | 10.23 ± 0.68b | 33.67 ± 0.84a | 54.81 ± 6.2b | 41.90 ± 6.33b | 101.08 ± 3.09c | 100.81 ± 1.72c | |
| MAP3 | 13.49 ± 0.62a | 28.48 ± 0.61b | 39.60 ± 7.3c | 55.83 ± 5.32a | 153.56 ± 0.62a | 117.46 ± 1.89a | |
| MAP4 | 8.96 ± 0.58c | 33.36 ± 0.71a | 43.27 ± 5.2c | 51.89 ± 6.84a | 134.75 ± 1.72b | 106.86 ± 2.17b | |
| Valine | Methionine | Isoleucine | Leucine | Tyrosine | Phenylalanine | ||
| On day 0 | 7.66 ± 0.25 | 4.48 ± 0.37 | 5.52 ± 0.28 | 8.76 ± 0.29 | 4.30 ± 0.21 | 4.68 ± 0.28 | |
| On 18th day | AP | 11.59 ± 0.52b | 6.51 ± 0.50b | 7.20 ± 0.24bc | 11.61 ± 0.32b | 7.65 ± 0.18d | 7.25 ± 0.23d |
| VP | 9.51 ± 0.46e | 4.83 ± 0.36c | 6.20 ± 0.75d | 9.74 ± 0.43d | 5.44 ± 0.36a | 5.68 ± 0.46e | |
| MAP1 | 13.28 ± 0.19a | 6.71 ± 0.16b | 8.91 ± 0.29a | 10.59 ± 0.28c | 9.56 ± 0.17b | 9.60 ± 0.26b | |
| MAP2 | 10.34 ± 0.34d | 7.17 ± 0.63ab | 7.12 ± 0.88bc | 10.95 ± 0.25c | 7.87 ± 0.23b | 7.09 ± 0.31d | |
| MAP3 | 11.05 ± 0.27bc | 7.69 ± 0.82a | 7.25 ± 0.27bc | 12.73 ± 0.31a | 8.47 ± 0.25c | 9.67 ± 0.27b | |
| MAP4 | 10.56 ± 0.22cd | 6.58 ± 0.27b | 8.17 ± 0.18ab | 12.36 ± 0.28a | 10.22 ± 0.31a | 9.88 ± 0.35a | |
| On 36th day | AP | 11.14 ± 0.27d | 6.42 ± 0.32d | 6.70 ± 1.57c | 10.1 ± 0.34d | 5.01 ± 0.29b | 7.03 ± 0.48d |
| VP | 10.26 ± 0.33e | 6.33 ± 0.48d | 7.84 ± 1..32c | 10.01 ± 0.65d | 9.75 ± 0.36a | 5.96 ± 0.44e | |
| MAP1 | 20.44 ± 0.23b | 11.85 ± 0.37c | 17.21 ± 1.59a | 28.54 ± 0.44a | 19.98 ± 0.54b | 15.28 ± 0.48a | |
| MAP2 | 19.87 ± 0.29 | 12.13 ± 0.31bc | 13.84 ± 1.39b | 23.45 ± 0.63b | 16.58 ± 0.65b | 12.81 ± 0.39b | |
| MAP3 | 23.08 ± 0.18a | 15.32 ± 0.35a | 12.84 ± 0.96b | 21.63 ± 0.48c | 13.59 ± 0.53b | 11.18 ± 0.57c | |
| MAP4 | 18.22 ± 0.28c | 12.74 ± 0.31b | 17.56 ± 1.84a | 28.84 ± 0.61a | 18.54 ± 0.62 | 15.55 ± 0.48a | |
| Lysine | Histidine | Arginine | Proline | Total | |||
| On day 0 | 78.26 ± 0.44 | 24.59 ± 0.45 | 14.20 ± 0.32 | 8.09 ± 0.26 | 432.90 ± 3.37 | ||
| On 18th day | AP | 36.49 ± 2.758c | 29.84 ± 0.77a | 15.24 ± 0.35c | 7.61 ± 0.58b | 410.82 ± 4.76d | |
| VP | 31.68 ± 0.47d | 25.63 ± 0.32b | 12.14 ± 0.43e | 5.82 ± 0.83c | 380.99 ± 5.84e | ||
| MAP1 | 45.94 ± 0.98b | 23.84 ± 0.58c | 17.19 ± 0.48a | 10.23 ± 0.24a | 450.25 ± 3.28b | ||
| MAP2 | 36.23 ± 0.37c | 22.96 ± 0.38c | 13.13 ± 0.35d | 9.55 ± 0.85a | 434.04 ± 4.98c | ||
| MAP3 | 47.96 ± 2.01a | 17.23 ± 0.53d | 16.28 ± 0.23b | 8.02 ± 0.67b | 462.27 ± 4.22a | ||
| MAP4 | 28.04 ± 0.29e | 18.33 ± 0.66c | 11.64 ± 0.42e | 5.42 ± 0.33c | 415.27 ± 5.25d | ||
| On 36th day | AP | 34.21 ± 0.74e | 14.38 ± 0.63e | 8.25 ± 1.35 d | 5.60 ± 0.64d | 326.17 ± 4.18e | |
| VP | 29.51 ± 0.87f | 14.66 ± 0.59e | 9.87 ± 0.94d | 6.28 ± 0.68d | 344.17 ± 5.34d | ||
| MAP1 | 81.22 ± 0.56c | 50.55 ± 0.46a | 33.46 ± 0.69a | 23.88 ± 0.72a | 648.66 ± 4.52c | ||
| MAP2 | 103.46 ± 1.04a | 48.71 ± 0.63b | 27.48 ± 0.73b | 21.36 ± 0.83b | 655.09 ± 5.83bc | ||
| MAP3 | 91.46 ± 0.73b | 30.78 ± 0.58d | 23.87 ± 0.53c | 18.43 ± 0.77c | 682.33 ± 5.07a | ||
| MAP4 | 75.19 ± 0.87d | 32.12 ± 0.52c | 28.52 ± 0.81b | 22.01 ± 0.68b | 660.26 ± 4.78b | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, P.; Fang, S.; Mei, J.; Xie, J. Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Molecules 2020, 25, 871. https://doi.org/10.3390/molecules25040871
Zhou Q, Li P, Fang S, Mei J, Xie J. Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Molecules. 2020; 25(4):871. https://doi.org/10.3390/molecules25040871
Chicago/Turabian StyleZhou, Qianqian, Peiyun Li, Shiyuan Fang, Jun Mei, and Jing Xie. 2020. "Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage" Molecules 25, no. 4: 871. https://doi.org/10.3390/molecules25040871
APA StyleZhou, Q., Li, P., Fang, S., Mei, J., & Xie, J. (2020). Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Molecules, 25(4), 871. https://doi.org/10.3390/molecules25040871








