Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization
Abstract
1. Introduction
2. Results
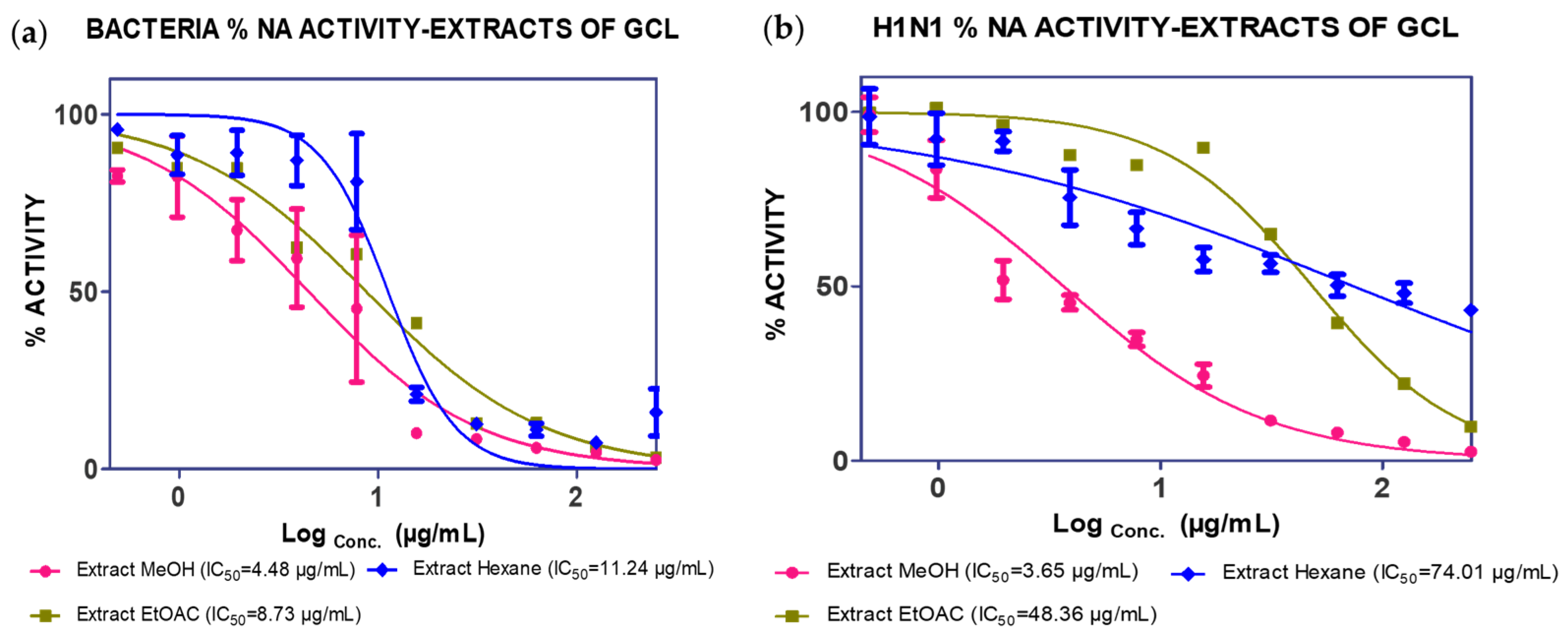
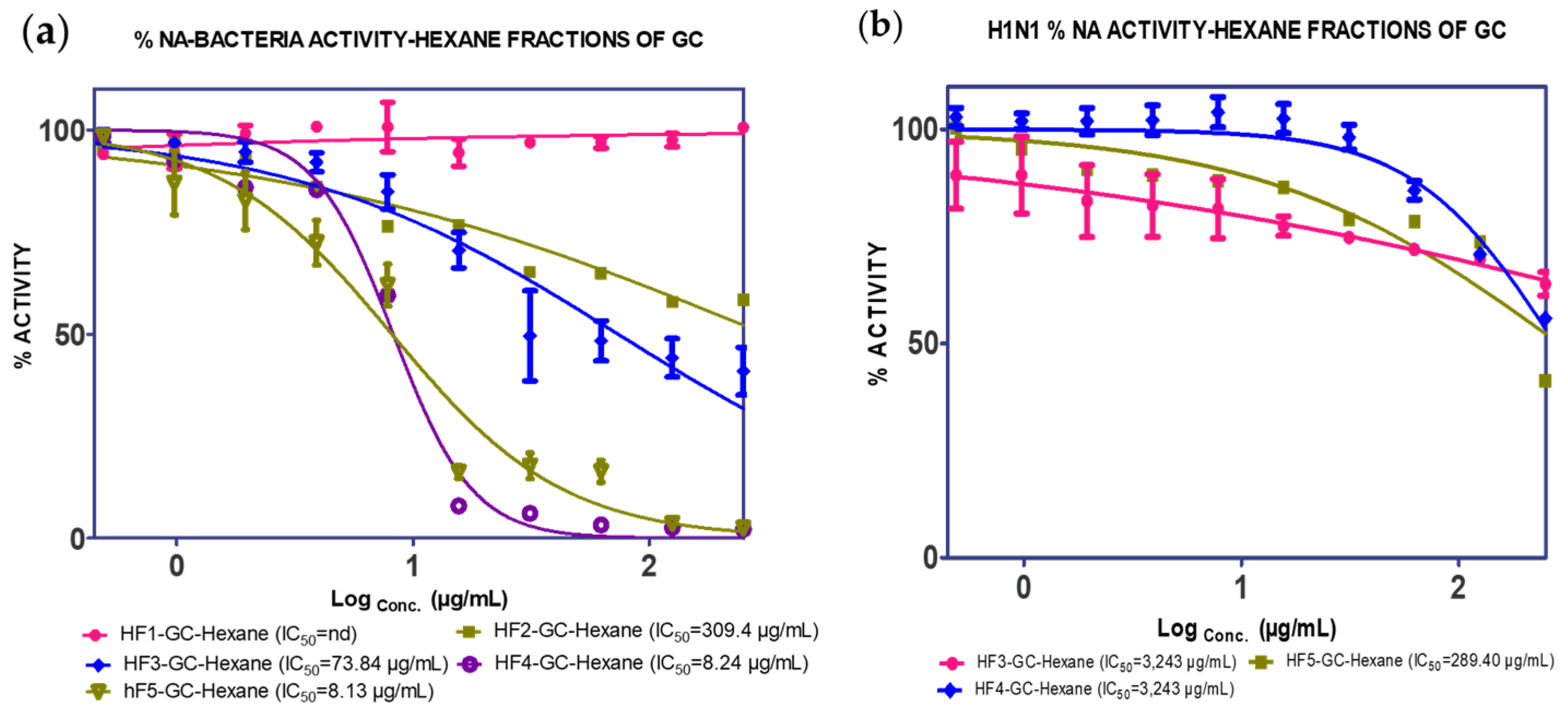
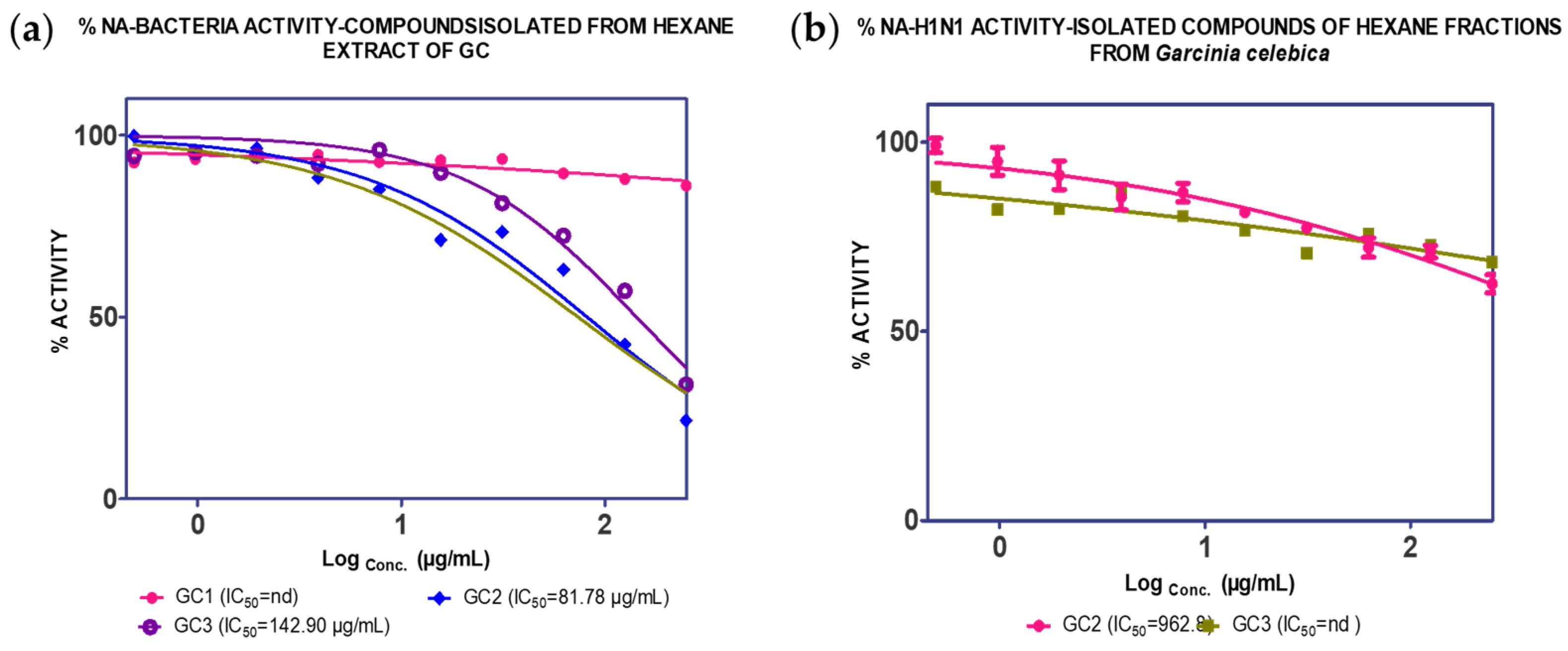
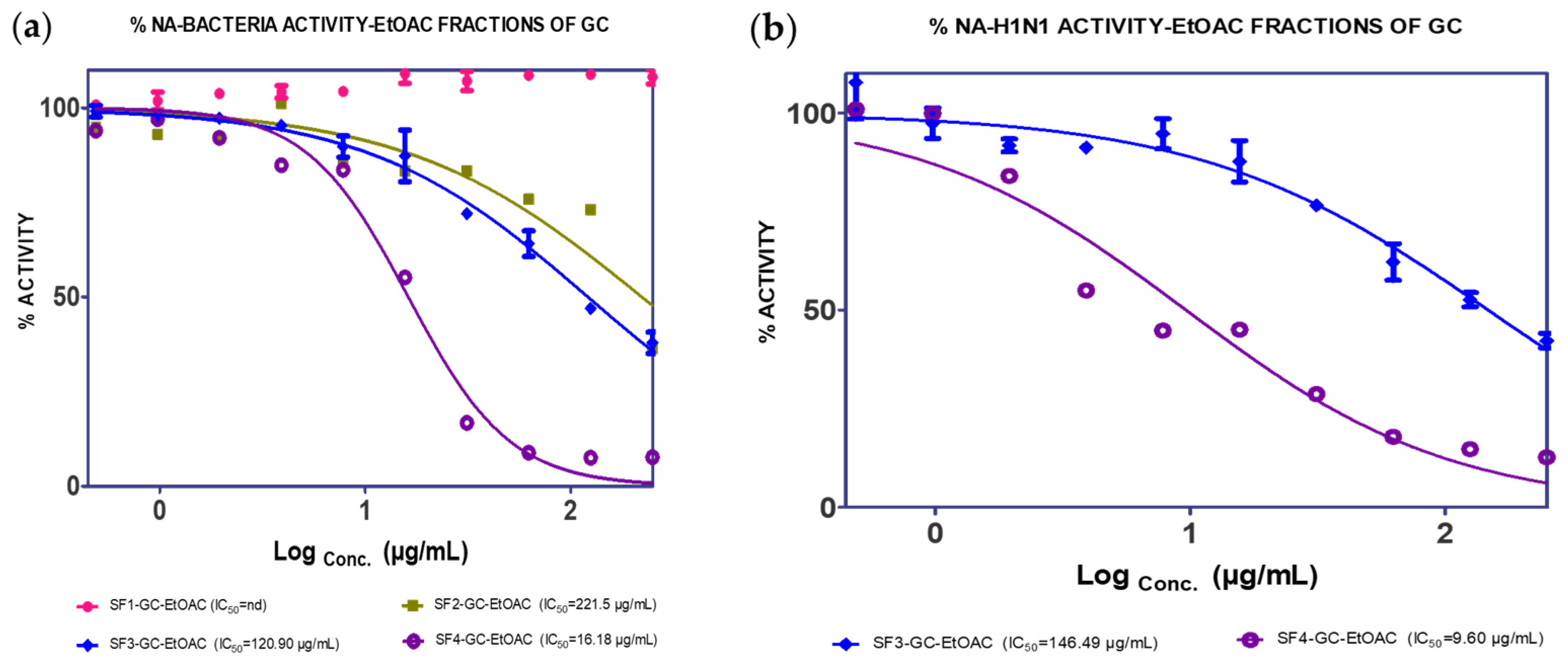
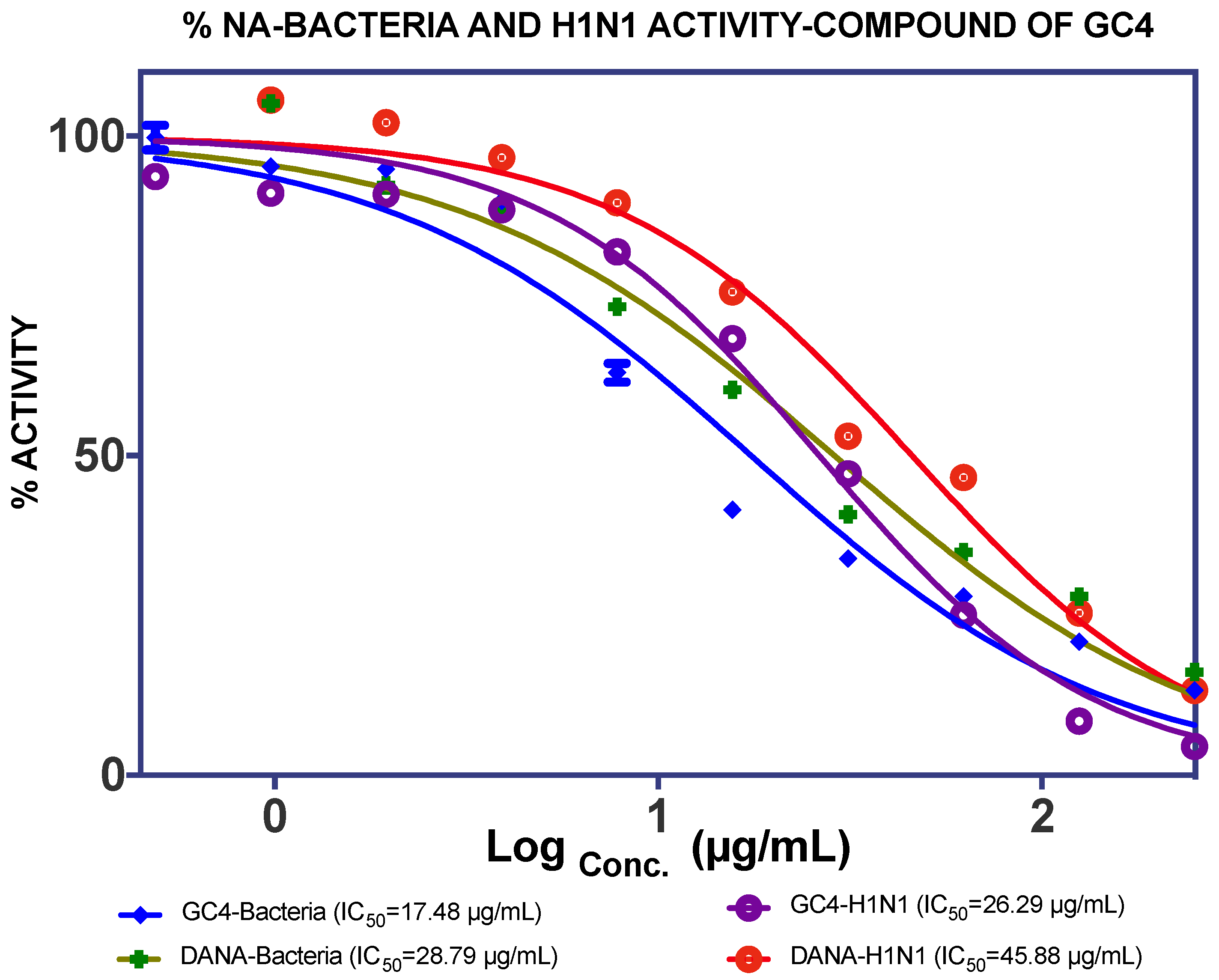
2.1. Extraction, Isolation and Bioassay
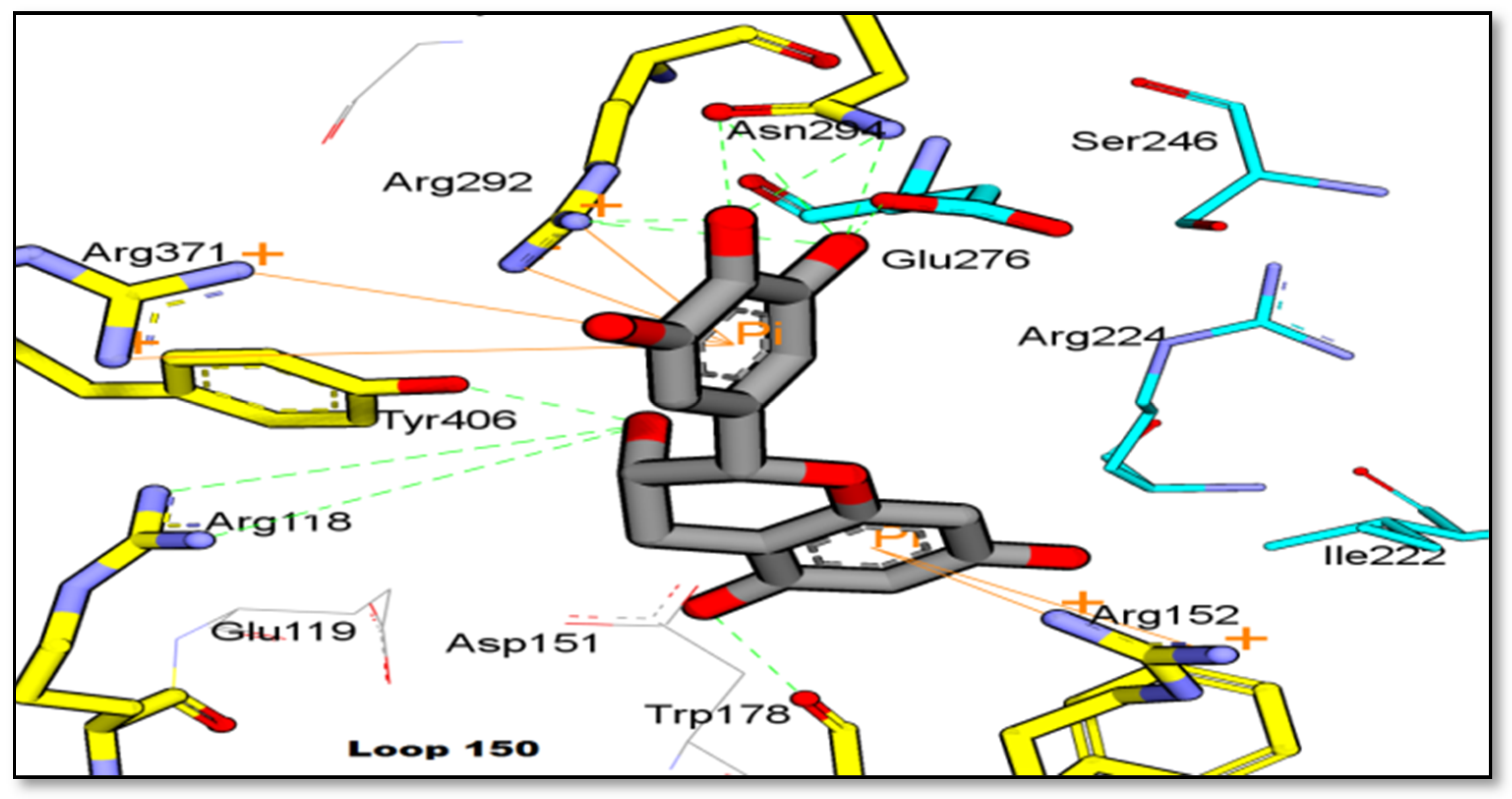
2.2. Binding Interaction of the Isolated Compound from Garcinia celebica
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of Compounds from n-Hexane Extract of GCL
4.3. Isolation of Compounds from EtOAc Extract of GCL
4.4. General Experiments and Spectroscopy Methods
4.5. Neuraminidase (NA) Activity
4.6. Molecular Docking Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, R.B.; McCombs, J.E.; Kohler, J.J. Sialidase specificity determined by chemoselective modification of complex sialylated glycans. ACS Chem. Biol. 2012, 7, 1509–1514. [Google Scholar] [CrossRef]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef]
- Schwerdtfeger, S.M.; Melzig, M.F. Sialidases in biological systems. Pharmazie 2010, 65, 551–561. [Google Scholar]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Byrd-Leotis, L.; Cummings, R.D.; Steinhauer, D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017, 18, 1541. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza a virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Greenway, K.T.; LeGresley, E.B.; Pinto, B.M. The influence of 150-cavity binders on the dynamics of influenza a neuraminidases as revealed by molecular dynamics simulations and combined clustering. PLoS ONE 2013, 8, e59873. [Google Scholar] [CrossRef]
- Rudrawar, S.; Dyason, J.C.; Rameix-Welti, M.A.; Rose, F.J.; Kerry, P.S.; Russell, R.J.; van der Werf, S.; Thomson, R.J.; Naffakh, N.; von Itzstein, M. Novel sialic acid derivatives lock open the 150-loop of an influenza a virus group-1 sialidase. Nat. Commun. 2010, 1, 113. [Google Scholar] [CrossRef] [PubMed]
- Laborda, P.; Wang, S.Y.; Voglmeir, J. Influenza neuraminidase inhibitors: Synthetic approaches, derivatives and biological activity. Molecules 2016, 21, 1513. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A. Method of Isolating Shikimic Acid from a Plant. U.S. Patent 8,203,020, 19 June 2012. [Google Scholar]
- Ikram, N.K.K.; Durrant, J.D.; Muchtaridi, M.; Zalaludin, A.S.; Purwitasari, N.; Mohamed, N.; Rahim, A.S.A.; Lam, C.K.; Normi, Y.M.; Rahman, N.A.; et al. A virtual screening approach for identifying plants with anti h5n1 neuraminidase activity. J. Chem. Inf. Modeling 2015, 55, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Parikesit, P. Tree species distribution along the environmental gradients in pananjung pangandaran nature reserve, west java. Biodivesritas 2008, 9, 275–279. [Google Scholar]
- Subarnas, A. Antiproliferative activity of primates-consumed plants against mcf-7 human breast cancer cell lines. E3 J. Med. Res. 2012, 1, 038–043. [Google Scholar]
- Dahlan, Z.; Hanum, L.; Zahar, E. Exploration and study of the diversity of garcinia l. Based sources of evidence and their use for lectures macromorphology plant morphology. Forum Kependidikan 2009, 28, 163–171. (in indonesian). [Google Scholar]
- Sari, R.; Hanan, A. Garcinia (clusiaceae) in Bogor Botanical Garden: Physiognomy, Diversity and Potential; Seminar Sehari Hari Cinta Puspa dan Satwa Nasional: Bogor, Indonesia, 2000; pp. 65–75. (in indonesian) [Google Scholar]
- Elfita, E.; Muharni, M.; Latief, M.; Darwati, D.; Widiyantoro, A.; Supriyatna, S.; Bahti, H.H.; Dachriyanus, D.; Cos, P.; Maes, L.; et al. Antiplasmodial and other constituents from four indonesian garcinia spp. Phytochemistry 2009, 70, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Schmidtke, M.; von Grafenstein, S.; Kirchmair, J.; Liedl, K.R.; Rollinger, J.M. Influenza neuraminidase: A druggable target for natural products. Nat. Prod. Rep. 2012, 29, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Sari, R. Collection of Garcinia in Bogor Botanical Garden: Conservation and Potential; LIPI, Research Insitute of Indonesia: Bogor, Indonesia; pp. 217–221. (in indonesiaan)
- Ee, G.C.L.; Lim, C.K.; Cheow, Y.L.; Sukari, M.A. Xanthones and triterpenoids from mesua daphnifolia and garcinia maingayi. Malays. J. Sci. 2005, 24, 183–185. [Google Scholar]
- Elya, B.; He, H.P.; Kosela, S.; Hanafi, H.; Hao, X.J. Triterpenoids from garcinia rigida. Rec. Nat. Prod. 2011, 5, 56–59. [Google Scholar]
- Susanti, D.; Amiroudine, M.Z.A.M.; Rezali, M.F.; Taher, M. Friedelin and lanosterol from garcinia prainiana stimulated glucose uptake and adipocytes differentiation in 3t3-l1 adipocytes. Nat. Prod. Res. 2013, 27, 417–424. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Saelim, S.; Karnsomchoke, P.; Phongpaichit, S. Friedolanostanes and lanostanes from the leaves of garcinia hombroniana. J. Nat. Prod. 2005, 68, 1222–1225. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Adair, A.; Dampawan, P.; Taylor, W.C.; Turner, P.C. Lanostanes and friedolanostanes from the pericarp of garcinia hombroniana. Phytochemistry 2000, 55, 183–188. [Google Scholar] [CrossRef]
- Klaiklay, S.; Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S. Friedolanostanes and xanthones from the twigs of garcinia hombroniana. Phytochemistry 2013, 85, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2017, 34, 90–122. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; Kijjoa, A.; Silva, A.M.S.; Mondranondra, I.-O.; Kengthong, S.; Gales, L.; Damas, A.M.; Herz, W. Lanostanes and friedolanostanes from the bark of garcinia speciosa. Phytochemistry 2004, 65, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Trinh, B.T.; Tran, Q.N.; Pham, H.D.; Hansen, P.E.; Duus, F.; Connolly, J.D.; Nguyen, L.H. Friedolanostane, friedocycloartane and benzophenone constituents of the bark and leaves of garcinia benthami. Phytochemistry 2011, 72, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Chapter 22—Methods for profiling drug-like properties: General concepts. In Drug-like Properties: Concepts, Structure Design and Methods; Academic Press: San Diego, CA, USA, 2008; pp. 257–259. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Walters, W.P. Going further than lipinski’s rule in drug design. Expert Opin. Drug Discov. 2012, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Kihlberg, J. Drug discovery beyond the rule of 5-opportunities and challenges. Expert Opin. Drug Discov. 2017, 12, 115–119. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, X.; Dwek, R.A.; Stevens, J.; Wilson, I.A. Structural characterization of the 1918 influenza virus h1n1 neuraminidase. J. Virol. 2008, 82, 10493–10501. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Ejele, A.E.; Iwu, I.C.; Enenebeaku, C.K.; Ukiwe, L.N.; Okolue, B.N. Bioassay-guided isolation, purification and partial characterization of antimicrobial compound from basic metabolite of garcinia kola. JETEAS 2012, 3, 668–672. [Google Scholar]
- Lim, C.K. Phytochemicals from Garcinia, Mesua and Jatropha Species and Their Biological Activities. Ph.D. Thesis, Univerisi Putra Malaysia, Kuala Lumpur, Selangor, 2005. [Google Scholar]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-M.; Seong, B.-L. Chapter 99—anti-influenza viral activity of catechins and derivatives. In Tea in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2013; pp. 1185–1193. [Google Scholar]
- Yamada, H.; Takuma, N.; Daimon, T.; Hara, Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: A prospective clinical study. J. Altern. Complement. Med. 2006, 12, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Echigo, N. Green tea catechins inhibit the endonuclease activity of influenza a virus rna polymerase. PLoS Curr. 2009, 1, RRN1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Wang, H.D.; Lee, S.M.; Wang, Y.T.; Du, G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorganic Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Ma, Y.; Wang, M.; Dong, Y. Recent advances in the structure-based design of neuraminidase inhibitors as antiinfluenza agents. Curr. Med. Chem. 2012, 19, 5885–5894. [Google Scholar] [CrossRef]
- Choi, H.M.; Kim, J.Y.; Li, Z.P.; Jenis, J.; Ban, Y.J.; Baiseitova, A.; Park, K.H. Effectiveness of prenyl group on flavonoids from epimedium koreanum nakai on bacterial neuraminidase inhibition. Molecules 2019, 24, 317. [Google Scholar] [CrossRef]
- Uchide, N.; Toyoda, H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef]
- Raab, M.; Tvaroska, I. The binding properties of the h5n1 influenza virus neuraminidase as inferred from molecular modeling. J. Mol. modeling 2011, 17, 1445–1456. [Google Scholar] [CrossRef][Green Version]
- Müller, P.; Downard, K.M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar] [CrossRef]
- Hurt, A. Fluorometric Neuraminidase Inhibition Assay; WHO Collaborating Centre for Reference and Research on Influenza: Melbourne, Australia, 2007; pp. 1–10. [Google Scholar]
- Muchtaridi, M.; Bing, C.S.; Abdurahinm, A.S.; Wahab, H.A. Evidence of combining pharmacophore modeling-docking simulation for screening on neuraminidase inhibitors activity of natural product compounds. Asian J. Chem. 2014, 26, 59–63. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods in Mol. Biol. 2008, 443, 365–382. [Google Scholar]
Sample Availability: Samples of the G. celebica leaves extracts and catechin (isolated compound) are available from the authors. |

| Extract | Partitions | Fractions | Compounds | IC50 * | |
|---|---|---|---|---|---|
| NA-C. perfringens a | NA-H1N1 b | ||||
| MeOH extract | 4.48 µg/mL | 3.65 µg/mL | |||
| (17.98% mass) | Hexane extract | 11.04 µg/mL | 74.01 µg/mL | ||
| (14.96% w/w) | F1 (5.07%) | nd | nd | ||
| F2 (4.5%) | 39.42 µg/mL | nd | |||
| F3 (21.40%) | 73.84 µg/mL | 3.243 µg/mL | |||
| GC1 (2.20%) | nd | nd | |||
| F4 (21.28%) | 8.24 µg/mL | 289.4 µg/mL | |||
| GC2 (0.55%) | 81.74 µg/mL | 962.80 µg/mL | |||
| GC3 (0.24%) | 142.49 µg/mL | nd | |||
| F5 (21.45%) | 277.0 µg/mL | nd | |||
| EtOAc extract | 38.39 µg/mL | 48.36 µg/mL | |||
| (25.86% w/w) | F1 (4.80%) | nd | nd | ||
| F2 (26.39%) | 120.90 µg/mL | nd | |||
| F3 (14.21%) | 221.50 µg/mL | 146.49 µg/m | |||
| F4 (30.4%) | 16.18 µg/mL | 9.60 µg/mL | |||
| GC4 (0.49%) | 17.48 µg/mL | 26.29 µg/mL | |||
| Compound Code | Molecular Structure | Molecular Formula | MW | HBD | HBA | Log P |
|---|---|---|---|---|---|---|
| GC1 | 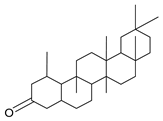 | C30H50O | 426 | 1 | 3 | 7.03 |
| GC2 | 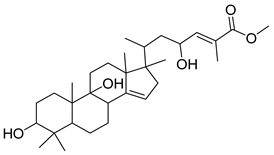 | C31H48O4 | 502 | 3 | 5 | 5.18 |
| GC3 | 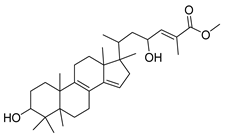 | C31H48O4 | 485 | 2 | 4 | 6.13 |
| GC4 | 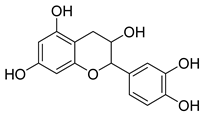 | C15H14O6 | 290 | 5 | 6 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muchtaridi, M.; Sugijanto, M.; Mohd Gazzali, A.; Wahab, H.A. Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization. Molecules 2020, 25, 821. https://doi.org/10.3390/molecules25040821
Muchtaridi M, Sugijanto M, Mohd Gazzali A, Wahab HA. Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization. Molecules. 2020; 25(4):821. https://doi.org/10.3390/molecules25040821
Chicago/Turabian StyleMuchtaridi, Muchtaridi, Milyadi Sugijanto, Amirah Mohd Gazzali, and Habibah A. Wahab. 2020. "Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization" Molecules 25, no. 4: 821. https://doi.org/10.3390/molecules25040821
APA StyleMuchtaridi, M., Sugijanto, M., Mohd Gazzali, A., & Wahab, H. A. (2020). Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization. Molecules, 25(4), 821. https://doi.org/10.3390/molecules25040821






