Abstract
The essential oil (EO) of plants of the Myrtaceae family has diverse chemical composition and several applications. However, data on the oil yield, its composition, and its complete chemistry are still unavailable for some species belonging to this family, such as Myrcia eximia DC. In this study, the chemical compositions of the EOs of Myrcia eximia were evaluated by using gas chromatography (GC) alone and gas chromatography coupled with mass spectrometry (GC–MS). Samples for both evaluations were collected from the city of Magalhães Barata, State of Pará, Brazil, in 2017 and 2018. For the plant material collected in 2017, EO was obtained by hydrodistillation (HD) only, while, for the material collected in 2018, EO was obtained by hydrodistillation and steam distillation (SD), in order to evaluate the differences in chemical composition and mass yield of the EO. The yields of (E)-caryophyllene were 15.71% and 20.0% for the samples collected by HD in 2017 and 2018, respectively, while the yield was 15.0% for the sample collected by SD in 2018. Hexanal was found to be the major constituent in the EO obtained by HD, with yield of up to 26.09%. The oil yields reached 0.08% by using SD, and 0.01% and 0.36% for the samples collected in 2017 and 2018, respectively, using HD. The results of this study provide new information about the mass yield and chemical composition of Myrcia eximia DC, and they can add value and income to traditional populations, as well as facilitate the preservation of this species.
1. Introduction
Myrtaceae is one of the most important families of the Brazilian flora, and it has representatives of significant medicinal interest [1]. This family is composed of approximately 150 genera and 4630 species, especially distributed in the tropical and subtropical regions. It is widely dispersed in the Americas and in Australia, although it is found all over the world [2]. In Brazil, there are 23 genera and approximately 1034 species present throughout the country [3].
Recent studies on essential oils (EOs) isolated from plants of the Myrtaceae family showed that they have important properties, such as insecticidal, parasiticidal, antifungal, antibacterial, antimicrobial, and antioxidant activities [4]. This demonstrates the great importance of this family with respect to the discovery of new techniques, which can solve problems in various sectors, such as health, food, and even agricultural production.
The genus Myrcia DC is the most representative of the Myrtaceae family. In Brazil, it is represented by 23 genera and 974 species [5]. Many species of Myrcia, such as M. Silvatica, M. punicifolia, and M. speciosa are used in folk medicine, usually as infusions, for treating diabetes [6,7]. Others, such as M. salicifolia and M. ovata are used in the treatment of gastric diseases, diarrhea, cold sores, and mouth ulcers [6,8]. In addition, plants of the Myrcia species are sources of EOs with antibacterial, antinociceptive, and anti-inflammatory activities [9,10]. Sesquiterpenes and monoterpenes are the most frequently found components of their EOs [6].
Some studies conducted on the EOs of Myrcia species revealed their chemical diversity; they contained a wide range of chemicals, such as β-caryophyllene, germacrene B, δ-cadinene [11], α-pinene, α-terpineol [12], caryophyllene oxide, globulol, (E)-nerolidyl acetate, ar-curcumene, δ-cadinene, and spathulenol [13]. Studies on the species Myrcia eximia DC only focused on its anatomy and taxonomy. This species, popularly known in Brazil as “goiabinha”, is geographically distributed in the northeast, midwest, and southeast regions of Brazil [14,15]. Apart from a small report on the existence of β-caryophyllene [16], there is no literature available on the yield and chemical composition of its EO. In this context, herein, we aim to analyze the mass yield of EOs of Myrcia eximia DC collected in 2017 and 2018 from the city of Magalhães Barata, northeast Pará-Brazil, Eastern Amazon. We aim to garner new information for the dissemination of knowledge related to the chemical profile of the EOs of this species.
2. Results and Discussion
2.1. Yields
Moisture contents of 9.43% and 11.95% were obtained for leaf samples of Myrcia eximia DC collected in 2017 and 2018, respectively. This variation may be related to the collection period, because the sample with the highest moisture content was that collected in the rainy season. For samples obtained by hydrodistillation (HD), yields ranged from 0.01% to 0.36% (w/w), and the sample collected in the dry period of 2017 presented the highest mass yield of EO. This yield was 0.08% (w/w) for the same sample, when collected using steam distillation (SD). Figure 1 shows the chromatograms of the EO fractions collected in 2017 and 2018. Differences in the yields of EO fractions may be associated with the extraction technique employed. Other authors studying other plants compared the extraction methods of HD and steam distillation (SD) and reported that they can influence and induce differences in mass yields and chemical compositions at the end of the extraction process [17,18,19,20,21].
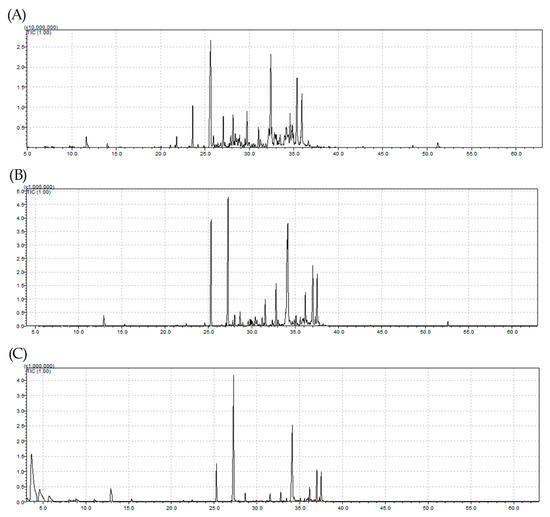
Figure 1.
Ion chromatograms of Myrcia eximia DC essential oils (Eos) injected in GC/MS: (A) sample collected in 2017 by hydrodistillation (HD), (B) sample collected in 2018 by HD, and (C) sample collected in 2018 by steam distillation (SD). The x-axis represents the retention time, while the y-axis represents the relative concentration.
2.2. Chemical Composition of the EO
The samples were quantified and identified by using gas chromatography (GC) alone and gas chromatography combined with mass spectrometry (GC–MS). In total, 93 chemical compounds were identified, and they are listed in Table 1. To obtain the EO, two different techniques were used, HD and SD. For the plant material collected in 2017, EO was obtained only by HD, while, for the material collected in 2018, EO was obtained by HD and SD.

Table 1.
Chemical composition of essential oils extracted from leaves of Myrcia eximia DC, at different periods, by hydrodistillation (HD) and steam distillation (SD).
The main classes of compounds found in the sample collected in 2017 (dry season) were aldehydes (2.38%), hydrocarbon sesquiterpenes (36.21%), oxygenated sesquiterpenes (53.41%), and other compounds (0.27%), whereas, in the sample collected in 2018 (rainy season), there was large quantitative variation with respect to the classes of compounds obtained in the 2017 sample, i.e., 40.5% aldehydes, 23% hydrocarbon sesquiterpenes, and 30.5% oxygenated sesquiterpenes, as well as other compounds (0.2%), were identified. The different collection periods influenced the composition of this specimen of M. eximia because the aldehyde content increased, while the contents of hydrocarbon sesquiterpenes and oxygenated sesquiterpenes decreased.
All the EO samples presented qualitative and quantitative variations depending on the season of collection. In the dry period, (E)-caryophyllene (15.71%), caryophyllene oxide (10.25%), 14-hydroxy-9-epi-(E)-caryophyllene (7.02%), α-cadinol (5%), allohimachalol (3.49%), caryophylla-4(12), 8(13)-dien-5-α-ol (3.31%), and α-copaene (3.25%) were obtained as the main components. In the rainy period, hexanal (26.1%), (E)-caryophyllene (20.3%), caryophyllene oxide (16.3%), (2E)-hexenal (6.63%), α-copaene (4.84%), 14-hydroxy-9-epi-(E)-caryophyllene (4.63%), and nonanal (3.24%) were obtained as the major constituents. Therefore, EOs of the same species may vary qualitatively and quantitatively in composition, depending on the location, time of the day, climate, and season of the year [24,25,26].
The chemical constituents of the EO samples obtained by HD in 2017 and 2018 were different, and 96.93% and 98.84% of their components were identified, respectively.
By comparing the chemical constituents of these oils, the differences among the molecules can be identified. For instance, there are molecules that were identified only in the 2017 material obtained by HD, such as hexanal (26.09%) and (2E)-hexenal (6.63%). The difference in chemical compositions of these oils can have a direct impact on their biological activities, as well as their industrial and food applications.
From 2018 samples, hexanal (26.09%) and (2E)-hexenal (6.63%) could be obtained as major compounds by HD. These compounds were not found in the EO of the same plant sample obtained by SD, and they were not present in the oil extracted by HD of the material collected in 2017, either. Hexanal and (2E)-hexenal have antimicrobial activity against Salmonella enteritidis, Escherichia coli, Listeria monocytogenes, and Aspergillus flavus [27,28,29]; therefore, they can be used to extend the shelf life of minimally processed foods, such as apples, which are sold to customers, on a regular basis, ready to be consumed [29].
Nonanal was identified in the EO obtained by HD of the samples collected in 2017 and 2018, with its contents being 1.28% and 3.24%, respectively. This compound was also identified in the oil extracted by SD of the 2018 sample. The presence of this substance enhances the antimicrobial activity of the EO against bacterial and fungal pathogens. The minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) against Penicillium cyclopium were investigated [30], and the results demonstrated that this volatile compound could alter the fungal hyphae morphology, leading to loss in cytoplasmic material and mycelial distortion. In addition, this substance caused severe changes in the permeability of fungal cell membranes. In this study, the authors obtained MIC = 0.3 mL/L and MFC = 0.4 mL/L, demonstrating that nonanal has suitable activity against P. cyclopium fungus. Zavala-Sánchez et al. [31] reported the antidiarrheal activity of nonanal, which showed significant inhibitory effects in mice with diarrhea induced by castor oil, magnesium sulfate, and arachidonic acid [31]. Nonanal was also used for alpha stimulation [32], and improvement of trap performance against Aedes aegypti [33].
α-Copaene was also identified, and its contents were 3.25% and 4.84% in the EO extracted from the samples collected in 2017 and 2018, respectively, by HD. The sample collected that same year was subjected to extraction by steam distillation, in which this compound was obtained in greater quantities (10.98%). This sesquiterpene has antioxidant and antigenotoxic activities [34]. There are also reports in the literature that host plants producing α-copaene are able to influence the mating of Ceratitis capitate, the male Mediterranean fruit fly [35].
Sesquiterpene (E)-caryophyllene was obtained as the major product in both the extraction methods used. When HD was used, (E)-caryophyllene contents were 15.71% and 20.27% in the 2017 and 2018 samples, respectively. When SD was used, (E)-caryophyllene content was 15.0%. (E)-Caryophyllene is a generally recognized as safe (GRAS) food cannabinoid, and its use is approved by the United States Food and Drug Administration (FDA). Its biological activities are widely reported in the literature, such as those against bacteria, [36], fungi [37], and viruses [38]. There are also reports of its anti-inflammatory [39], anticancer [40], analgesic [41], and antiphytoviral [42] activities. The analogs caryophyllene oxide and 14-hydroxy-9-epi-(E)-caryophyllene were also identified in the three extractions performed.
Caryophyllene oxide was identified in the sample oil obtained by HD with contents of 10.25% and 16.31% in 2017 and 2018, respectively. When the 2018 sample was subjected to SD, caryophyllene oxide was obtained in greater quantity (22.16%). 14-Hydroxy-9-epi-(E)-caryophyllene was also obtained from the three extractions. Its contents were 7.02% and 4.63% in the oil obtained by HD of samples collected in 2017 and 2018, respectively, and 7.84% of the EO obtained by SD of the sample collected in 2018. There are several reports in the literature on plants in which these compounds are the major components of the EOs, and these were investigated in relation to their property of inducing programmed cell death in Trypanosoma cruzi [43] and antioxidant activity [44].
α-Cadinol constituted 5.00% and 0.10% of the total EOs obtained by HD of the 2017 and 2018 samples, respectively, while the oil obtained by SD contained 0.46% of this compound. The EO of plants containing α-cadinol are reported to have cytotoxic [45], anti-tyrosinase [46], and antimicrobial activities [47].
3. Materials and Methods
3.1. Plant Material
Leaf samples of Myrcia eximia DC were collected in two different periods from the city of Magalhães Barata, Pará, Brazil. The first sample was collected during the dry season (Amazonian summer), on 12 June 2017, at geographic coordinates of 00°47′51.6″ south (S) and 047°33′38.4″ west (W). The samples were identified by Dr. Antonio Elielson Sousa da Rocha and the incorporation of an exsicata in the Herbarium of Emílio Goeldi Museum, in the city of Belém, Pará, Brazil, under the registration number MG-231868. The second sample was collected in the rainy season (Amazonian winter), on 10 March 2018, at geographic coordinates of 00°47′54.2″ S and 047°33′5.56″ W with the incorporation of an exsicata in the Herbarium of Emílio Goeldi Museum, in the city of Belém, Pará, Brazil, under the registration number MG-237469.
3.2. Preparation and Characterization of the Raw Material
The leaf samples of Myrcia eximia DC were dried in an air-circulation oven for five days, at 35 °C, and then crushed in a knife mill (Tecnal, model TE-631/3, Piracicaba/SP, Brazil) at a speed of 2251 rpm for 10 min. The moisture content was analyzed by using a moisture analyzer (model IV2500, GEHAKA, Duquesa de Goiás, Real Parque, São Paulo, Brazil).
3.3. Hydrodistillation
Hydrodistillation was performed on a Clevenger-type apparatus [48,49], using 176.29 g of the plant material collected in 2017 and 2018. The extraction period was 10,800 s with a temperature of 100 °C. After extraction, anhydrous sodium sulfate (Na2SO4) was added, and the EO was centrifuged to eliminate moisture. The mass yield of the EO was calculated on dry basis (db), by relating the oil mass obtained by HD and the dry mass used in the extraction process, according to Equation (1).
3.4. Steam Distillation
For extraction by SD [50], 100 g of MG-231868 (vegetable material collected in 2018) was used. The extraction time was 10,000 s, and the yield was calculated according to Equation (1).
3.5. Analysis of Volatile Compounds
The chemical composition of the EOs was evaluated according to a reported methodology [51], by using gas chromatography/mass spectrometry (Shimadzu, QP-2010 plus system, (City Kyoto, Japan), under the following conditions: silica capillary column Rtx-5MS (30 m × 0.25 mm, film thickness = 0.25 μm), program temperature of 60–240 °C (3 °C/min), injector temperature of 250 °C, helium as drag gas (linear velocity of 32 cm/s, measured at 100 °C), and splitless injection (1 μL of a 2:1000 hexane solution). Ionization was obtained by the electronic impact technique at 70 eV; the temperature of the ion source and other parts was 200 °C. The volatile compounds were quantified by gas chromatography using a flame ionization detector (FID) (Shimadzu, QP 2010 system), under the same conditions as GC/MS, except that nitrogen was used as the drag gas. The retention index was calculated for all the volatile constituents using a homologous series of n-alkanes (C8–C20). They were identified by comparison of their mass spectra and retention indices to those reported in the literature [22,52].
4. Conclusions
High concentrations of oxygenated sesquiterpenes were found in the EOs of Myrcia eximia DC specimens collected in 2017 and 2018, among which (E)-caryophyllene gained prominence in the chemical composition of both specimens. Aldehydes were responsible for the characterization of the 2018 sample (HD) oils, with emphasis on hexanal. Notably, hydrocarbon sesquiterpenes are commonly found in the chemical composition of EOs of the genus Myrcia, such as (E)-caryophyllene. The results of this study of M. eximia can contribute to dissemination of knowledge regarding the chemical composition of this species, which is almost incipient in the literature. As noted, important molecules were identified in the Myrcia eximia DC essential oil, which shows that this species can be a natural source of chemically active substances for a wide range of industrial applications.
Supplementary Files
Supplementary File 1Author Contributions
O.O.F. conceptualization, formal analysis, investigation, methodology, writing—original draft; J.N.d.C. and C.d.J.P.F. formal analysis, writing—original draft; S.G.S. and W.A.d.C. methodology, writing—original draft; S.G.S and W.A.d.C methodology; M.S.d.O. formal analysis, methodology, writing—original draft; E.H.d.A.A. formal analysis, supervision, visualization, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PAPQ (Programa de Apoio à Publicação Qualificada) Propesp (Edital 01/2020, PAPQ, Propesp, UFPa).
Acknowledgments
The authors thank CAPES, the Federal University of Pará, and the Paraense Museum Emílio Goeldi.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). An. Acad. Bras. Cienc. 2017, 89, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Dluzniewski, F.D.S.; Vettorato, J.G.; Ghellar Müller, N.T. Abordagem etnobotânica de Myrtaceae no município de Sete de Setembro, Rio Grande do Sul, Brasil. Rev. Interdiscip. Em Ciências Da Saúde E Biológicas – Ricsb 2018, 2, 21–31. [Google Scholar] [CrossRef]
- Santos, C.d.; Galaverna, R.S.; Angolini, C.F.F.; Nunes, V.V.A.; de Almeida, L.F.R.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Duarte, R.M.T.; Duarte, M.C.T.; Eberlin, M.N. Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three myrcia species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.P.; Alves, C.C.F.; Miranda, M.L.D.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; Martins, C.H.G.; Ambrosio, M.A.L.V.; de Souza Silva, T.; et al. Chemical composition and in vitro leishmanicidal, antibacterial and cytotoxic activities of essential oils of the Myrtaceae family occurring in the Cerrado biome. Ind. Crops Prod. 2018, 123, 638–645. [Google Scholar] [CrossRef]
- Stadnik, A.; Oliveira, M.I.U.; de Roque, N. Floristic survey of Myrtaceae in Jacobina municipality, Chapada Diamantina, Bahia State, Brazil. Hoehnea 2016, 43, 87–97. [Google Scholar] [CrossRef][Green Version]
- Cascaes, M.M.; Guilhon, G.M.S.P.; de Aguiar Andrade, E.H.; das Graças Bichara Zoghbi, M.; da Silva Santos, L. Constituents and pharmacological activities of Myrcia (Myrtaceae): A review of an aromatic and medicinal group of plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (myrtaceae) essential oil from amazonian Ecuador: A chemical characterization and bioactivity profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef]
- Cândido, C.S.; Portella, C.S.A.; Laranjeira, B.J.; da Silva, S.S.; Arriaga, A.M.C.; Santiago, G.M.P.; Gomes, G.A.; Almeida, P.C.; Carvalho, C.B.M. Effects of Myrcia ovata Cambess. essential oil on planktonic growth of gastrointestinal microorganisms and biofilm formation of Enterococcus faecalis. Brazilian J. Microbiol. 2010, 41, 621–627. [Google Scholar] [CrossRef]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; Ribeiro, A.D.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Brazilian J. Pharmacogn. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Jiménez, D.; Araque, M.; Rojas, L.; Cordero, A.; Briceño, B. Componentes volátiles y actividad antibacteriana del vástago de Myrcia splendens (Sw.) DC. Rev. la Fac. Farm. 2012, 54, 7–11. [Google Scholar]
- Do Silva, A.N.; Uetanabaro, A.P.T.; Lucchese, A.M. Chemical composition and antibacterial activity of essential oils from Myrcia alagoensis (Myrtaceae). Nat. Prod. Commun. 2013, 8, 269–271. [Google Scholar] [CrossRef]
- De Cerqueira, M.D.; Souza-Neta, L.C.; Passos, M.D.G.V.M.; Lima, E.D.O.; Roque, N.F.; Martins, D.; Guedes, M.L.S.; Cruz, F.G. Seasonal variation and antimicrobial activity of Myrcia myrtifolia essential oils. J. Braz. Chem. Soc. 2007, 18, 998–1003. [Google Scholar] [CrossRef]
- Limberger, R.P.; Sobral, M.; Henriques, A.T.; Menut, C.; Bessière, J.M. Óleos voláteis de espécies de Myrcia nativas do Rio Grande do Sul. Quim. Nova 2004, 27, 916–919. [Google Scholar] [CrossRef]
- Amaral, D.D.; Viera, I.C.G.; Salomão, R.P.; de Almeida, S.S.; Jardim, M.A.G. Checklist da Flora Arbórea de Remanescentes Florestais da Região Metropolitana de Belém, Pará, Brasil. Bol. do Mus. Para. Emilio Goeldi Ciências Nat. 2009, 4, 231–289. [Google Scholar]
- Grandtner, M.M.; Chevrette, J. Dictionary of Trees; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Maia, O.G.S.; Andrade, L.H.A. Database of the amazon aromatic plants and their essential oils. Quim. Nova. 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of Supercritical Fluid Extraction and Traditional Distillation on the Isolation of Aromatic Compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Abd El-Gaber, A.S.; El Gendy, A.N.G.; Elkhateeb, A.; Saleh, I.A.; El-Seedi, H.R. Microwave Extraction of Essential Oil from Anastatica hierochuntica (L): Comparison with Conventional Hydro-Distillation and Steam Distillation. J. Essent. Oil Bear. Plants 2018, 21, 1003–1010. [Google Scholar] [CrossRef]
- Khanavi, M.; Hadjiakhoondi, A.; Amin, G.; Amanzadeh, Y.; Rustaiyan, A.; Shafiee, A. Comparison of the Volatile Composition of Stachys persica Gmel. and Stachys byzantina C. Koch. Oils Obtained by Hydrodistillation and Steam Distillation. Zeitschrift für Naturforsch. C 2004, 59, 463–467. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.Á. CO2 -supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary ( Rosmarinus officinalis ). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database, 2nd ed.; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar]
- Silva, S.G.; Figueiredo, P.L.B.; Nascimento, L.D.; da Costa, W.A.; Maia, J.G.S.; Andrade, E.H.A. Planting and seasonal and circadian evaluation of a thymol-type oil from Lippia thymoides Mart. & Schauer. Chem. Cent. J. 2018, 12, 113. [Google Scholar] [PubMed]
- Ribeiro, A.F.; Andrade, E.H.A.; Salimena, F.R.G.; Maia, J.G.S. Circadian and seasonal study of the cinnamate chemotype from Lippia origanoides Kunth. Biochem. Syst. Ecol. 2014, 55, 249–259. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.; de Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.B.; Silva, M.P.; da Costa, W.A.; Pinto, R.H.H.; Cordeiro, R.M.; da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of bioactive compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Academic Press: Cambridge, MA, USA, 2020; pp. 149–167. [Google Scholar]
- Gardini, F.; Lanciotti, R.; Guerzoni, M.E. Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett. Appl. Microbiol. 2001, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Mazzanti, G.; Uccella, N.; Castelli, F. Study on the mechanisms of the antibacterial action of some plant alpha,beta-unsaturated aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of Hexanal, (E)-2-Hexenal, and Hexyl Acetate To Improve the Safety of Fresh-Sliced Apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Chen, S.; Zeng, L.; Wang, T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9. [Google Scholar] [CrossRef]
- Zavala-Sánchez, M.A.; Pérez-Gutiérrez, S.; Pérez-González, C.; Sánchez-Saldivar, D.; Arias-García, L. Antidiarrhoeal activity of nonanal, an aldehyde isolated from Artemisia ludoviciana. Pharm. Biol. 2002, 40, 263–268. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Choi, H.J.; Park, S.J.; Kim, S. Olfactory Stimulation Effect of Aldehydes, Nonanal, and Decanal on the Human Electroencephalographic Activity, According to Nostril Variation. Biomedicines 2019, 7, 57. [Google Scholar] [CrossRef]
- Barbosa, R.M.R.; Furtado, A.; Regis, L.; Leal, W.S. Evaluation of an oviposition-stimulating kairomone for the yellow fever mosquito, Aedes aegypti, in Recife, Brazil. J. Vector Ecol. 2010, 35, 204–207. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Tatar, A.; Geyıkoglu, F.; Hacımuftuoglu, A. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia 2014, 69, 936–942. [Google Scholar] [CrossRef]
- Shelly, T.E. Exposure to α-Copaene and α-Copaene-Containing Oils Enhances Mating Success of Male Mediterranean Fruit Flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 94, 497–502. [Google Scholar] [CrossRef]
- Pieri, F.A.; de Castro Souza, M.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef]
- Cipriano, M.; Neta, S.; Vittorazzi, C.; Guimarães, A.C.; Damasceno, J.; Martins, L.; Fronza, M.; Coutinho Endringer, D.; Scherer, R.; Guimar, A.C.; et al. Pharmaceutical Biology Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm. Biol. 2017, 55. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Pharmaceutical Biology Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spa. John M. Pezzuto Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.B.M.; Neves Selis, N.; Souza, C.L.S.; Júnior, M.N.S.; Souza, E.P.; Silva, L.S.C.d.; Souza Nascimento, F.; Amorim, A.T.; Campos, G.B.; et al. Anti-inflammatory activity of β-caryophyllene combined with docosahexaenoic acid in a model of sepsis induced by Staphylococcus aureus in mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β -caryophyllene and β -caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Vuko, E.; Rusak, G.; Dunkic, V.; Kremer, D.; Kosalec, I.; Rada, B.; Bezic, N. Inhibition of satellite RNA associated cucumber mosaic virus infection by essential oil of micromeria croatica (pers.) schott. Molecules 2019, 24, 1342. [Google Scholar] [CrossRef]
- Moreno, É.M.; Leal, S.M.; Stashenko, E.E.; García, L.T. Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide). BMC Complement. Altern. Med. 2018, 18, 225. [Google Scholar] [CrossRef]
- de Souza Araújo, C.; Paula de Oliveira, A.; Nascimento Lima, R.; Barreto Alves, P.; Coimbra Diniz, T.; Roberto Guedes da Silva Almeida, J. Chemical constituents and antioxidant activity of the essential oil from leaves of Annona vepretorum Mart. (Annonaceae). Pharmacogn. Mag. 2015, 11, 615–618. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Grandini, A.; Spagnoletti, A.; Asanza, M.; Scalvenzi, L. Cytotoxic Effect and TLC Bioautography-Guided Approach to Detect Health Properties of Amazonian Hedyosmum sprucei Essential Oil. Evidence-based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zardi-Bergaoui, A.; Jelizi, S.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Comparative study of the chemical composition and bioactivities of essential oils of fresh and dry seeds from Myoporum insulare R. Br. Ind. Crops Prod. 2018, 111, 232–237. [Google Scholar] [CrossRef]
- Ali, N.; Chhetri, B.; Dosoky, N.; Shari, K.; Al-Fahad, A.; Wessjohann, L.; Setzer, W. Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Cruz, J.N.; Gomes Silva, S.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; Teixeira, E.; da Silva, N.J.N.; de Aguiar Andrade, E.H.; de Jesus Chaves Neto, A.M.; et al. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Silva, S.G.; da Costa, R.A.; de Oliveira, M.S.; da Cruz, J.N.; Figueiredo, P.L.B.; Brasil, D.d.S.B.; Nascimento, L.D.; Chaves Neto, A.M.d.J.; de Carvalho Junior, R.N.; Andrade, E.H.d.A. Chemical profile of Lippia thymoides, evaluation of the acetylcholinesterase inhibitory activity of its essential oil, and molecular docking and molecular dynamics simulations. PLoS ONE 2019, 14, e0213393. [Google Scholar] [CrossRef]
- Lopes, N.P.; Kato, M.J.; de Aguiar Andrade, E.H.; Soares Maia, J.G.; Yoshida, M. Circadian and seasonal variation in the essential oil from Virola surinamensis leaves. Phytochemistry 1997, 46, 689–693. [Google Scholar] [CrossRef]
- Gurgel, E.S.C.; de Oliveira, M.S.; Souza, M.C.; da Silva, S.G.; de Mendonça, M.S.; Souza Filho, A.P.d.S. Chemical compositions and herbicidal (phytotoxic) activity of essential oils of three Copaifera species (Leguminosae-Caesalpinoideae) from Amazon-Brazil. Ind. Crops Prod. 2019, 142, 111850. [Google Scholar] [CrossRef]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. The NIST Mass Spectral Search Program for the Nist/Epa/Nih Mass Spectra Library; Standard Reference Data Program of the National Institute of Standards and Technology: Gaithers-burg, MD, USA, 2011. [Google Scholar]
Sample Availability: Samples of the compounds (essential oil extracted from Myrcia eximia DC) are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).