Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties †
Abstract
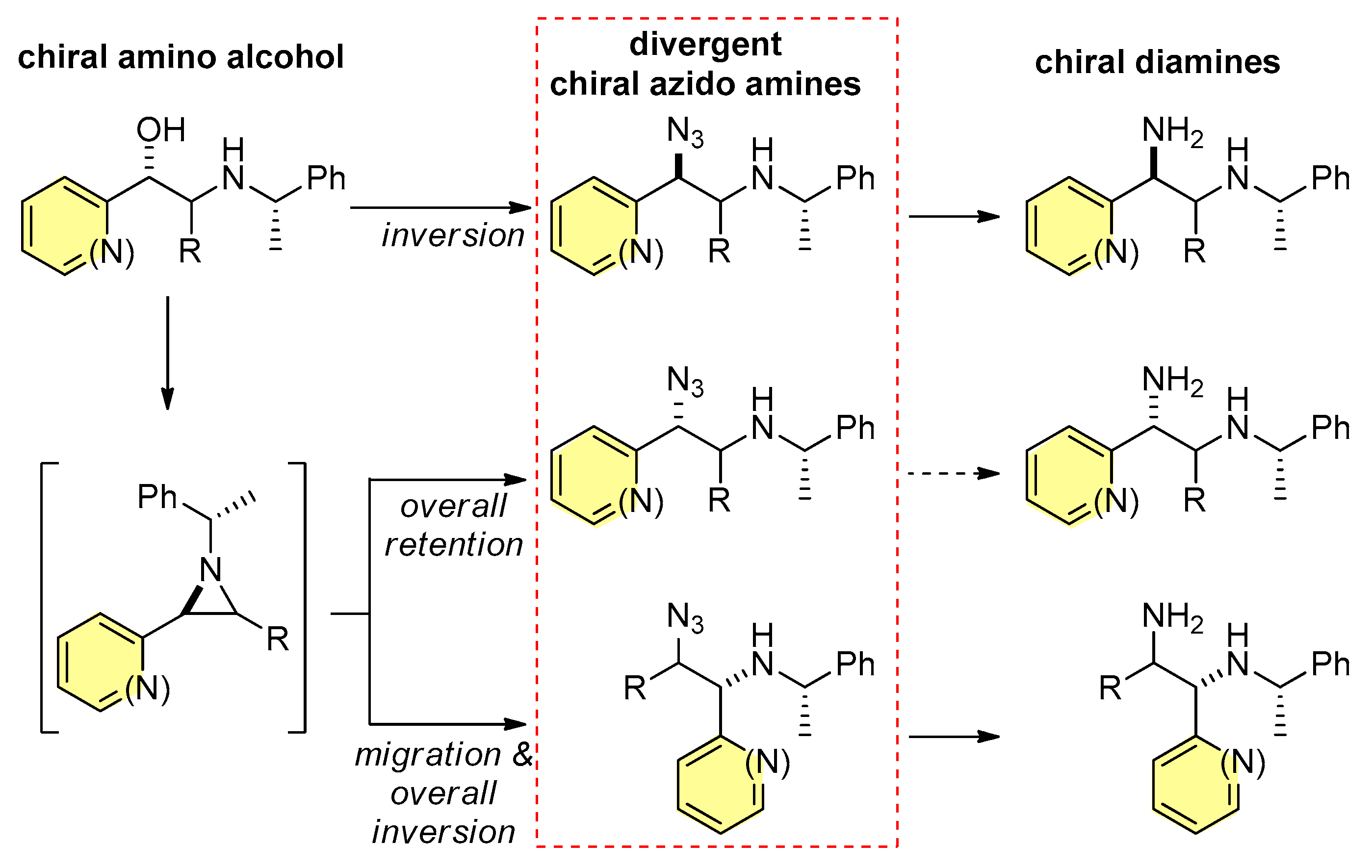
1. Introduction
2. Results and Discussion
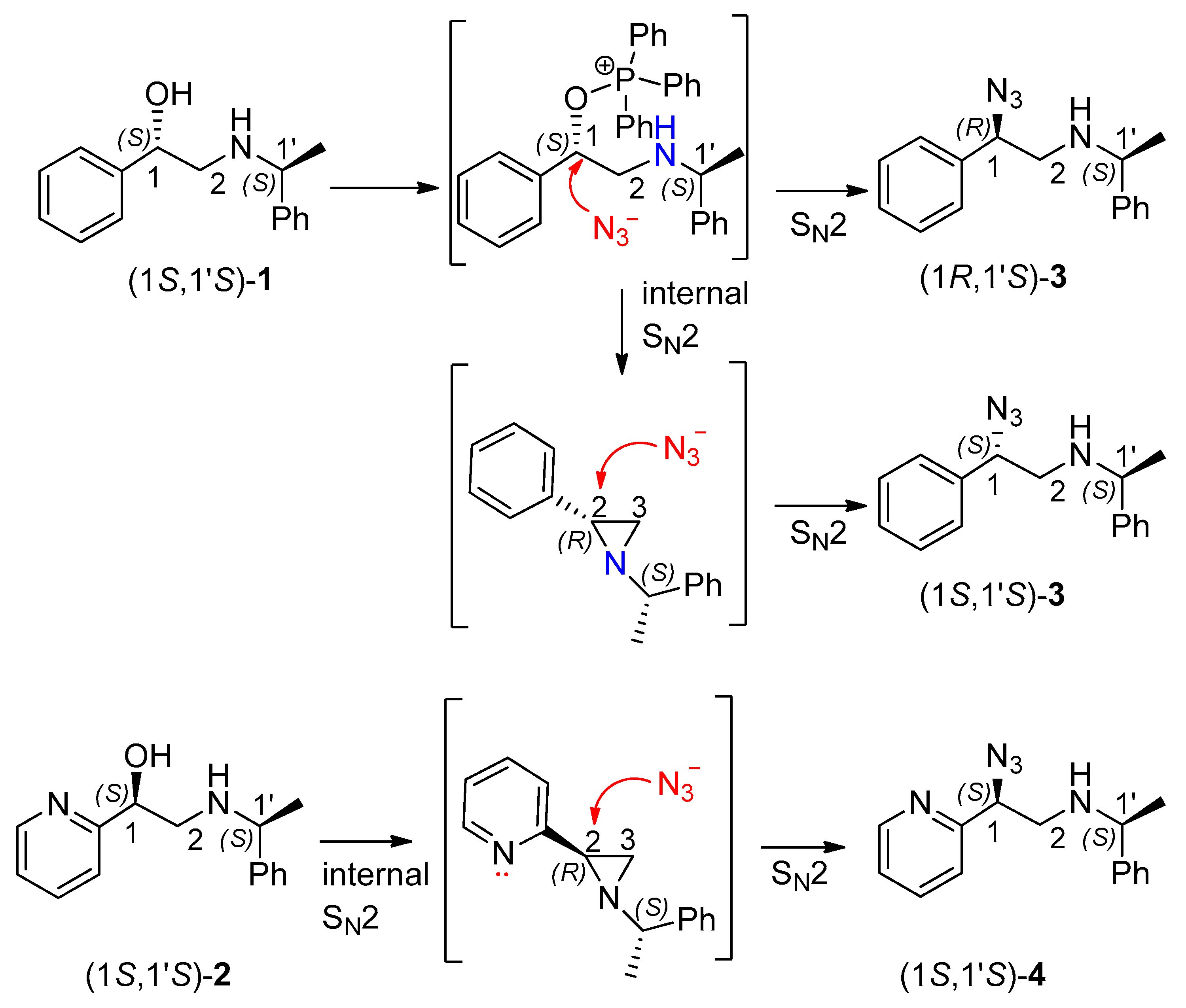
2.1. Attempted Direct Substitution of the Hydroxyl Group by the External Nucleophile
2.2. Synthesis of Aziridines
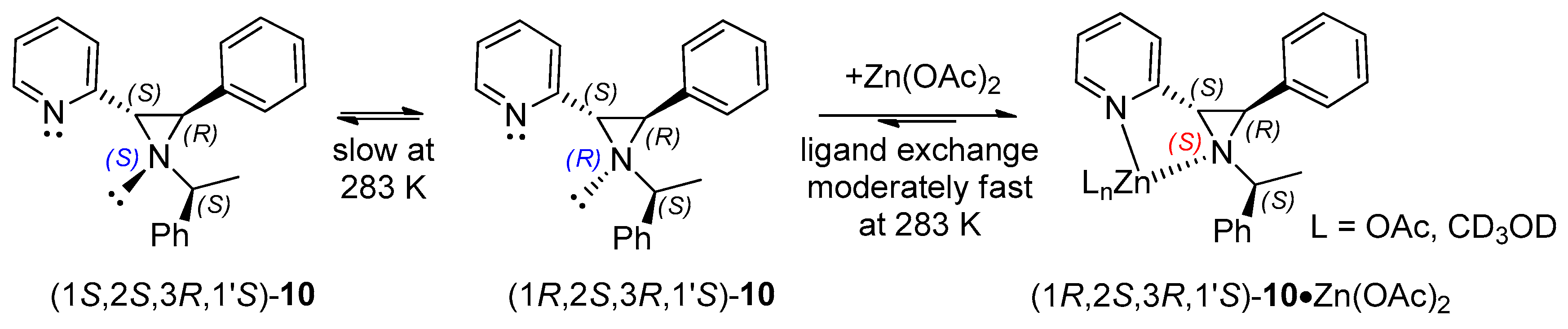
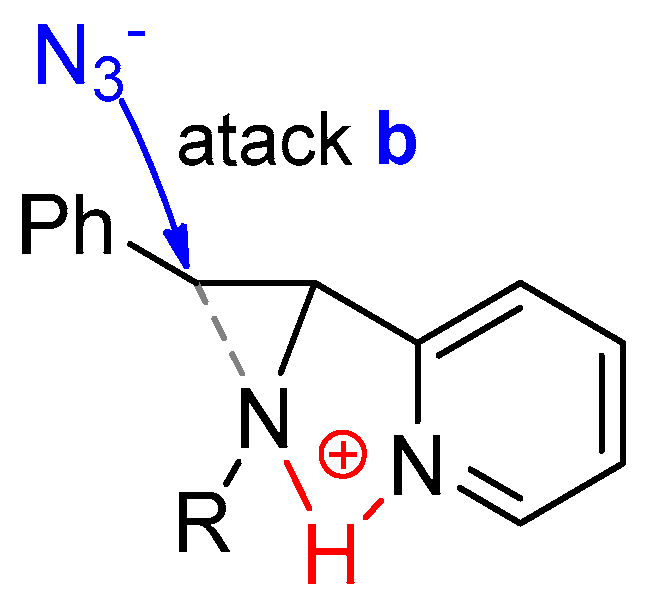
2.3. Aziridine Structural Investigation by NMR
2.4. Synthesis of Diamines
2.4.1. Synthesis of Diamines by Ring Opening of Aziridines with Hydrazoic Acid
2.4.2. Synthesis of Diamines by Nucleophilic Substitution of Sulfamidates
2.4.3. Reduction of Azido Amines to Vic-Diamines
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of Aziridines
3.3. General Procedure for the Synthesis of Cyclic Sulfamidates
3.4. Synthesis of Azides
3.5. General Procedure for the Synthesis of Diamines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakano, H.; Owolabi, I.A.; Chennapuram, M.; Okuyama, Y.; Kwon, E.; Seki, C.; Tokiwa, M.; Takeshita, M. β-Amino Alcohol Organocatalysts for Asymmetric Additions. Heterocycles 2018, 97, 647–667. [Google Scholar] [CrossRef]
- Reddy, U.V.S.; Chennapuram, M.; Seki, C.; Kwon, E.; Okuyama, Y.; Nakano, H. Catalytic Efficiency of Primary β-Amino Alcohols and Their Derivatives in Organocatalysis. Eur. J. Org. Chem. 2016, 4124–4143. [Google Scholar] [CrossRef]
- Kacprzak, K.M. Chemistry and Biology of Cinchona Alkaloids. In Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 605–641. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Sargsyan, E. Ephedra Alkaloids-Alkaloids Derived by Amination Reaction: Phenylalanine Derived. In Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 909–922. [Google Scholar] [CrossRef]
- Wosińska-Hyrydczuk, M.; Skarżewski, J. 2-Oxiranyl-pyridines: Synthesis and Regioselective Epoxide Ring Openings with Chiral Amines as a Route to Chiral Ligands. Heteroatom Chem. 2019, 2381208. [Google Scholar] [CrossRef]
- Shao, X.; Li, K.; Malcolmson, S.J. Enantioselective Synthesis of anti-1,2-Diamines by Cu-Catalyzed Reductive Couplings of Azadienes with Aldimines and Ketimines. J. Am. Chem. Soc. 2018, 140, 7083–7087. [Google Scholar] [CrossRef]
- Métro, T.X.; Gomez, P.D.; Cossy, J. Highly Enantioselective Synthesis of Linear β-Amino Alcohols. Chem. Eur. J. 2009, 15, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Métro, T.-X.; Duthion, B.; Pardo, D.G.; Cossy, J. Rearrangement of β-amino alcohols via aziridiniums: A review. Chem. Soc. Rev. 2010, 39, 89–102. [Google Scholar] [CrossRef]
- Stankovic, S.; D’hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Speybroeck, V.; Kimpe, N.D.; Ha, H.-J. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.-J.; Jung, J.-H.; Lee, W.K. Application of Regio- and Stereoselective Functional Group Transformations of Chiral Aziridine-2-carboxylates. Asian J. Org. Chem. 2014, 3, 1020–1035. [Google Scholar] [CrossRef]
- Panday, S.K. Advances in the Mitsunobu Reaction: An Excellent Organic Protocol with Versatile Applications. Org. Chem. 2019, 16, 127–140. [Google Scholar] [CrossRef]
- Fletcher, S. The Mitsunobu reaction in the 21st century. Org. Chem. Front. 2015, 2, 739–752. [Google Scholar] [CrossRef]
- Hughes, D.L. The Mitsunobu Reaction. Org. React. 1992, 42, 335. [Google Scholar] [CrossRef]
- Savoia, D.; Alvaro, A.; Fabio, R.D.; Gualandi, A. Asymmetric Route to Pyridines Bearing a Highly Functionalized 2-Alkyl Substituent by Aziridine Ring-Opening Reactions. J. Org. Chem. 2007, 72, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Alker, D.; Doyle, K.J.; Harwood, L.M.; McGregor, A. The direct synthesis of the cyclic sulfamidate of (S)-Prolinol: Simultaneous N-Protection and activation towards nucleophilic displacement of oxygen. Tetrahedr. Asymmetry 1990, 1, 877–880. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Spivey, A.C.; Schofield, C.J. Cyclic sulfamidates: New synthetic precursors for/8-functionalized a-amino acids. Tetrahedr. Asymmetry 1990, 1, 881–884. [Google Scholar] [CrossRef]
- White, G.J.; Garst, M.E. Cyclic sulfamate from N-substituted-2-amino-3-phenyl-2-propanol and its nucleophilic reactions. J. Org. Chem. 1991, 56, 3177–3178. [Google Scholar] [CrossRef]
- Skarżewski, J.; Gupta, A. Synthesis of C2 symmetric primary vicinal diamines. Double stereospecific Mitsunobu reaction on the heterocyclic diols derived from tartaric acid. Tetrahedr. Asymmetry 1997, 8, 1861–1867. [Google Scholar] [CrossRef]
- Loibner, H.; Zbiral, E. Reaktionen mit phosphororganischen Verbindungen. XLI. Neuartige synthetische Aspekte des Systems Triphenylphosphin-Azodicarbonsäureester-Hydroxyverbindung. Helv. Chim. Acta 1976, 59, 2100–2113. [Google Scholar] [CrossRef]
- Sweeney, J.B. Aziridines: Epoxides ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef]
- Sweeney, J.B. Synthesis of Aziridines. In Aziridines and Epoxides in Organic Synthesis; Yudin, A.K., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Olofsson, B.; Wijtmans, R.; Somfai, P. Synthesis of N-H vinylaziridines: A comparative study. Tetrahedron 2002, 58, 5979–5982. [Google Scholar] [CrossRef]
- Olivo, H.F.; Hemenway, M.S.; Hartwig, A.C.; Chan, R. New Preparation of Activated 2-Vinylaziridines from 1,4-Aminoalcohols. Synlett 1998, 247–248. [Google Scholar] [CrossRef]
- Testa, L.; Akssira, M.; Zaballos-Garcıa, E.; Arroyo, P.; Domingo, L.R.; Sepu’lveda-Arques, J. Experimental and theoretical investigations for the regio and stereoselective transformation of trans 1,2,3-trisubstituted aziridines into trans oxazolidin-2-ones. Tetrahedron 2003, 59, 677–683. [Google Scholar] [CrossRef]
- Sternativo, S.; Marini, F.; Verme, F.-D.; Calandriello, A.; Testaferri, L.; Tiecco, M. One-pot synthesis of aziridines from vinyl selenones and variously functionalized. Tetrahedron 2010, 66, 6851–6857. [Google Scholar] [CrossRef]
- Falorni, M.; Lardicc, L. Alkylmetal Asymmetric Reduction. 18.1 Starting Materials in the Preparation of New Chiral Reducing Agents: Synthetic Approach to Primary Alkyl Halides Derived from (+)-Camphor. J. Org. Chem. 1986, 51, 5291–5294. [Google Scholar] [CrossRef]
- Stephens, D.; Zhang, Y.; Cormier, M.; Chavez, G.; Arman, H.; Larionov, O.-V. Three-component reaction of small-ring cyclic amines with arynes and acetonitrile. Chem. Commun. 2013, 49, 6558–6560. [Google Scholar] [CrossRef] [PubMed]
- Drakenberg, T.; Lehn, J.M. Nuclear magnetic resonance studies of rate processes and conformations. Part XX. Nitrogen inversion in the gas phase. J. Chem Soc. Perkin II 1972, 532–535. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.W.; Shipman, M.; Tucker, J.H.R.; Walsh, T.R. Control of pyramidal inversion rates by redox switching. J. Am. Chem. Soc. 2006, 128, 14260–14261. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.W.; Clarke, A.J.; Clarkson, G.J.; Shipman, M.; Tucker, J.H.R. Umbrella motion in aziridines: Use of simple chemical inputs to reversibly control the rate of pyramidal inversion. Chem. Commun. 2007, 5078–5080. [Google Scholar] [CrossRef]
- Fiore, K.; Martelli, G.; Monari, M.; Savoia, D. Design and synthesis of enantiopure 1-[1(S)-(2-pyridyl)alkyl]-2(R)-isopropylaziridines, new ligands for asymmetric catalysis. Tetrahedr. Asymmetry 1999, 10, 4803–4810. [Google Scholar] [CrossRef]
- Savoia, D.; Alvaro, G.; Di Fabio, R.; Fiorelli, C.; Gualandi, A.; Monari, M.; Piccinelli, F. Highly diastereoselective synthesis of 2,6-di[1-(2-alkylaziridin-1-yl)alkyl]pyridines, useful ligands in palladium-catalyzed asymmetric allylic alkylation. Adv. Synth. Catal. 2006, 348, 1883–1893. [Google Scholar] [CrossRef]
- Niu, J.-L.; Wang, M.C.; Kong, P.-P.; Chen, Q.-T.; Zhu, Y.; Song, M.-P. Origin of enantioselectivity with heterobidentate sulfide-tertiary amine (sp3) ligands in palladium-catalyzed allylic substitution. Tetrahedron 2009, 65, 8869–8878. [Google Scholar] [CrossRef]
- Bartnik, R.; Lesniak, L. Dibromo[α-(tert-butyl-1 aziridinyl-2)benzylideneamine]zinc(II), [ZnBr2(C13H18N2)] (1), et Dibromo[α-(tert-butyl-1 aziridinyl-2)benzylamine zinc(II), [ZnBr2(C13H20N2)] (2). Acta Cryst. 1983, C39, 1034–1036. [Google Scholar] [CrossRef]
- Bánai, I. Dynamic NMR for coordination chemistry. New J. Chem. 2018, 42, 7569–7581. [Google Scholar] [CrossRef]
- Polat-Cakir, S.; Beksultanova, N.; Dogan, O. Synthesis of Functionalized Novel α-Amino-β-alkoxyphosphonates through Regioselective Ring Opening of Aziridine-2-phosphonates. Helv. Chim. Acta 2019, 102, e1900199. [Google Scholar] [CrossRef]
- Van Dort, M.E.; Jung, Y.-W.; Sherman, P.S.; Kilbourn, M.R.; Wieland, D.M. Fluorine for Hydroxy Substitution in Biogenic Amines: Asymmetric Synthesis and Biological Evaluation of Fluorine-18-Labeled -Fluorophenylalkylamines as Model Systems. J. Med. Chem. 1995, 38, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.C.; Maria, E.J.; Suárez Ordóñeza, R.M.; Thierry, J.; Cariou, K.; Dodd, R.H. Synthesis of Orthogonally N-Protected, C-4 Functionalized Cyclic Guanidines from l-Serine. Synlett 2017, 28, 815–818. [Google Scholar] [CrossRef]
- Wang, Z.-P.A.; Tiana, C.-L.; Zheng, J.-S. The recent developments and applications of the traceless-Staudinger reaction in chemical biology study. RSC Adv. 2015, 5, 107192–107199. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 20–24 are available from the authors. |


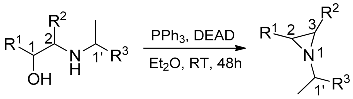
| Configuration of Amino Alcohol | R1 | R2 | R3 | Configuration of Products | Yield a (%) |
|---|---|---|---|---|---|
| (1R,1′S)-1 | Ph | H | Ph | (2S,1′S)-8 | 76 |
| (1S,1′S)-1 | Ph | H | Ph | (2R,1′S)-8 | 72 |
| (1S,1′S)-2 | Py | H | Ph | (2R,1′S)-9 | 45, 82 b |
| (1S,2S,1′S)-5 | Py | Ph | Ph | (2R,3S,1′S)-10 | 33, 74 b |
| (1R,2R,1′S)-5 | Py | Ph | Ph | (2S,3R,1′S)-10 | 33, 76 b |
| (1R,2R,1′R)-6 | Py | Ph | cyclohexyl | (2S,3R,1′R)-11 | 45 |
| (1S,2S,1′S)-7 | Bpy | Ph | Ph | (2R,3S,1′S)-12 | 65 |
| (1R,2R,1′S)-7 | Bpy | Ph | Ph | (2S,3R,1′S)-12 | 70 |
| Signal | DFT δ, ppm | Experiment δ, ppm | ||
|---|---|---|---|---|
| (1SN)-10 | (1RN)-10 | Major | Minor | |
| H-2 | 3.16 | 3.74 | 3.27 | 3.52 |
| H-3 | 4.20 | 3.49 | 3.97 | 3.49 |
| 1-CH | 4.75 | 3.10 | 3.91 | 3.12 |
| CH3 | 1.59 | 1.46 | 1.50 | 1.44 |
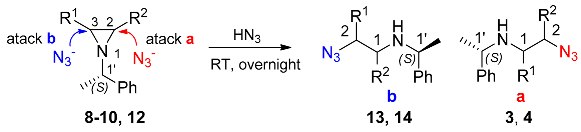
| Configuration of Aziridines | R1 | R2 | Configuration of Azides | Yield of Azides (%) |
|---|---|---|---|---|
| (2S,1′S)-8 | H | Ph | (1R,1′S)-3 | 97 |
| (2R,1′S)-8 | H | Ph | (1S,1′S)-3 | 90 |
| (1R,1′S)-9 | H | Py | (2S,1′S)-4 | 93 a |
| (2R,3S,1′S)-10 | Ph | Py | (1R,2R,1′S)-13 | 95 |
| (2S,3R,1′S)-12 | Ph | Bpy | (1S,2S,1′S)-14 | 94 |

| Configuration of Azides | R1 | R2 | Configuration of Diamines | Yield of Diamines (%) |
|---|---|---|---|---|
| (1R,1′S)-3 | Ph | H | (1R,1′S)-20 | 74 |
| (1S,1′S)-4 | Py | H | (1S,1′S)-21 | 75 |
| (1R,2R,1′S)-13 | Ph | Py | (1R,2R,1′S)-22 | 60 |
| (1S,2S,1′S)-14 | Ph | Bpy | (1S,2S,1′S)-23 | 50 |
| (1R,2S,1′S)-19 | Py | Ph | (1R,2S,1′S)-24 | 70 |
| (1S,2R,1′S)-19 | Py | Ph | (1S,2R,1′S)-24 | 73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wosińska-Hrydczuk, M.; Boratyński, P.J.; Skarżewski, J. Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties. Molecules 2020, 25, 727. https://doi.org/10.3390/molecules25030727
Wosińska-Hrydczuk M, Boratyński PJ, Skarżewski J. Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties. Molecules. 2020; 25(3):727. https://doi.org/10.3390/molecules25030727
Chicago/Turabian StyleWosińska-Hrydczuk, Marzena, Przemysław J. Boratyński, and Jacek Skarżewski. 2020. "Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties" Molecules 25, no. 3: 727. https://doi.org/10.3390/molecules25030727
APA StyleWosińska-Hrydczuk, M., Boratyński, P. J., & Skarżewski, J. (2020). Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties. Molecules, 25(3), 727. https://doi.org/10.3390/molecules25030727








