Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water
Abstract
1. Introduction
2. Results and Discussion
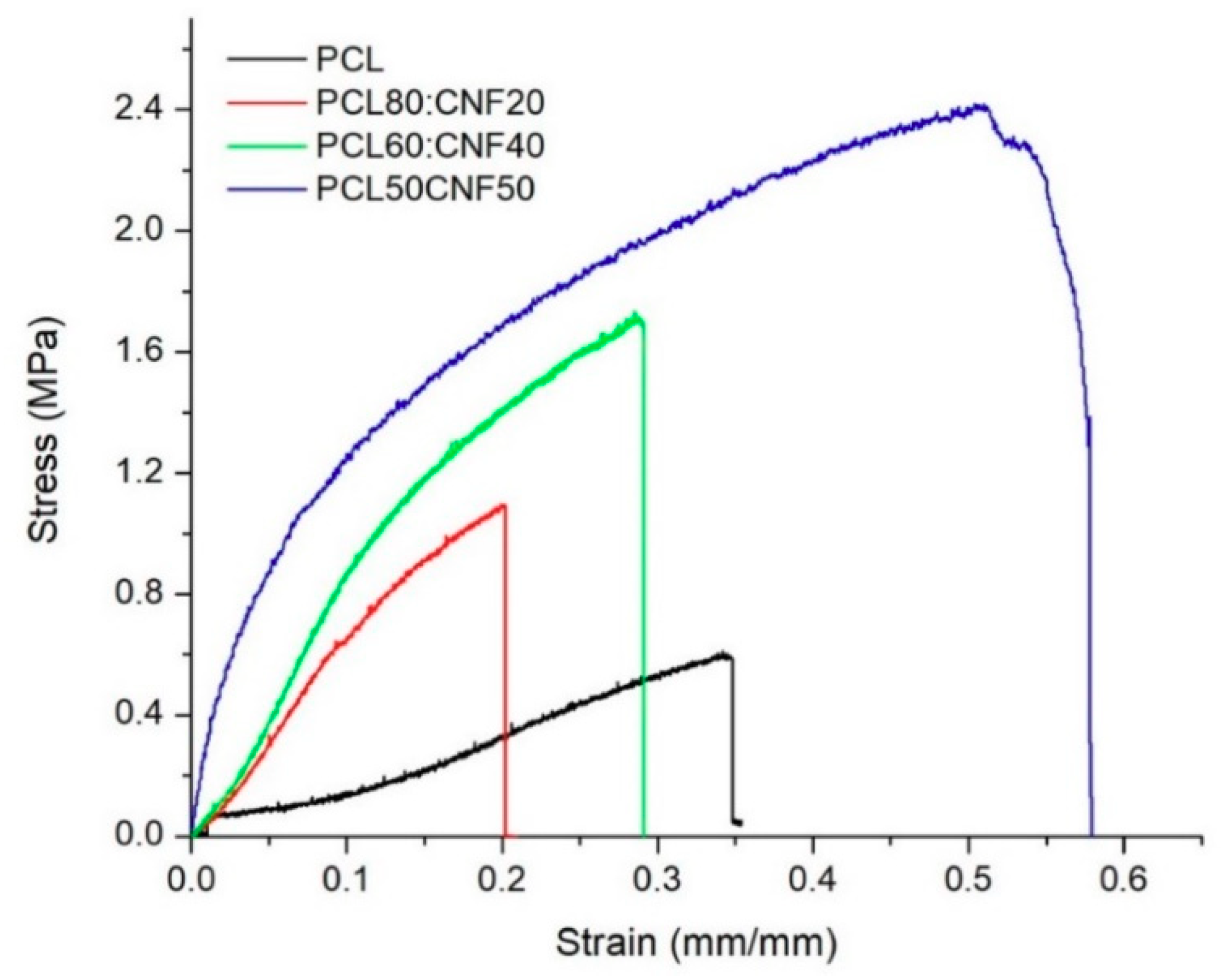
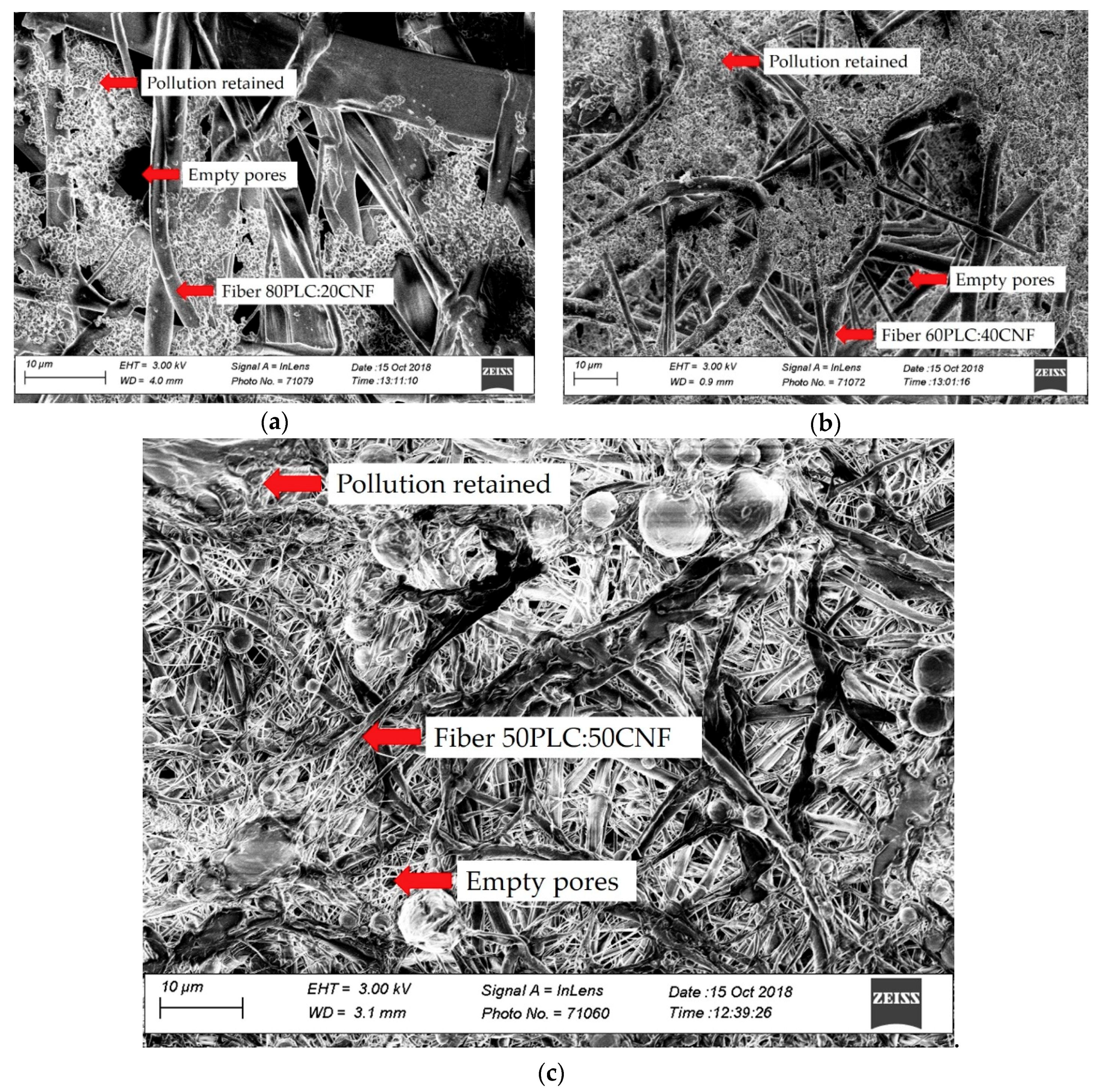
2.1. Characterization of the Electro-spun Agave Bagasse Membranes
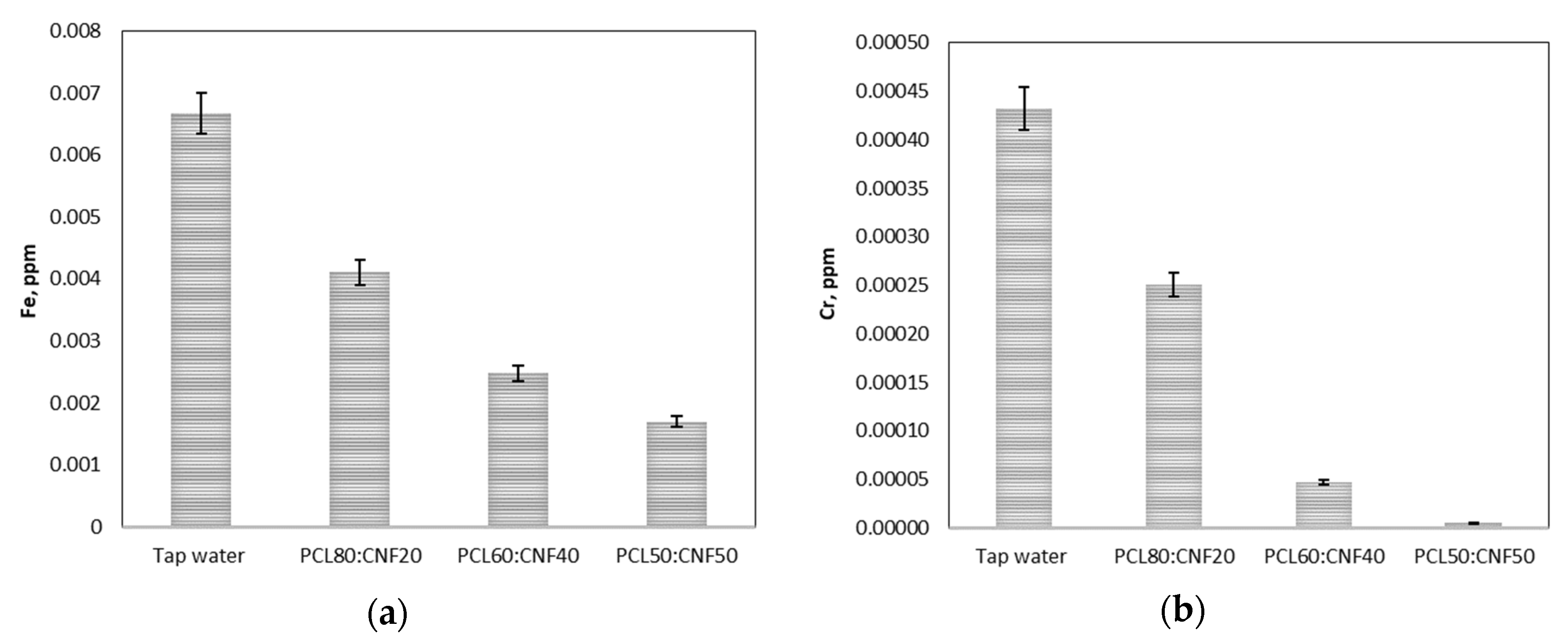
2.2. Filtration Performance of the PCL:CNF Composite Electro-spun Membranes
3. Materials and Methods
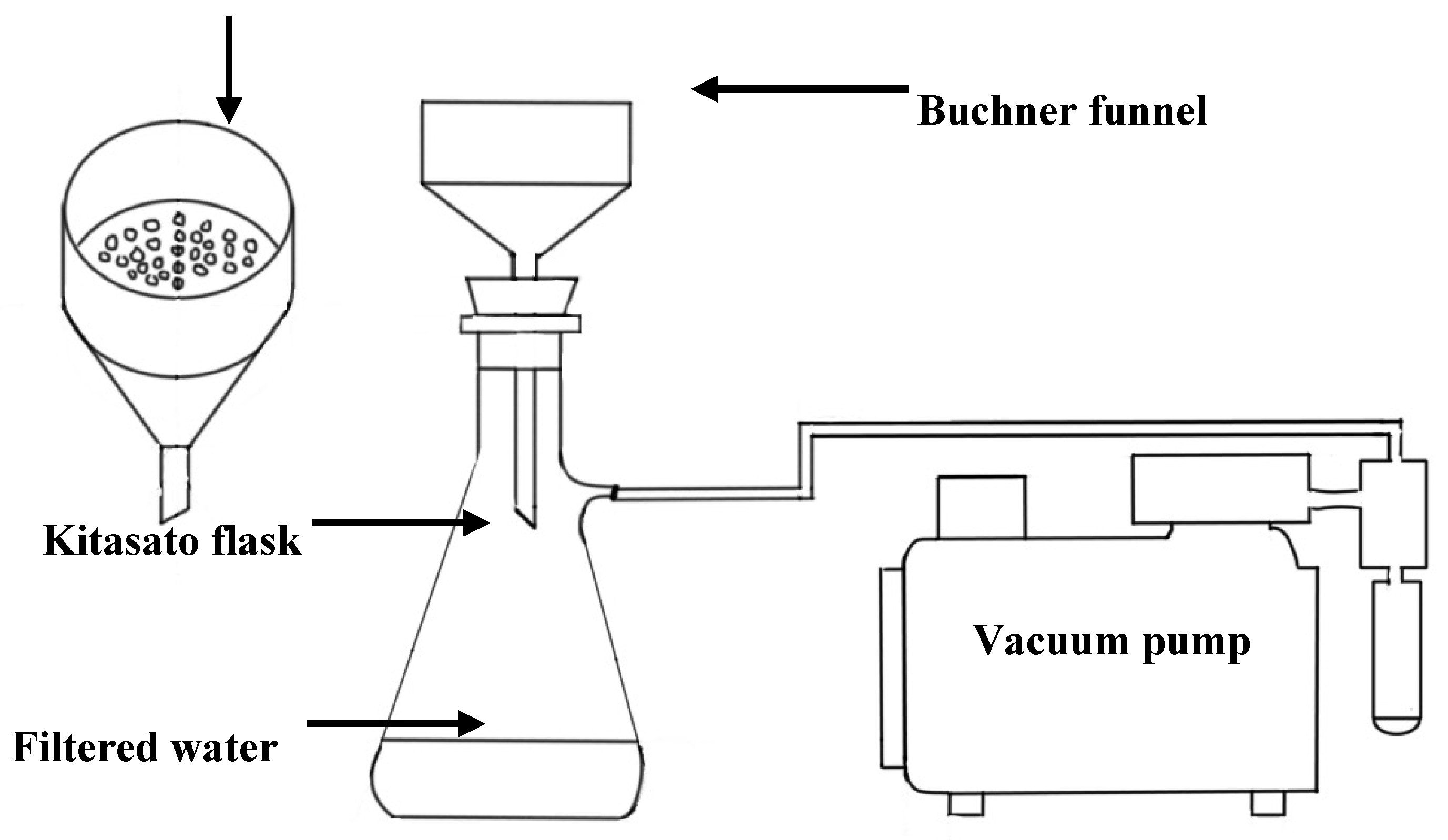
3.1. Preparation of Membranes by Electro-Spinning
3.2. Characterization of the Obtained Membranes
3.3. Filtration Performance of the PCL:CNF Composite Electro-Spun Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nation Development Programme. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals.html (accessed on 14 December 2019).
- Garcia-Alonso, J.A.; Zurita-Martinez, F.; Guzmán-González, C.A.; Del Real-Olvera, J.; Sulbarán-Rangel, B. Nanostructured diatomite and its potential for the removal of an antibiotic from water. Bioinspir. Biomimet. 2018, 7, 167–173. [Google Scholar] [CrossRef]
- Tejeda, A.; Torres-Bojorges, A.X.; Zurita, F. Carbamazepine removal in three pilot-scale hybrid wetlands planted with ornamental species. Ecol. Eng. 2017, 98, 410–417. [Google Scholar] [CrossRef]
- Bonilla-Lemus, P.; Ramírez-Bautista, G.A.; Zamora-Muñoz, C.; Ibarra-Montes, M.D.R.; Ramírez-Flores, E.; Hernández-Martínez, M.D. Acanthamoeba spp. in domestic tap water in houses of contact lens wearers in the metropolitan area of Mexico City. Exp. Parasitol. 2010, 126, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choo, G.; Kim, H.; Oh, J.-E. Evaluation of the current contamination status of PFASs and OPFRs in South Korean tap water associated with its origin. Sci. Total Environ. 2018, 634, 1505–1512. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.-F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Palacios, H.H.; Hernandez, D.J.A.; Esquivel, A.M.; Toriz, G.; Rojas, O.J.; Sulbaran-Rangel, B.C. Isolation and Characterization of Nanofibrillar Cellulose from Agave tequilana Weber Bagasse. Adv. Mater. Sci. Eng. 2019, 2019, 7. [Google Scholar] [CrossRef]
- Hernández, J.A.; Romero, V.H.; Escalante, A.; Toriz, G.; Rojas, O.J.; Sulbarán, B. Agave tequilana Bagasse as Source of Cellulose Nanocrystals via Organosolv Treatment. BioResources 2018, 13, 3603–3614. [Google Scholar] [CrossRef]
- Robles, E.; Fernández-Rodríguez, J.; Barbosa, A.M.; Gordobil, O.; Carreño, N.L.V.; Labidi, J. Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. Carbohydr. Polym. 2018, 183, 294–302. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Gatti, I.A.; Parra-Saldivar, R.; Dallemand, J.-F.; Rittmann, B.E.; Iqbal, H.M.N. Biotechnological revalorization of Tequila waste and by-product streams for cleaner production—A review from bio-refinery perspective. J. Clean. Prod. 2018, 172, 3713–3720. [Google Scholar] [CrossRef]
- National Regulator Council for Tequila Industry. Available online: https://www.crt.org.mx/ (accessed on 5 June 2019).
- Gobeille, A.; Yavitt, J.; Stalcup, P.; Valenzuela, A. Effects of soil management practices on soil fertility measurements on Agave tequilana plantations in Western Central Mexico. Soil Tillage Res. 2006, 87, 80–88. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jiménez, J.F.; del Real, J.I.; Salcedo, E.; Zamora, J.F.; Íñiguez, G. Utilización de subproductos de la industria tequilera. Parte 11. Compostaje de bagazo de agave crudo y biosólido provenientes de una planta de tratamiento de vinazas tequileras. Rev. Int. Contam. Ambient. 2013, 29, 303–313. [Google Scholar]
- Torres-Tello, E.V.; Robledo-Ortíz, J.R.; González-García, Y.; Pérez-Fonseca, A.A.; Jasso-Gastinel, C.F.; Mendizábal, E. Effect of agave fiber content in the thermal and mechanical properties of green composites based on polyhydroxybutyrate or poly(hydroxybutyrate-co-hydroxyvalerate). Ind. Crops Prod. 2017, 99, 117–125. [Google Scholar] [CrossRef]
- Langhorst, A.E.; Burkholder, J.; Long, J.; Thomas, R.; Kiziltas, A.; Mielewski, D. Blue-agave fiber-reinforced polypropylene composites for automotive applications. BioResources 2018, 13, 820–835. [Google Scholar] [CrossRef]
- Íñiguez, G.; Valadez, A.; Manríquez, R.; Moreno, M.V. Utilization of by-products from the tequila industry. Part 10: Characterization of different decomposition stages of Agave tequilana webber bagasse using FTIR spectroscopy, thermogravimetric analysis and scanning electron microscopy. Rev. Int. Contam. Ambient. 2011, 27, 61–74. [Google Scholar]
- Ramírez-Cortina, C.R.; Alonso-Gutiérrez, M.S.; Rigal, L. Valorización de residuos agroindustriales del tequila para alimentación de rumiantes. Rev. Chapingo Ser. Cienc. For. Ambiente 2012, 18, 449–457. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Vargas-Tah, A.; López-Ortega, K.M.; Medina-López, Y.N.; Mendoza-Pérez, J.A.; Avila, S.; Singh, S.; Simmons, B.A.; Loaces, I.; Martinez, A. Sequential enzymatic saccharification and fermentation of ionic liquid and organosolv pretreated agave bagasse for ethanol production. Bioresour. Technol. 2017, 225, 191–198. [Google Scholar] [CrossRef]
- Annandarajah, C.; Li, P.; Michel, M.; Chen, Y.; Jamshidi, R.; Kiziltas, A.; Hoch, R.; Grewell, D.; Montazami, R. Study of Agave Fiber-Reinforced Biocomposite Films. Materials 2019, 12, 99. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Valdez-Fausto, E.M.; Rentería-Urquiza, M.; Jiménez-Amezcua, R.M.; Anzaldo Hernández, J.; Torres-Rendon, J.G.; García Enriquez, S. Study of green nanocomposites based on corn starch and cellulose nanofibrils from Agave tequilana Weber. Carbohydr. Polym. 2018, 201, 9–19. [Google Scholar] [CrossRef]
- Pech-Cohuo, S.-C.; Canche-Escamilla, G.; Valadez-González, A.; Fernández-Escamilla, V.V.A.; Uribe-Calderon, J. Production and Modification of Cellulose Nanocrystals from Agave tequilana Weber Waste and Its Effect on the Melt Rheology of PLA. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Gunasekaran, S. Electrospun plant mucilage nanofibers as biocompatible scaffolds for cell proliferation. Int. J. Biol. Macromol. 2018, 115, 1218–1224. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Preparation of various nanofiber layers using wire electrospinning system. Arab. J. Chem. 2019, 12, 5162–5172. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Massara, T.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef]
- Molodkina, L.; Kolosova, D.; Leonova, E.; Kudoyarov, M.; Patrova, M.; Vedmetskii, Y. Track membranes in post-treatment of domestic wastewater. Pet. Chem. 2012, 52, 487–493. [Google Scholar] [CrossRef]
- Goncharuk, V. Drinking Water: Factors Affecting the Quality of Drinking Water. In Drinking Water: Physics, Chemistry and Biology; Goncharuk, V.V., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 105–245. [Google Scholar]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 385–392. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Vega Erramuspe, I.; Peresin, M. Natural polymers as alternative adsorbents and treatment agents for water remediation. BioResourses 2019, 14, 10093–10160. [Google Scholar] [CrossRef]
- Pervez, N.M.; Stylios, K.G. An Experimental Approach to the Synthesis and Optimisation of a ‘Green’ Nanofibre. Nanomaterials 2018, 8, 383. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Hruza, J. Effect of Laminating Pressure on Polymeric Multilayer Nanofibrous Membranes for Liquid Filtration. Nanomaterials 2018, 8, 272. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, R.; Huang, X.; Wang, X.; Tang, S. Fabrication and biocompatibility of agarose acetate nanofibrous membrane by electrospinning. Carbohydr. Polym. 2018, 197, 237–245. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Correa, E.; Moncada, M.E.; Zapata, V.H. Electrical characterization of an ionic conductivity polymer electrolyte based on polycaprolactone and silver nitrate for medical applications. Mater. Lett. 2017, 205, 155–157. [Google Scholar] [CrossRef]
- Hamad, A.; Hassouna, M.; Shalaby, T.; Elkady, M.; Abd Elkawi, M.; Hamad, H. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Hamad, H.; Abd El-latif, M.; Kashyout, A.; Sadik, W.; Feteha, M. Optimizing the preparation parameters of mesoporous nanocrystalline titania and its photocatalytic activity in water: Physical properties and growth mechanisms. Process Saf. Environ. Prot. 2015, 98, 390–398. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.; Maldonado-Hódar, F. Physicochemical properties of new cellulose-TiO2 composites for the removal of water pollutants: Developing specific interactions and performances by cellulose functionalization. J. Environ. Chem. Eng. 2018, 6, 5032–5041. [Google Scholar] [CrossRef]
- World Health Organization. Water Quality and Health-Review of Turbidity: Information for Regulators and Water Suppliers (No. WHO/FWC/WSH/17.01); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Domínguez, A.; Couto, S.R.; Sanroman, M.A. Dye decolorization by Trametes hirsuta immobilized into alginate beads. World J. Microbiol. Biotechnol. 2005, 21, 405–409. [Google Scholar] [CrossRef]
- Astuti, N.; Wibowo, N.; Ayub, M.R.S.S.N. The porosity calculation of various types of paper using image analysis. J. Pendidik. Fisika Indones. 2018, 14, 46–51. [Google Scholar] [CrossRef]
- Technical Association of the Pulp Paper (TAPPI). Industry TAPPI Test Methods; TAPPI: Atlanta, GA, USA, 1988. [Google Scholar]
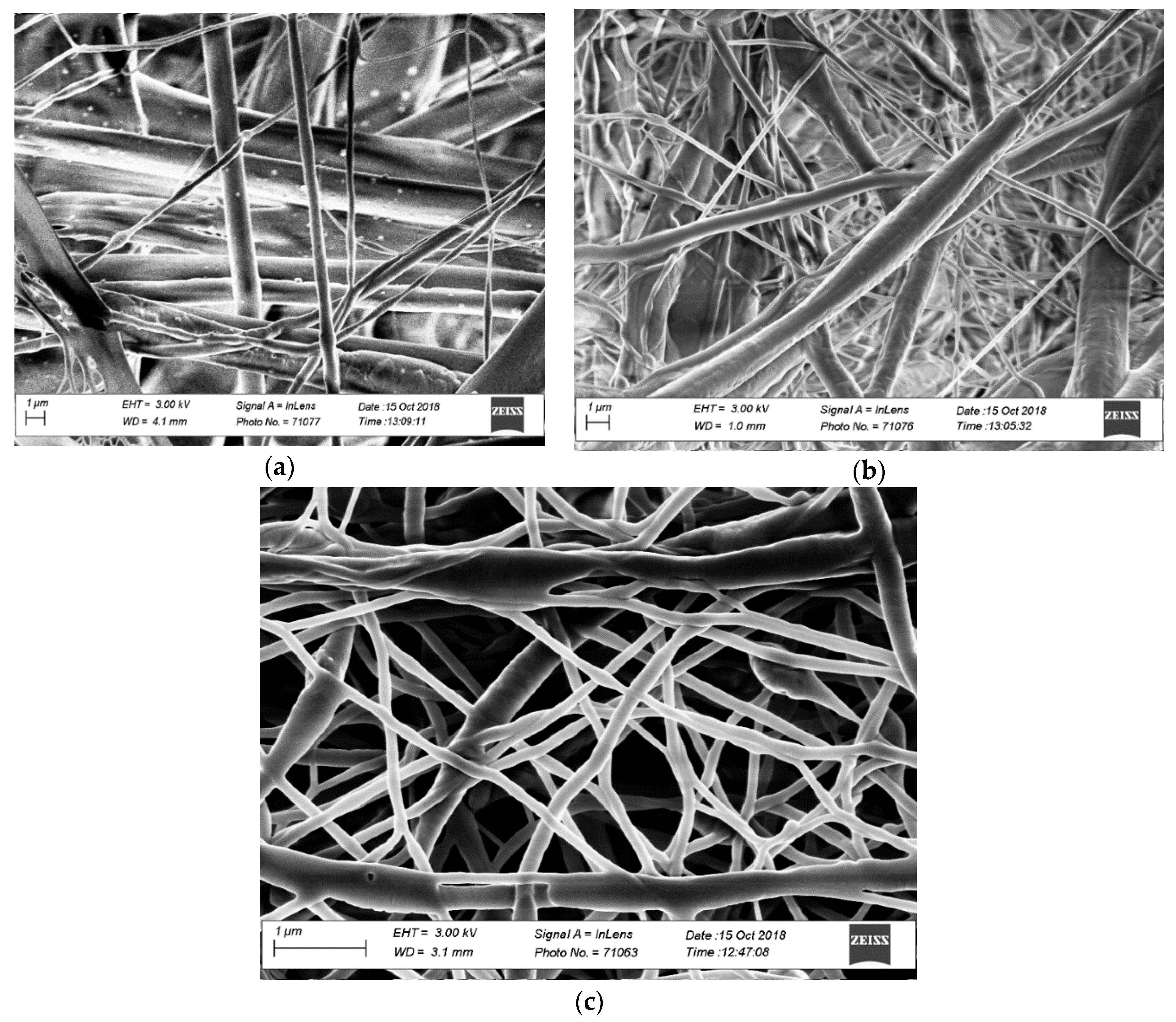
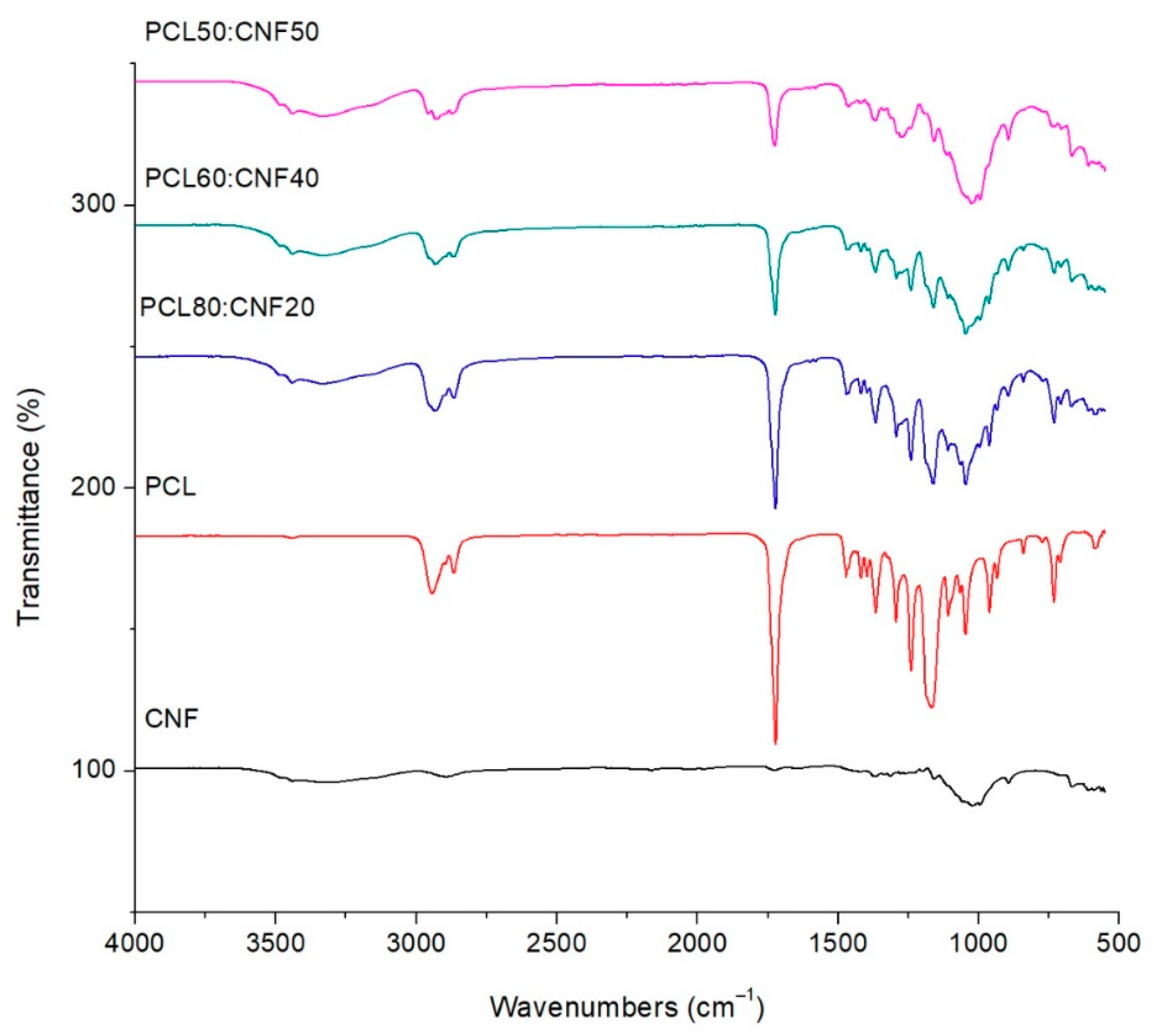
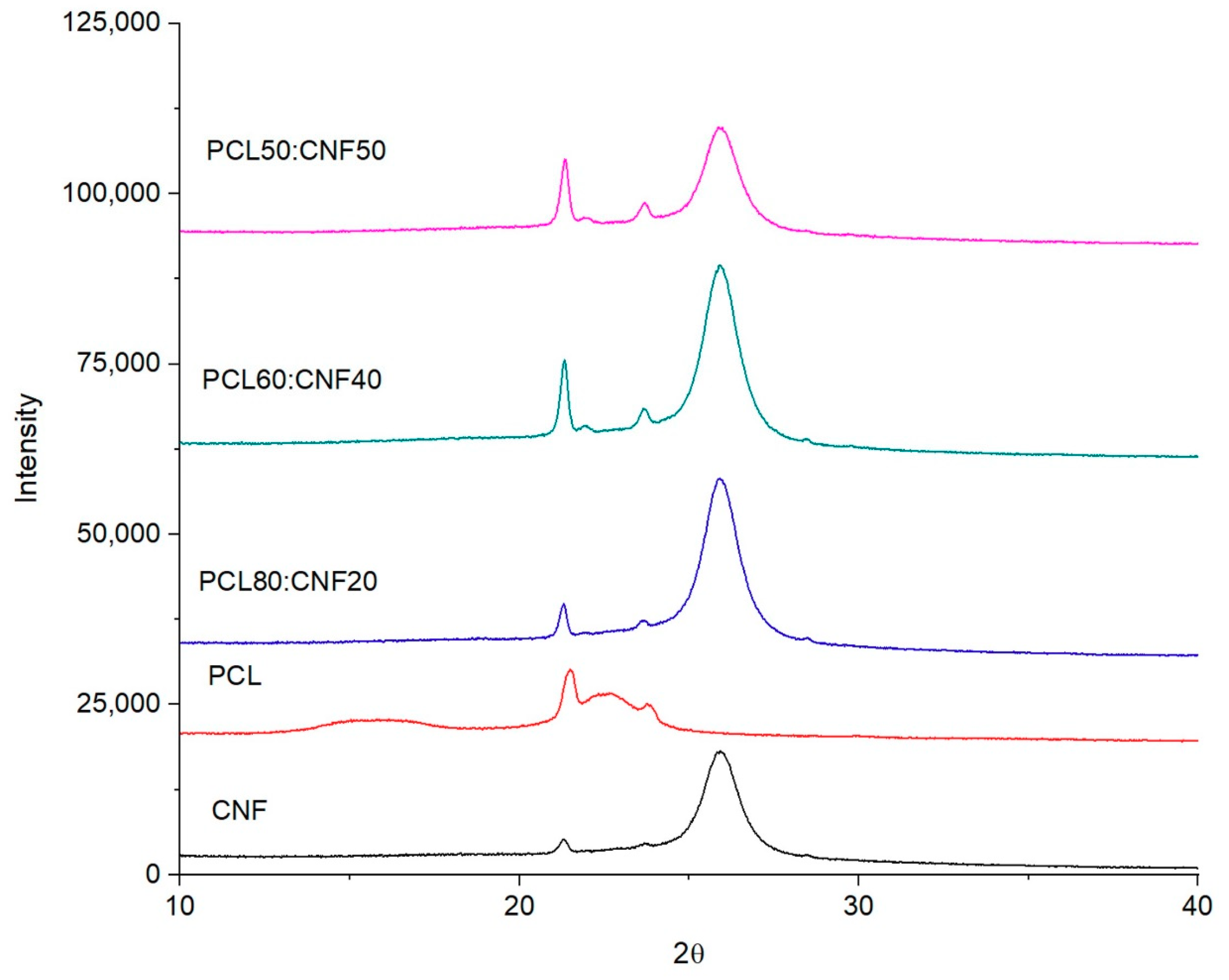
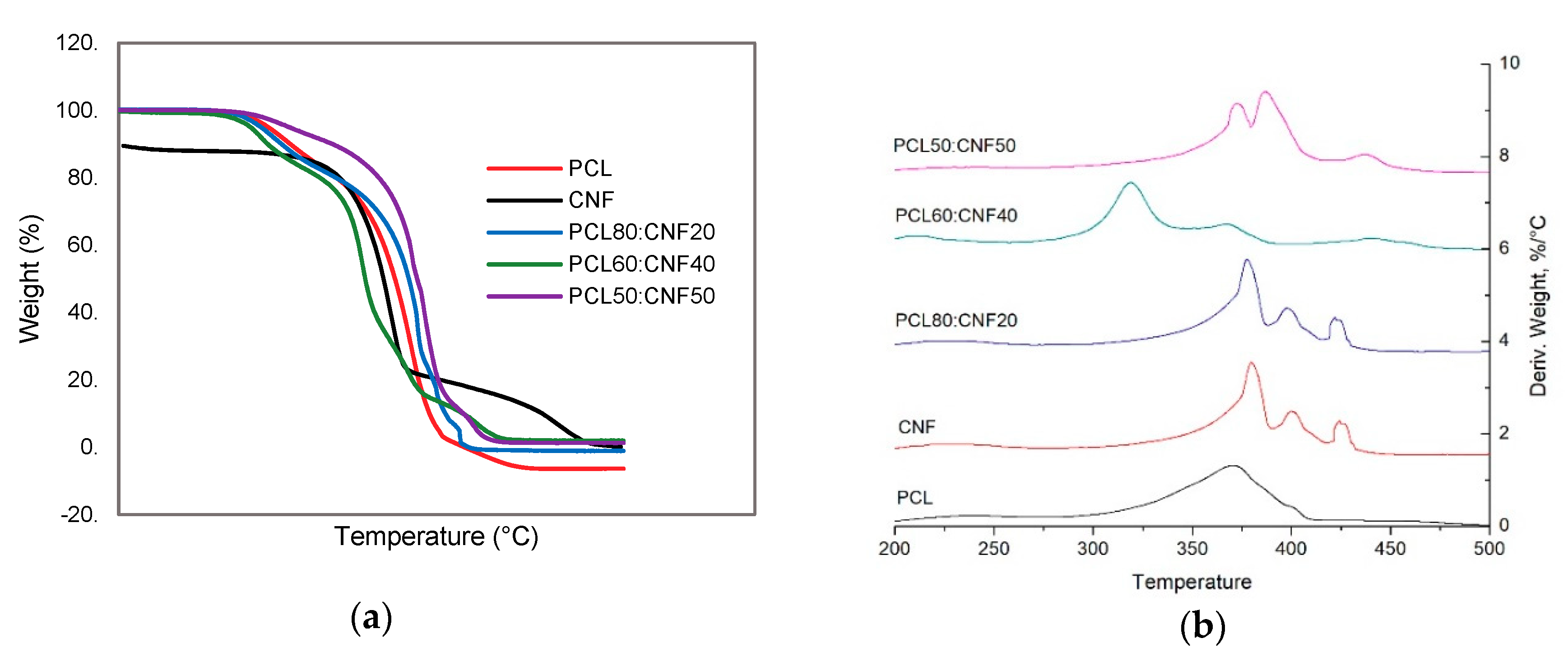
| Membrane | Fiber Diameter (nm) | Permeability (µm/Pa/s) | Porosity |
|---|---|---|---|
| PCL80:CNF20 | 1049 ± 98 | 0.635 ± 0.01 | 0.455 ± 0.03 |
| PCL60:CNF40 | 1747 ± 153 | 0.317 ± 0.02 | 0.468 ± 0.02 |
| PCL50:CNF50 | 216 ± 12 | 0.317 ± 0.04 | 0.302 ± 0.05 |
| Commercial cellulose membrane | 938 ± 20 | 1.270 ± 0.04 | 0.592 ± 0.01 |
| Membrane | Turbidity (NTU) | Conductivity (S/m) | Iron Reduction (%) | Chromium Reduction (%) | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| PCL80:CNF20 | 70 ± 3.5 | 0.91 | 669 ± 3.5 | 0 | 38 ± 1.9 | 42 ± 2.1 |
| PCL60:CNF40 | 83 ± 4.1 | 0.00 | 669 ± 3.5 | 0 | 62 ± 3.1 | 90 ± 4.5 |
| PCL50:CNF50 | 83 ± 4.1 | 0.00 | 669 ± 3.5 | 0 | 75 ± 3.5 | 99 ± 3.5 |
| pH | Temperature (°C) | Conductivity (μS/cm) | Turbidity (NTU) | Heavy Metal Concentration (ppb) | ||
|---|---|---|---|---|---|---|
| Chlorine | Iron | Chromium | ||||
| 8.69 ± 0.17 | 27.5 ± 0.5 | 669 ± 0.18 | 81 ± 1.62 | 57.041 ± 0.11 | 6.676 ± 0.13 | 0.432 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios Hinestroza, H.; Urena-Saborio, H.; Zurita, F.; Guerrero de León, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules 2020, 25, 683. https://doi.org/10.3390/molecules25030683
Palacios Hinestroza H, Urena-Saborio H, Zurita F, Guerrero de León AA, Sundaram G, Sulbarán-Rangel B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules. 2020; 25(3):683. https://doi.org/10.3390/molecules25030683
Chicago/Turabian StylePalacios Hinestroza, Hasbleidy, Hilary Urena-Saborio, Florentina Zurita, Aida Alejandra Guerrero de León, Gunasekaran Sundaram, and Belkis Sulbarán-Rangel. 2020. "Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water" Molecules 25, no. 3: 683. https://doi.org/10.3390/molecules25030683
APA StylePalacios Hinestroza, H., Urena-Saborio, H., Zurita, F., Guerrero de León, A. A., Sundaram, G., & Sulbarán-Rangel, B. (2020). Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules, 25(3), 683. https://doi.org/10.3390/molecules25030683









