New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative
Abstract
1. Introduction
2. Results and Discussion
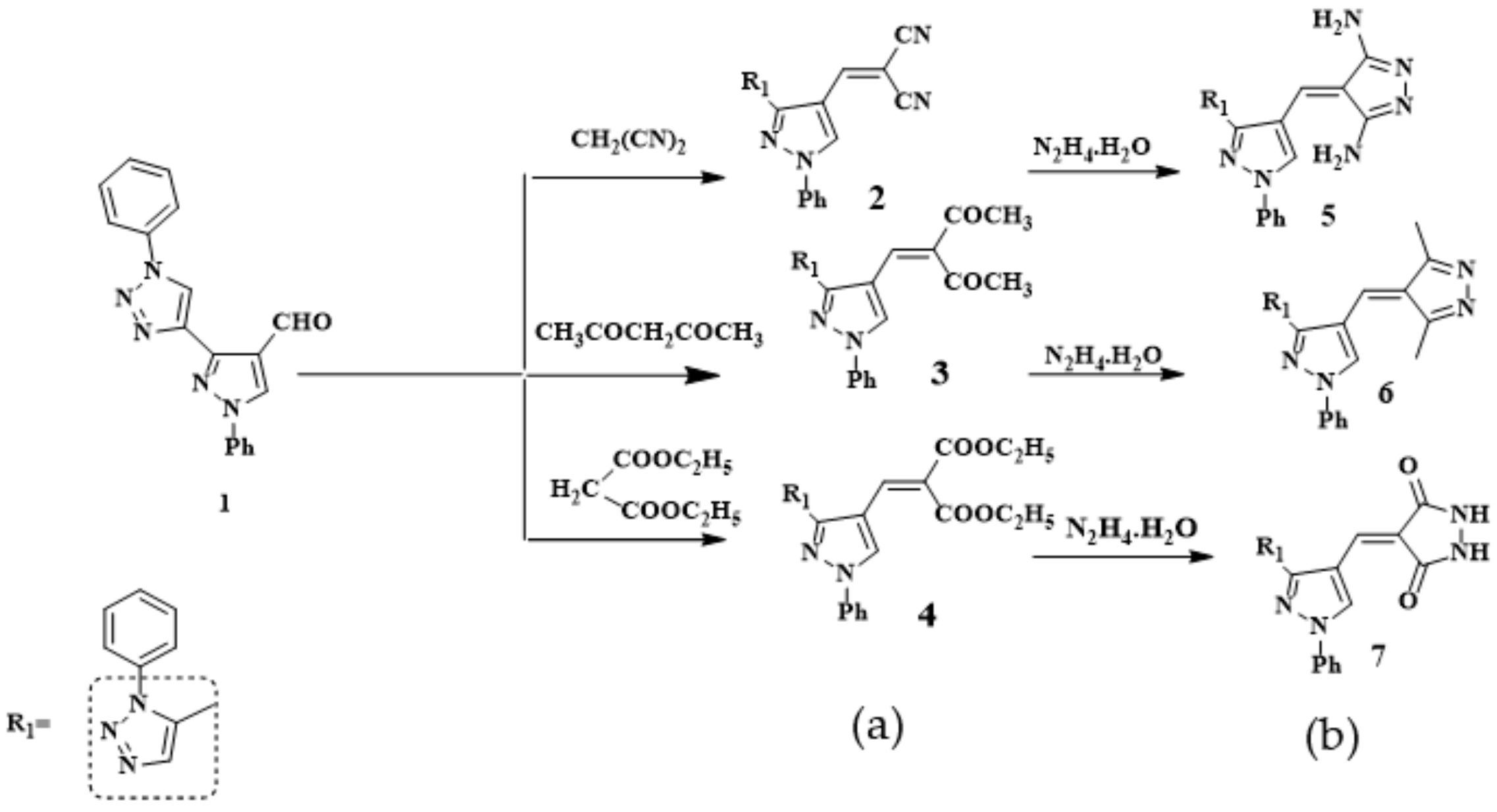
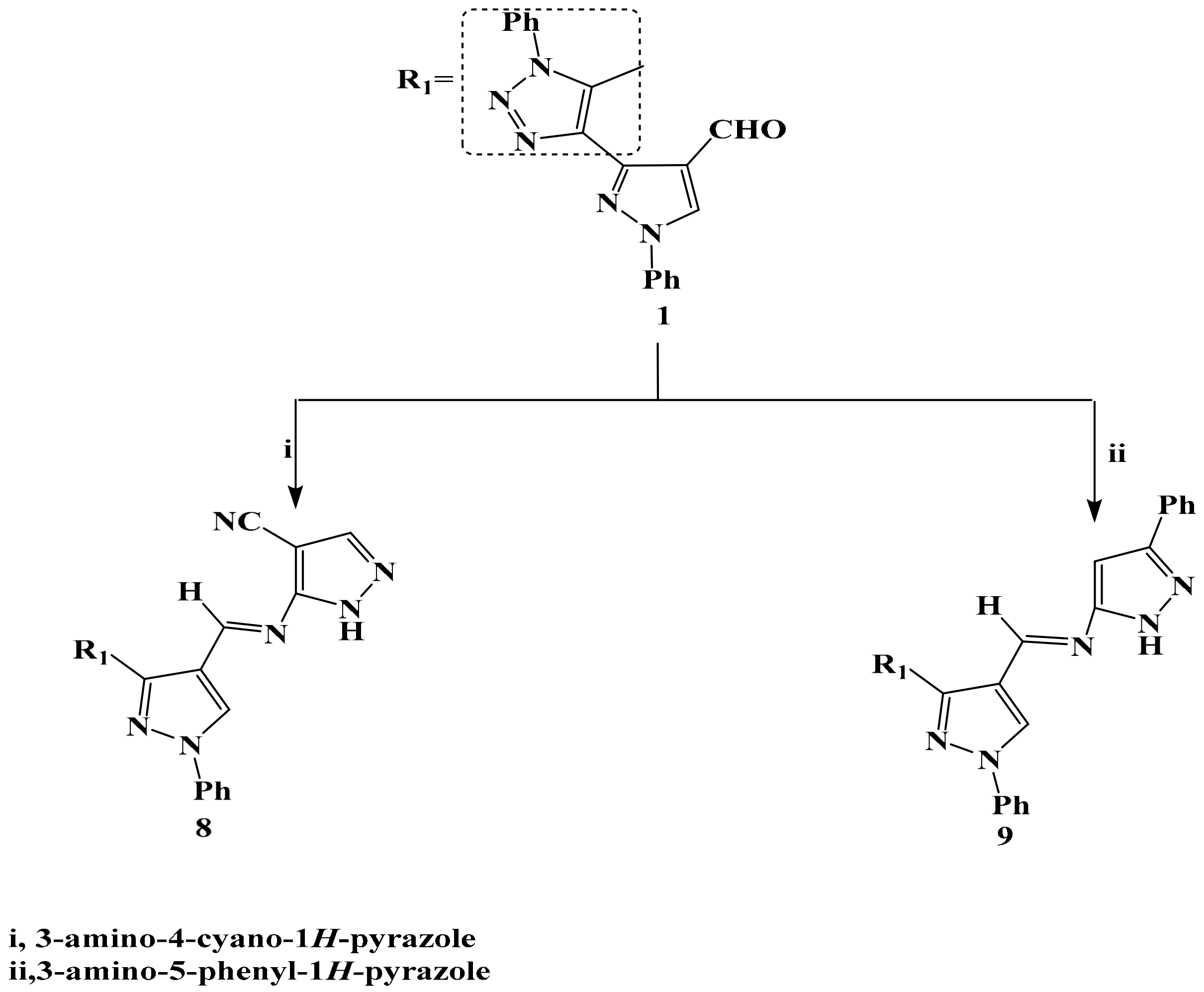
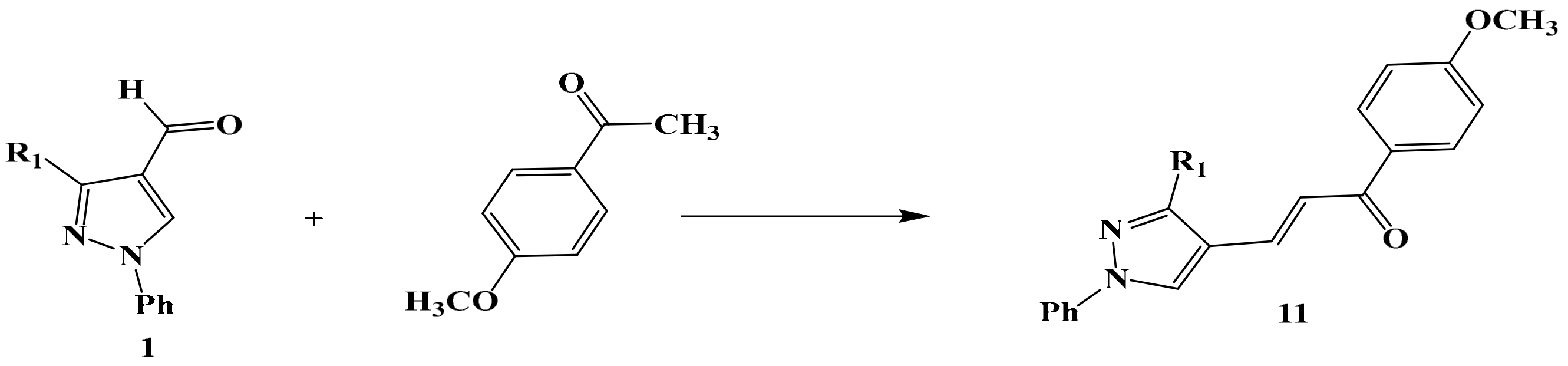
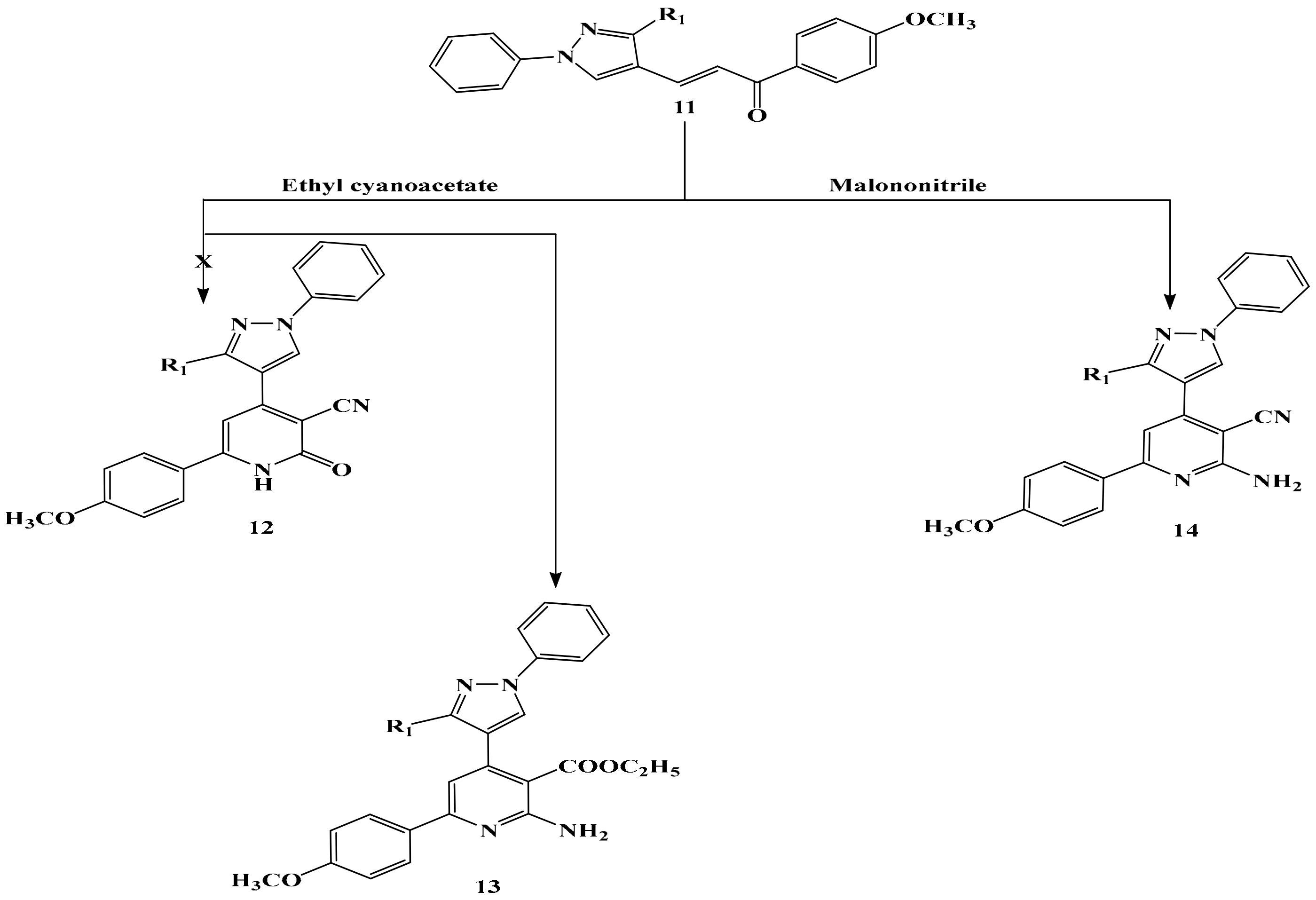
2.1. Chemistry
2.2. Pharmacological Screening
2.2.1. α-Reductase Inhibitors
2.2.2. Aromatase Activity Assay
3. Experimental
3.1. General Procedures
3.2. Synthesis of Compounds 2–4
General Producer
3.3. Synthesis of Compounds 5–7
General Producer
3.4. Synthesis of Compounds 8 and 9
General Producer
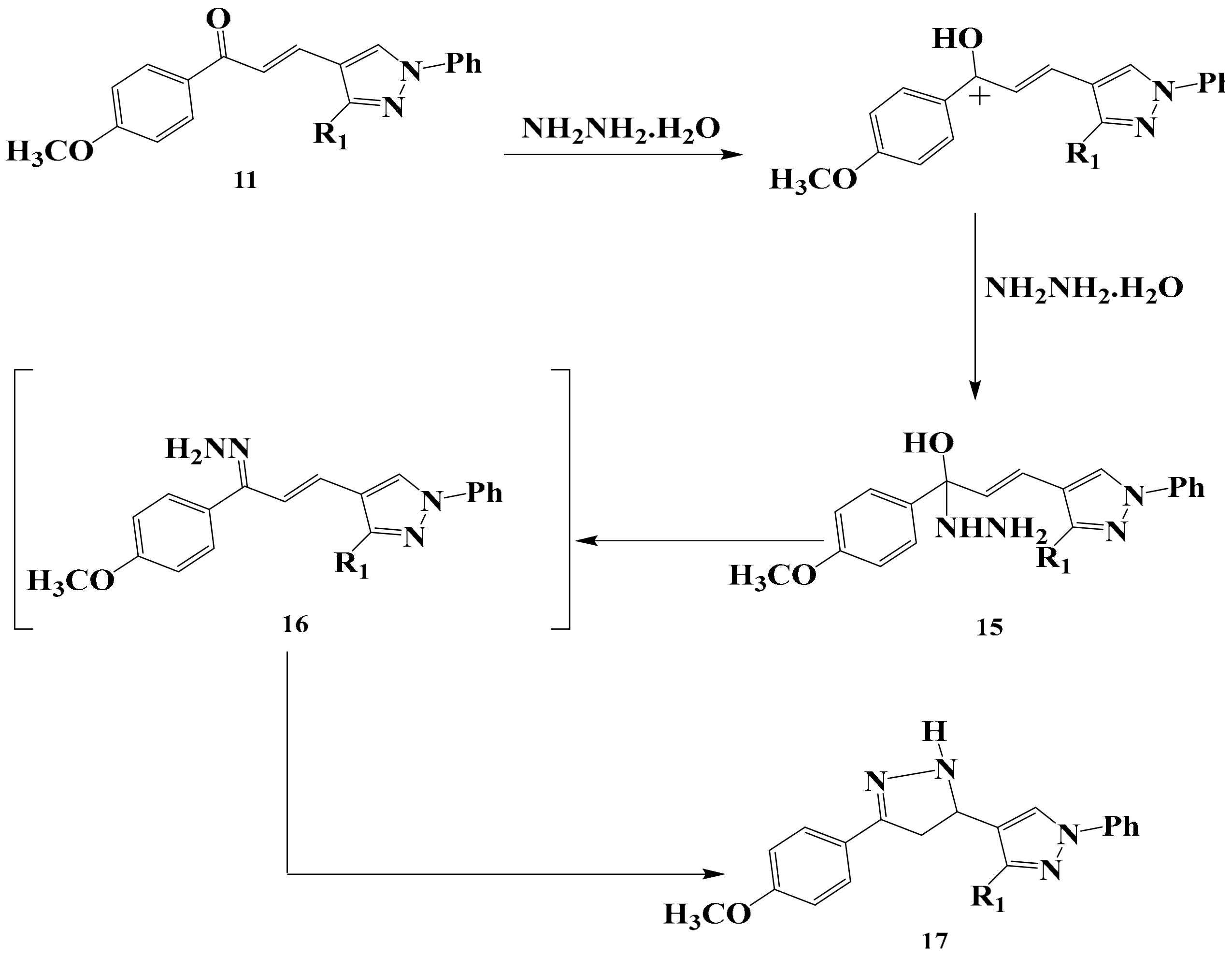
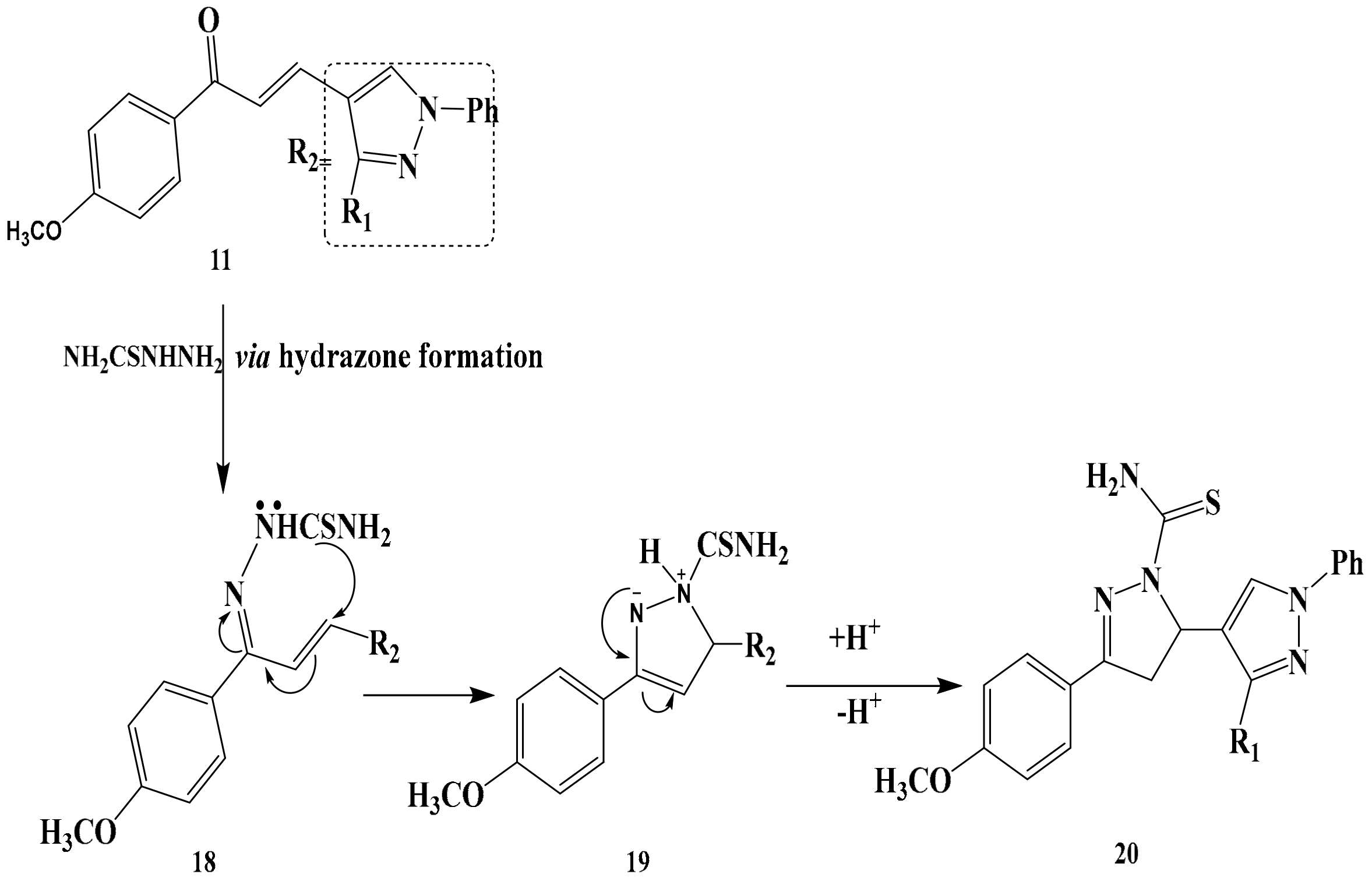
3.5. Synthesis of Compounds 13 and 14
General Producer
4. Biological Experiments
4.1. α-Reductase Inhibitors
4.1.1. Treatment of Animals
4.1.2. Aromatase Activity Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Wang, X.; Zhao, X.; Liu, B.; Yi, L.; Zuo, L.; Wen, Q.; Liu, F.; Xu, J.; Hu, S. A 2010 update of National Land Use/Cover Database of China at 1: 100000 scale using medium spatial resolution satellite images. Remote Sens. Environ. 2014, 149, 142–154. [Google Scholar] [CrossRef]
- Shoombuatong, W.; Prachayasittikul, V.; Prachayasittikul, V.; Nantasenamat, C. Prediction of aromatase inhibitory activity using the efficient linear method (ELM). Excli J. 2015, 14, 452. [Google Scholar] [PubMed]
- Njar, V.C.; Kato, K.; Nnane, I.P.; Grigoryev, D.N.; Long, B.J.; Brodie, A.M. Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17α-hydroxylase-C17, 20-lyase (P45017α): Potential agents for the treatment of prostate cancer. J. Med. Chem. 1998, 41, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Pet, C.; Batta, G.; Györgydeák, Z.; Sztaricskai, F. Glycoside Synthesis with Anomeric 1-N-Glycobiosyl-1, 2, 3-triazoles1. J. Carbohydr. Chem. 1996, 15, 465–483. [Google Scholar]
- Rashdan, H.R.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo [1, 2-b] phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Rashdan, H.R.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1, 3, 4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions. Molecules 2019, 24, 2371. [Google Scholar] [CrossRef]
- Khatri, P.; Yeatts, S.D.; Mazighi, M.; Broderick, J.P.; Liebeskind, D.S.; Demchuk, A.M.; Amarenco, P.; Carrozzella, J.; Spilker, J.; Foster, L.D. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: An analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014, 13, 567–574. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Alam, S.; Tanaka, M.; Lee, J.-C. Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 2007, 89, 82–88. [Google Scholar] [CrossRef]
- Polkam, N.; Rayam, P.; Anireddy, J.S.; Yennam, S.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Kotapalli, S.S.; Ummanni, R.; Balasubramanian, S. Synthesis, in vitro anticancer and antimycobacterial evaluation of new 5-(2, 5-dimethoxyphenyl)-1, 3, 4-thiadiazole-2-amino derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 1398–1402. [Google Scholar] [CrossRef]
- Malladi, S.; Isloor, A.M.; Isloor, S.; Akhila, D.; Fun, H.-K. Synthesis, characterization and antibacterial activity of some new pyrazole based Schiff bases. Arab. J. Chem. 2013, 6, 335–340. [Google Scholar] [CrossRef]
- Gallardo, A.; Marui, A. Modeling the dynamics of the freshwater-saltwater interface in response to construction activities at a coastal site. Int. J. Environ. Sci. Technol. 2007, 4, 285–294. [Google Scholar] [CrossRef][Green Version]
- Helser, T.E.; Stewart, I.; Fleischer, G.; Martell, S. Stock assessment of Pacific hake (whiting) in US and Canadian waters in 2006. Pac. Fish. Manag. Counc. 2006, 7700, 97220-1384. [Google Scholar]
- Merolla, P.; Arthur, J.; Akopyan, F.; Imam, N.; Manohar, R.; Modha, D.S. A digital neurosynaptic core using embedded crossbar memory with 45pJ per spike in 45nm. In Proceedings of the 2011 IEEE custom integrated circuits conference (CICC), San Jose, CA, USA, 19–21 September 2011; pp. 1–4. [Google Scholar]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Ribeiro, K.L.; Shaban, Y.R. Theoretical Studies no Laser Pumping for the 1.15 {mu} m Wavelength from the He {sup 3}(n, p) H {sup 3} Reaction; Estudos Teoricos do Bombeamento laser Para o Comprimento de onda 1.15 {mu} ma Partir da Reacao He {sup 3}(n, p) H {sup 3}; Associacao Brasileira de Energia Nuclear: Rio de Janeiro, RJ, Brazil, 1997. [Google Scholar]
- Kume, S.; Muto, A.; Aruga, J.; Nakagawa, T.; Michikawa, T.; Furuichi, T.; Nakade, S.; Okano, H.; Mikoshiba, K. The Xenopus IP3 receptor: Structure, function, and localization in oocytes and eggs. Cell 1993, 73, 555–570. [Google Scholar] [CrossRef]
- Moltzen, E.K.; Pedersen, H.; Boegesoe, K.P.; Meier, E.; Frederiksen, K.; Sanchez, C.; Lemboel, H.L. Bioisosteres of arecoline: 1, 2, 3, 6-tetrahydro-5-pyridyl-substituted and 3-piperidyl-substituted derivatives of tetrazoles and 1, 2, 3-triazoles. Synthesis and muscarinic activity. J. Med. Chem. 1994, 37, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.A.; Nasr, A.Z. The chemistry of C-nucleosides and their analogs I: C-nucleosides of hetero monocyclic bases. Adv. Heterocycl. Chem. 1997, 68, 225. [Google Scholar]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences (Online) 2014, 11. [Google Scholar] [CrossRef]
- Khloya, P.; Kumar, P.; Mittal, A.; Aggarwal, N.K.; Sharma, P.K. Synthesis of some novel 4-arylidene pyrazoles as potential antimicrobial agents. Org. Med. Chem. Lett. 2013, 3, 9. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Miron, A.; Liu, Q.; Wang, Y.; Su, D.; Yang, H.; Li, L.; Kuca, K. Trichothecenes: Immunomodulatory effects, mechanisms, and anti-cancer potential. Arch. Toxicol. 2017, 91, 3737–3785. [Google Scholar] [CrossRef]
- Bouazzi, H. Contribution à l’identification de nouveaux gènes impliqués dans la Déficience intellectuelle liée au Sexe (X-LID) par séquençage à haut débit de l’exome du chromosome X avec la technologie SOLiD; Inserm: Paris, France, 2016. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Facial synthesis of some new pyrazolopyridine, barbituric and thiobarbituric acid derivatives with antimicrobial activities. Inter. J. Adv. Res 2014, 2, 900–913. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of some new antimicrobial 5, 6, 7, 8-tetrahydro-pyrimido [4, 5-b] quinolone derivatives. Der Pharm. Chem 2014, 6, 23–29. [Google Scholar]
- Rashdan, H.R.; Roaiah, H.M.; Muhammad, Z.A.; Wietrzyk, J.; Milczarek, M.; Soliman, A.M. Design, efficient synthesis, mechanism of reaction and antiproliferative activity against cancer and normal cell lines of a novel class of fused pyrimidine derivatives. Acta Pol. Pharm. 2018, 75, 679–688. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of Potent Antimicrobial Pyrrole Derivatives. Int. J. 2014, 2, 1022–1035. [Google Scholar]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; El-Idreesy, T.T.; Rashdan, H.R. Synthesis of some new azolotriazine, 4-arylazopyrazole and pyridine derivatives containing 1, 2, 3-triazole moiety. Int. J. 2013, 1, 729–745. [Google Scholar]
- Rashdan, H.R.; Abdel-Aziem, A.; El-Naggar, D.H.; Nabil, S. synthesis and biological evaluation of some new pyridines, isoxazoles and isoxazolopyridazines bearing 1, 2, 3-triazole moiety. Acta Pol. Pharm. 2019, 76, 469–482. [Google Scholar]
- El-Naggar, M.; Mohamed, M.E.; Mosallam, A.M.; Salem, W.; Rashdan, H.R.; Abdelmonsef, A.H. Synthesis, Characterization, Antibacterial Activity, and Computer-Aided Design of Novel Quinazolin-2, 4-dione Derivatives as Potential Inhibitors Against Vibrio cholerae. Evol. Bioinform. 2020, 16, 1176934319897596. [Google Scholar] [CrossRef]
- Elnaggar, D.H.; Abdel Hafez, N.A.; Rashdan, H.R.; Abdelwahed, N.A.; Awad, H.M.; Ali, K.A. Synthesis, Antimicrobial and Antitumor Evaluations of a New Class of Thiazoles Substituted on the Chromene Scaffold. Mini Rev. Med. Chem. 2019, 19, 1717–1725. [Google Scholar] [CrossRef]
- Rashdan, H.; Nasr, S.; El-Refai, H.; Abdel-Aziz, M. A novel approach of potent antioxidant and antimicrobial agents containing coumarin moiety accompanied with cytotoxicity studies on the newly synthesized derivatives. J. Appl. Pharm. Sci. 2017, 7, 186–196. [Google Scholar]
- Anniyappan, M.; Muralidharan, D.; Perumal, P.T. Synthesis of Hantzsch 1, 4-dihydropyridines under microwave irradiation. Synth. Commun. 2002, 32, 659–663. [Google Scholar] [CrossRef]
- Shepard, R.N. Attention and the metric structure of the stimulus space. J. Math. Psychol. 1964, 1, 54–87. [Google Scholar] [CrossRef]
- George, A. Western State Terrorism; Polity Press Cambridge: Cambridge, UK, 1991. [Google Scholar]
- Lo, D.C.; McAllister, A.K.; Katz, L.C. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 1994, 13, 1263–1268. [Google Scholar] [CrossRef]
- Das, S.; Gautam, N.; Dey, S.K.; Maiti, T.; Roy, S. Oxidative stress in the brain of nicotine-induced toxicity: Protective role of Andrographis paniculata Nees and vitamin E. Appl. Physiol. Nutr. Metab. 2009, 34, 124–135. [Google Scholar] [CrossRef] [PubMed]

| Compound | IC50 (µM) |
|---|---|
| 1 | 0.00761 |
| 2 | 0.00454 |
| 3 | 0.00343 |
| 4 | 0.00122 |
| 5 | 0.00213 |
| 6 | 0.00192 |
| 7 | 0.00091 |
| 8 | 0.00021 |
| 9 | 0.00192 |
| 10 | 0.00171 |
| 11 | 0.00026 |
| 13 | 0.00015 |
| 14 | 0.00074 |
| 17 | 0.00063 |
| 20 | 0.00053 |
| Anastrozole | 0.00241 |
| Compound | IC50 (µM) |
|---|---|
| 1 | 0.00913 |
| 2 | 0.00724 |
| 3 | 0.00635 |
| 4 | 0.00566 |
| 5 | 0.00454 |
| 6 | 0.00342 |
| 7 | 0.00253 |
| 8 | 0.00144 |
| 9 | 0.00233 |
| 10 | 0.00142 |
| 11 | 0.00033 |
| 13 | 0.00024 |
| 14 | 0.00093 |
| 17 | 0.00084 |
| 20 | 0.00064 |
| Letrozole | 0.00280 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, M.; Abd El-All, A.S.; El-Naem, S.I.A.; Abdalla, M.M.; Rashdan, H.R.M. New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative. Molecules 2020, 25, 672. https://doi.org/10.3390/molecules25030672
El-Naggar M, Abd El-All AS, El-Naem SIA, Abdalla MM, Rashdan HRM. New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative. Molecules. 2020; 25(3):672. https://doi.org/10.3390/molecules25030672
Chicago/Turabian StyleEl-Naggar, Mohamed, Amira S. Abd El-All, Shweekar I. A. El-Naem, Mohamed M. Abdalla, and Huda R. M. Rashdan. 2020. "New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative" Molecules 25, no. 3: 672. https://doi.org/10.3390/molecules25030672
APA StyleEl-Naggar, M., Abd El-All, A. S., El-Naem, S. I. A., Abdalla, M. M., & Rashdan, H. R. M. (2020). New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative. Molecules, 25(3), 672. https://doi.org/10.3390/molecules25030672





