TBAB-Catalyzed 1,6-Conjugate Sulfonylation of para-Quinone Methides: A Highly Efficient Approach to Unsymmetrical gem-Diarylmethyl Sulfones in Water †
Abstract
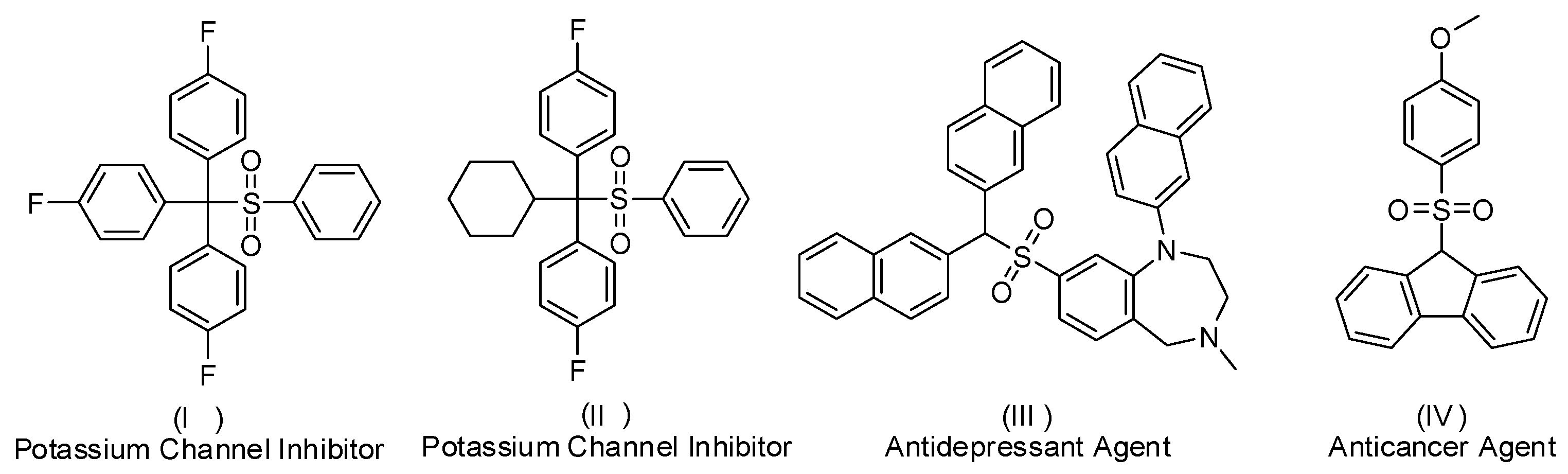
1. Introduction
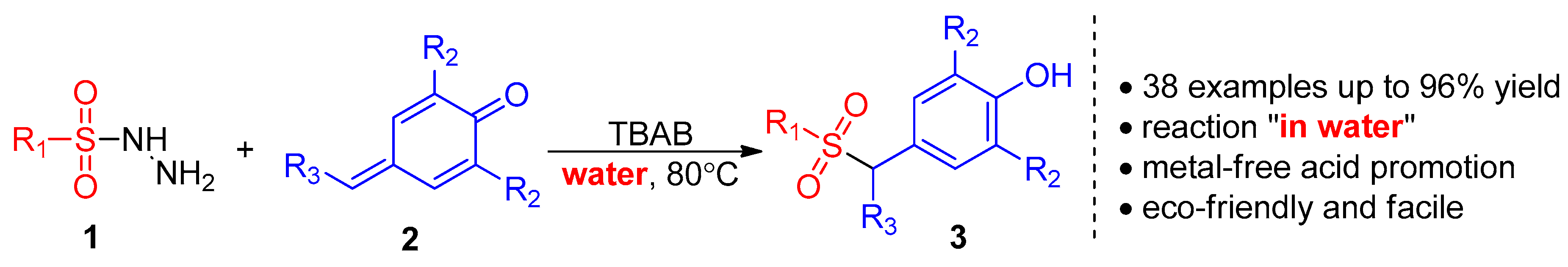
2. Results and Discussion
2.1. Optimization of Reaction Conditions
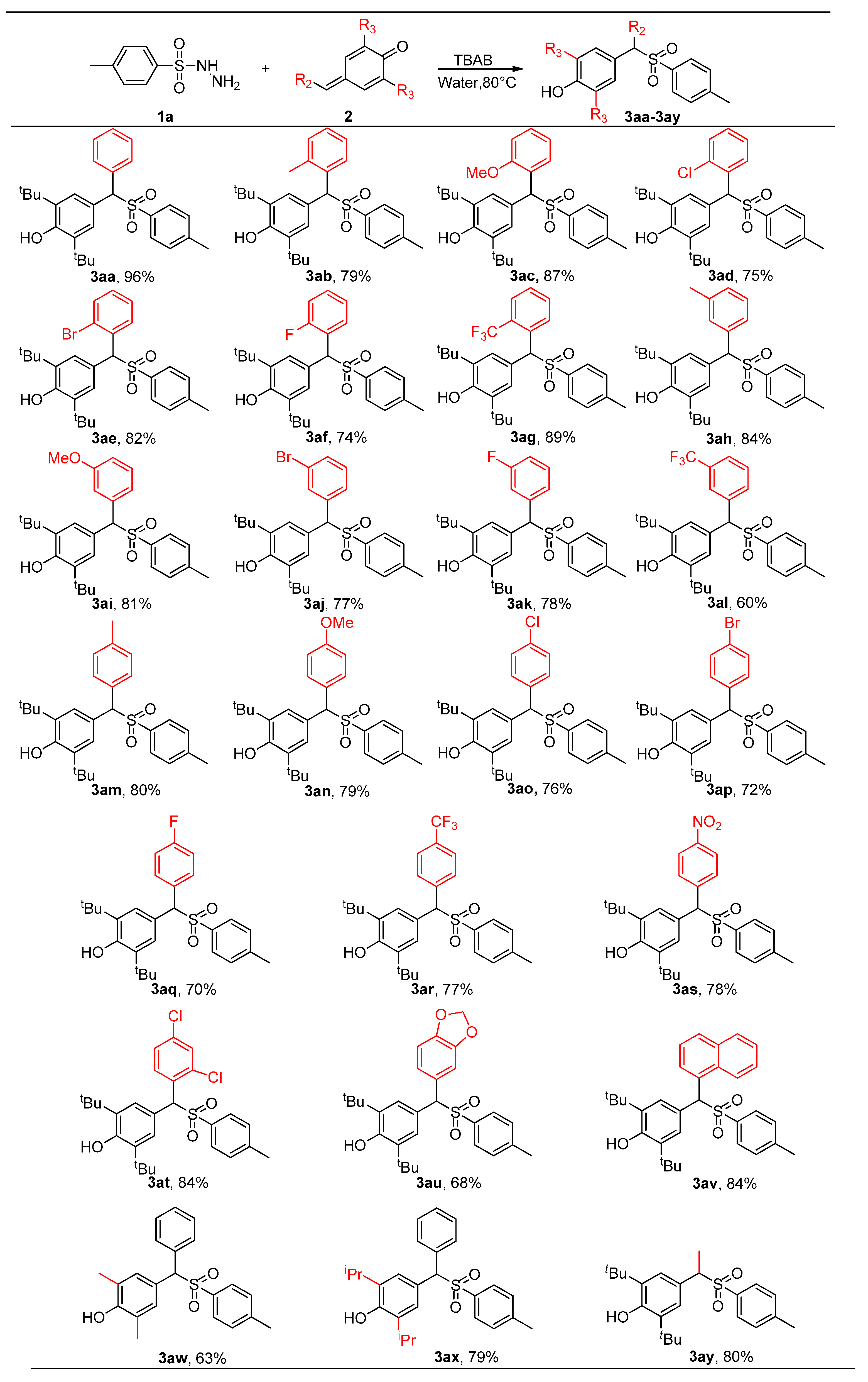
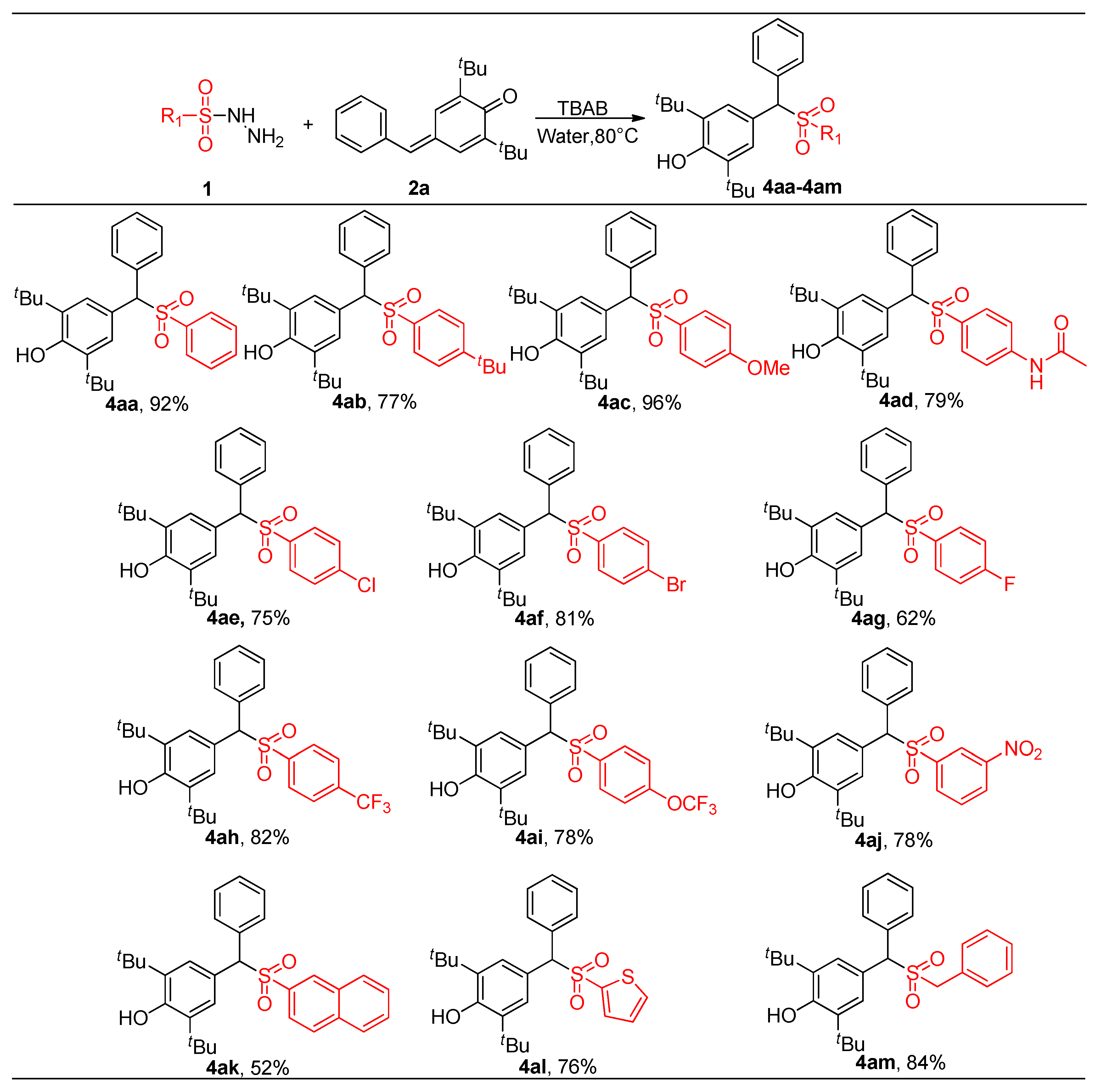
2.2. Reaction Scope
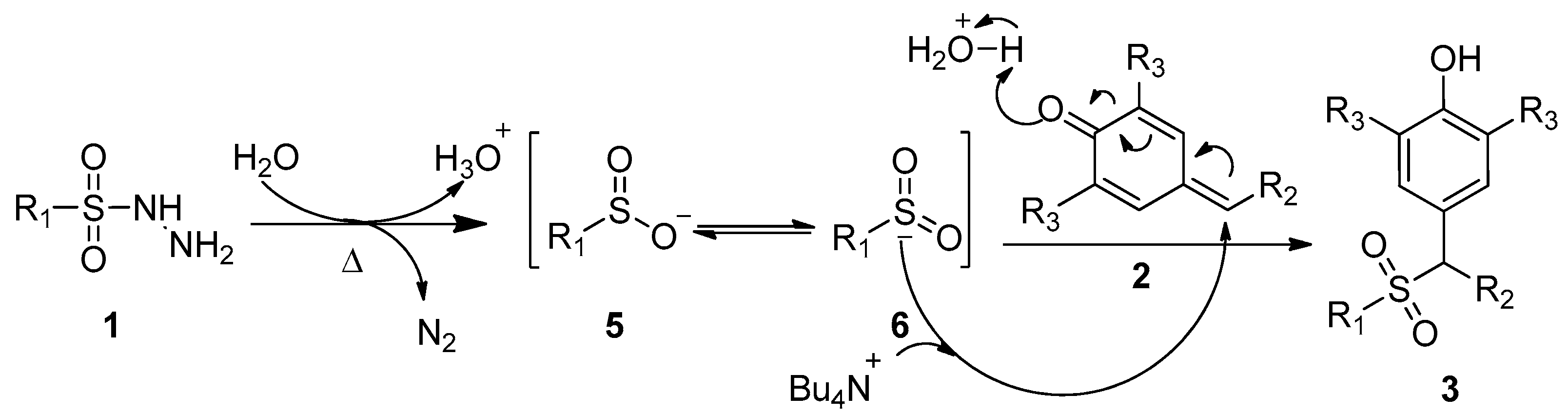
2.3. Proposed Mechanism
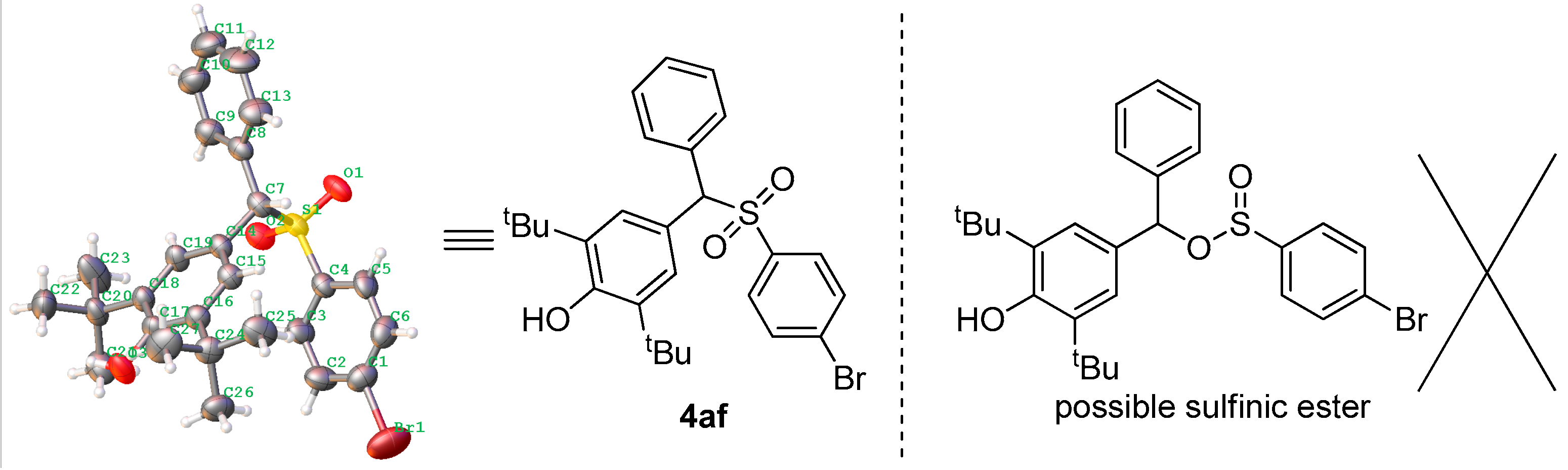
2.4. Derivatives of Products
3. Materials and Methods
3.1. General Information
3.2. Experiment
3.2.1. Representative Procedure for Synthesis of Gem-Diarylmethyl Sulfones
3.2.2. Representative Procedure for General Reaction Procedure for Synthesis of Unsymmetrical Triarylmethane 7
3.2.3. Representative Procedure for General Reaction Procedure for Synthesis of Unsymmetrical Gem-Diarylmethyl Thioether 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| THF | Tetrahydrofuran |
| DCE | Dichloroethane |
| DMSO | Dimethylsulfoxide |
| CTAB | Cetyltrimethyl Ammonium Bromide |
| TBAI | Tetrabutyl Ammonium Iodide |
| TEBAC | Triethylbenzyl Ammonium Chloride |
| TBAB | Tetrabutyl Ammonium Bromide |
| SDS | Sodium Dodecyl Sulfate |
| HRMS | High Resolution Mass Spectroscopy |
References
- Simpkins, N.S. Sulphones in Organic Synthesis; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Liang, S.; Shaaban, S.; Liu, N.-W.; Hofman, K.; Manolikakes, G. Chapter Three-Recent Advances in the Synthesis of C-S Bonds via Metal-Catalyzed or -Mediated Functionalization of C-H Bonds. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: San Diego, CA, USA, 2018; Volume 69, pp. 135–207. [Google Scholar]
- Hofman, K.; Liu, N.-W.; Manolikakes, G. Radicals and sulfur dioxide: A versatile combination for the construction of sulfonyl-containing molecules. Chem. Eur. J. 2018, 24, 11852–11863. [Google Scholar] [CrossRef] [PubMed]
- Román, R.; Barrio, P.; Mateu, N.; Sedgwick, D.M.; Fustero, S. Copper-catalyzed regioselective synthesis of (E)-β-fluorovinyl sulfones. Molecules 2019, 24, 1569. [Google Scholar]
- Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA Design for the optimization of TiO2 coating on polyether sulfone membranes. Molecules 2019, 24, 2924. [Google Scholar] [CrossRef] [PubMed]
- Alsaedi, A.M.R.; Farghaly, T.A.; Shaaban, M.R. Synthesis and antimicrobial evaluation of novel pyrazolopyrimidines incorporated with mono- and diphenylsulfonyl groups. Molecules 2019, 24, 4009. [Google Scholar] [CrossRef]
- Cohen, A.; Crozet, M.D.; Rathelot, P.; Azas, N.; Vanelle, P. Synthesis and promising in vitro antiproliferative activity of sulfones of a 5-nitrothiazole series. Molecules 2013, 18, 97–113. [Google Scholar] [CrossRef]
- Gouliaev, A.H.; Slok, F.A.; Teuber, L.; Demnitz, J. Potassium Channel Modulators. U.S. Patent 742,961,8B2, 30 September 2008. [Google Scholar]
- Langler, R.F.; Paddock, R.L.; Thompson, D.B.; Crandall, I.; Ciach, M.; Kain, K.C. Selected sulfonyl compounds as anticancer/antimalarial agents. Aust. J. Chem. 2003, 56, 1127–1133. [Google Scholar] [CrossRef]
- Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T. Substituted benzo[1,4]diazepine Derivatives as Antidepressants and Their Preparation. WO Patent WO2009145357A1, 3 November 2009. [Google Scholar]
- Schoenebeck, F.; Murphy, J.A.; Zhou, S.-Z.; Uenoyama, Y.; Miclo, Y.; Tuttle, T. Reductive cleavage of sulfones and sulfonamides by a neutral organic super-electron-donor (S.E.D.) reagent. J. Am. Chem. Soc. 2007, 129, 13368–13369. [Google Scholar] [CrossRef]
- Nambo, M.; Ariki, Z.T.; Canseco-Gonzalez, D.; Beattie, D.D.; Crudden, C.M. Arylative desulfonation of diarylmethyl phenyl sulfone with arenes catalyzed by scandium triflate. Org. Lett. 2016, 18, 2339–2342. [Google Scholar] [CrossRef]
- Nambo, M.; Crudden, C.M. Modular synthesis of triarylmethanes through palladium-catalyzed sequential arylation of methyl phenyl sulfone. Angew. Chem., Int. Ed. 2014, 53, 742–746. [Google Scholar] [CrossRef]
- Meyers, C.Y.; Chan-Yu-King, R.; Hua, D.H.; Kolb, V.M.; Matthews, W.S.; Parady, T.E.; Horii, T.; Sandrock, P.B.; Hou, Y.; Xie, S. Unexpected differences in the α-halogenation and related reactivity of sulfones with perhaloalkanes in KOH-t-BuOH. J. Org. Chem. 2003, 68, 500–511. [Google Scholar] [CrossRef]
- Guan, X.-Y.; Zhang, L.-D.; You, P.-S.; Liu, S.-S.; Liu, Z.-Q. 1,6-Conjugate sulfonylation of para-quinone methides: An expedient approach to unsymmetrical gem-diarylmethyl sulfones. Tetrahedron Lett. 2019, 60, 244–247. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. Catalyst-free 1,6-conjugate addition/aromatization/aulfonylation of para-quinone methides: Facile access to diarylmethyl sulfones. ACS Omega 2018, 3, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.A.; Reddy, P.S.; Sreedhar, B. Iron(III) chloride-catalyzed direct sulfonylation of alcohols with sodium arenesulfinates. Adv. Synth. Catal. 2010, 352, 1861–1869. [Google Scholar] [CrossRef]
- Feng, X.-W.; Wang, J.; Zhang, J.; Yang, J.; Wang, N.; Yu, X.-Q. Copper-catalyzed nitrogen loss of Sulfonylhydrazones: A reductive strategy for the synthesis of sulfones from carbonyl compounds. Org. Lett. 2010, 12, 4408–4411. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Luo, X.; Deng, G.; Liang, Y.; Liu, J. A catalyst-free and additive-free method for the synthesis of benzothiazolethiones from o-iodoanilines, DMSO and potassium sulfide. Green Chem. 2018, 20, 1970–1974. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, W.; Liu, N.; Zhang, Q.; Liu, Y.; Li, X.; Wei, Y.; Feng, L. Antioil Ag3PO4 nanoparticle/polydopamine/Al2O3 sandwich structure for complex wastewater treatment: Dynamic catalysis under natural light. ACS Sustainable Chem. Eng. 2018, 6, 8019–8028. [Google Scholar] [CrossRef]
- Bhaumik, P.; Chou, H.-J.; Lee, L.-C.; Chung, P.-W. Chemical transformation for 5-hydroxymethylfurfural production from saccharides using molten salt system. ACS Sustainable Chem. Eng. 2018, 6, 5712–5717. [Google Scholar] [CrossRef]
- Ishizuka, T.; Ohkawa, S.; Ochiai, H.; Hashimoto, M.; Ohkubo, K.; Kotani, H.; Sadakane, M.; Fukuzumi, S.; Kojima, T. A supramolecular photocatalyst composed of a polyoxometalate and a photosensitizing water-soluble porphyrin diacid for the oxidation of organic substrates in water. Green Chem. 2018, 20, 1975–1980. [Google Scholar] [CrossRef]
- Teng, Q.-H.; Peng, X.-J.; Mo, Z.-Y.; Xu, Y.-L.; Tang, H.-T.; Wang, H.-S.; Sun, H.-B.; Pan, Y.-M. Transition-metal-free C-N and C-C formation: Synthesis of benzo [4,5] imidazo [1–a] pyridines and 2-pyridones from ynones. Green Chem. 2018, 20, 2007–2012. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.N.; Coyne, A.G. Water: Nature’s reaction enforcer-comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef] [PubMed]
- Lindström, U.M. Stereoselective organic reactions in water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Carril, M.; SanMartin, R.; Tellitu, I.; Domínguez, E. On-water chemistry: copper-catalyzed straightforward synthesis of benzo[b]furan derivatives in neat water. Org. Lett. 2006, 8, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, D.; Coufourier, S.; Mbaye, M.D.; Gaillard, S.; Renaud, J.-L. Cyclopentadienone iron tricarbonyl complexes-catalyzed hydrogen transfer in water. Molecules 2020, 25, 421. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Saha, S.; Sengupta, D.; Chattopadhyay, S.; De, G.; Basu, B. Stabilized Cu2O nanoparticles on macroporous polystyrene resins [Cu2O@ARF]: Improved and reusable heterogeneous catalyst for on-water synthesis of triazoles via click reaction. Ind. Eng. Chem. Res. 2017, 56, 11726–11733. [Google Scholar] [CrossRef]
- Pesnot, T.; Gershater, M.C.; Edwards, M.; Ward, J.M.; Hailes, H.C. One-pot phosphate-mediated synthesis of novel 1,3,5-trisubstituted pyridinium salts: A new family of S. aureus inhibitors. Molecules 2017, 22, 626. [Google Scholar] [CrossRef]
- Hajra, S.; Singha Roy, S.; Aziz, S.M.; Das, D. Catalyst-Free “On-Water” Regio- and stereospecific ring-opening of spiroaziridine oxindole: Enantiopure synthesis of unsymmetrical 3,3′-bisindoles. Org. Lett. 2017, 19, 4082–4085. [Google Scholar] [CrossRef]
- Ibacache, J.A.; Valderrama, J.A.; Faúndes, J.; Danimann, A.; Recio, F.J.; Zúñiga, C.A. Green synthesis and electrochemical properties of mono- and dimers derived from phenylaminoisoquinolinequinones. Molecules 2019, 24, 4378. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef] [PubMed]
- Osako, T.; Ueno, Y.; Tachi, Y.; Itoh, S. C-S bond formation reaction between a phenolate and disulfide-bridged dicopper (I) complexes. Inorg. Chem. 2004, 43, 6516–6518. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.-Y.; Colvin, A.J.; Chen, Y.; Zhang, X.P. Synthesis of meso-arylsulfanyl-and alkylsulfanyl-substituted porphyrins via palladium-mediated C-S bond formation. J. Org. Chem. 2004, 69, 8886–8892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q.; Guan, X.-Y.; Liu, S.-S.; Ma, X.-B.; Li, Y.-Q.; Zhang, L.-D. Preparation of gem-diarylmethyl Amines Derivatives. China Patent CN201710847133, 28 May 2019. [Google Scholar]
- Liu, Z.-Q.; Zhang, L.-D.; Ma, X.-B.; Li, Y.-Q.; Guan, X.-Y. Preparation of gem-diarylmethyl Sulfones in Water. China Patent CN201710848209, 18 January 2019. [Google Scholar]
- Gao, S.; Xu, X.; Yuan, Z.; Zhou, H.; Yao, H.; Lin, A. 1,6-Addition arylation of para-quinone methides: An approach to unsymmetrical triarylmethanes. Eur. J. Org. Chem. 2016, 17, 3006–3012. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Anand, R.V. Triflic acid catalyzed 1,6-conjugate addition of thiols to p-quinone methides under continuous-flow conditions. Eur. J. Org. Chem. 2017, 25, 3716–3721. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds compounds 3aa–3ay and 4aa–4am are available from the authors. |

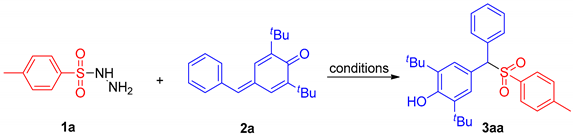
| Entry | Catalyst | Solvent | T (°C) | Time (h) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | - | Et2O | 40 | 10 | 0 |
| 2 | - | Toluene | 80 | 10 | 0 |
| 3 | - | THF | 70 | 10 | 50 |
| 4 | - | DCE | 80 | 10 | trace |
| 5 | - | Dioxane | 80 | 10 | trace |
| 6 | - | DMSO | 80 | 10 | trace |
| 7 | - | H2O | 80 | 10 | 61 |
| 8 | - | EtOH | 80 | 10 | 73 |
| 9 | Pd/C | H2O | 80 | 10 | 75 |
| 10 | Pd/TiO2 | H2O | 80 | 10 | 53 |
| 11 | Au/TiO2 | H2O | 80 | 10 | 43 |
| 12 | Pt/C | H2O | 80 | 10 | 70 |
| 13 | CTAB | H2O | 80 | 10 | 51 |
| 14 | TBAI | H2O | 80 | 10 | 78 |
| 15 | TEBAC | H2O | 80 | 10 | 80 |
| 16 | TBAB | H2O | 80 | 10 | 82 |
| 17 | SDS | H2O | 80 | 10 | 18 |
| 18 | TBAB | H2O | 90 | 10 | 81 |
| 19 | TBAB | H2O | 60 | 10 | 63 |
| 20 | TBAB | H2O | 30 | 10 | 15 |
| 21 | TBAB | H2O | 80 | 12 | 90 |
| 22 c | TBAB | H2O | 80 | 12 | 96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-Q.; You, P.-S.; Zhang, L.-D.; Liu, D.-Q.; Liu, S.-S.; Guan, X.-Y. TBAB-Catalyzed 1,6-Conjugate Sulfonylation of para-Quinone Methides: A Highly Efficient Approach to Unsymmetrical gem-Diarylmethyl Sulfones in Water. Molecules 2020, 25, 539. https://doi.org/10.3390/molecules25030539
Liu Z-Q, You P-S, Zhang L-D, Liu D-Q, Liu S-S, Guan X-Y. TBAB-Catalyzed 1,6-Conjugate Sulfonylation of para-Quinone Methides: A Highly Efficient Approach to Unsymmetrical gem-Diarylmethyl Sulfones in Water. Molecules. 2020; 25(3):539. https://doi.org/10.3390/molecules25030539
Chicago/Turabian StyleLiu, Zhang-Qin, Peng-Sheng You, Liang-Dong Zhang, Da-Qing Liu, Sheng-Shu Liu, and Xiao-Yu Guan. 2020. "TBAB-Catalyzed 1,6-Conjugate Sulfonylation of para-Quinone Methides: A Highly Efficient Approach to Unsymmetrical gem-Diarylmethyl Sulfones in Water" Molecules 25, no. 3: 539. https://doi.org/10.3390/molecules25030539
APA StyleLiu, Z.-Q., You, P.-S., Zhang, L.-D., Liu, D.-Q., Liu, S.-S., & Guan, X.-Y. (2020). TBAB-Catalyzed 1,6-Conjugate Sulfonylation of para-Quinone Methides: A Highly Efficient Approach to Unsymmetrical gem-Diarylmethyl Sulfones in Water. Molecules, 25(3), 539. https://doi.org/10.3390/molecules25030539






