Comparative Study of the Effect of 5,6-Dihydroergosterol and 3-epi-5,6-dihydroergosterol on Chemokine Expression in Human Keratinocytes
Abstract
1. Introduction
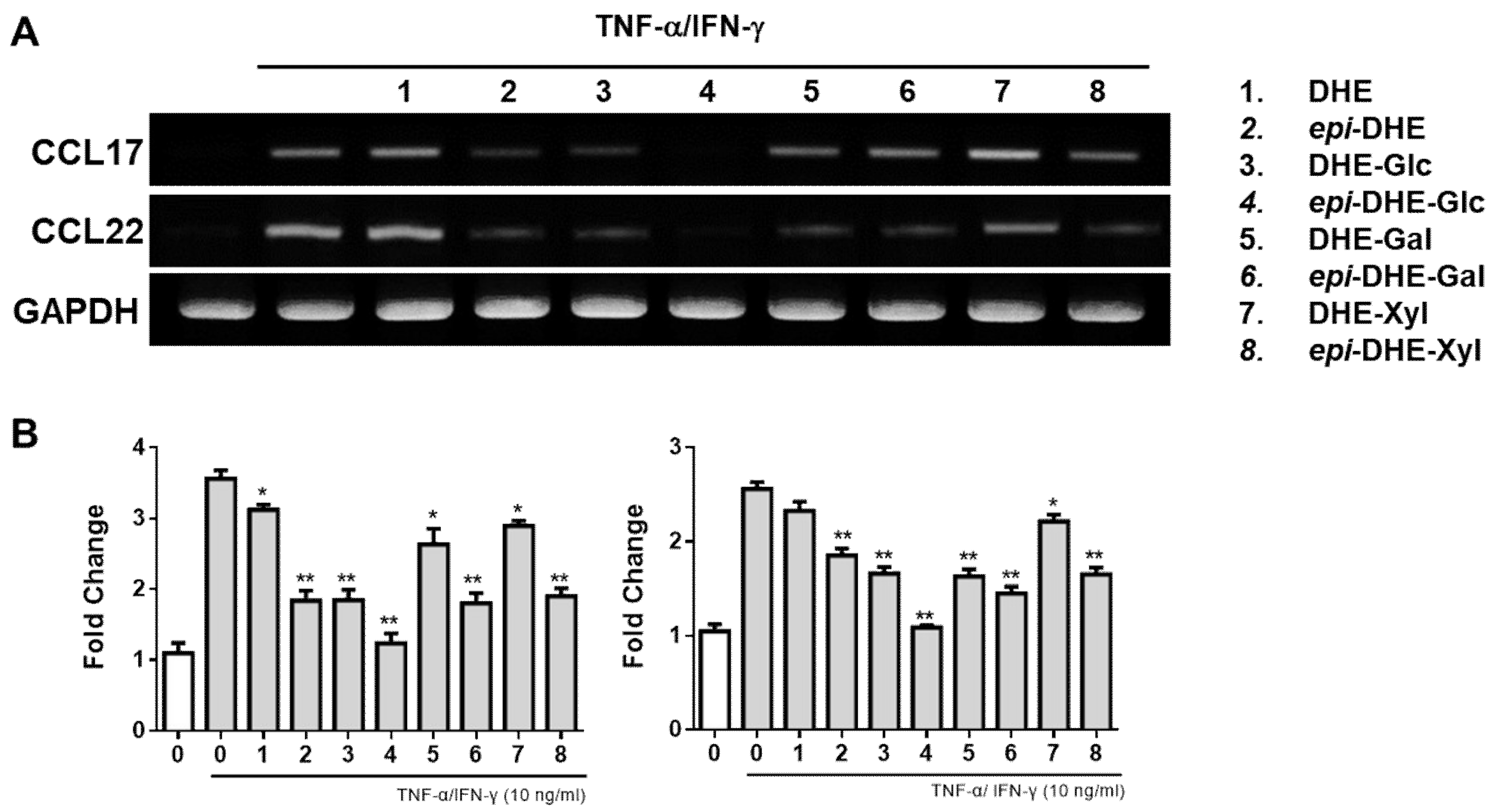
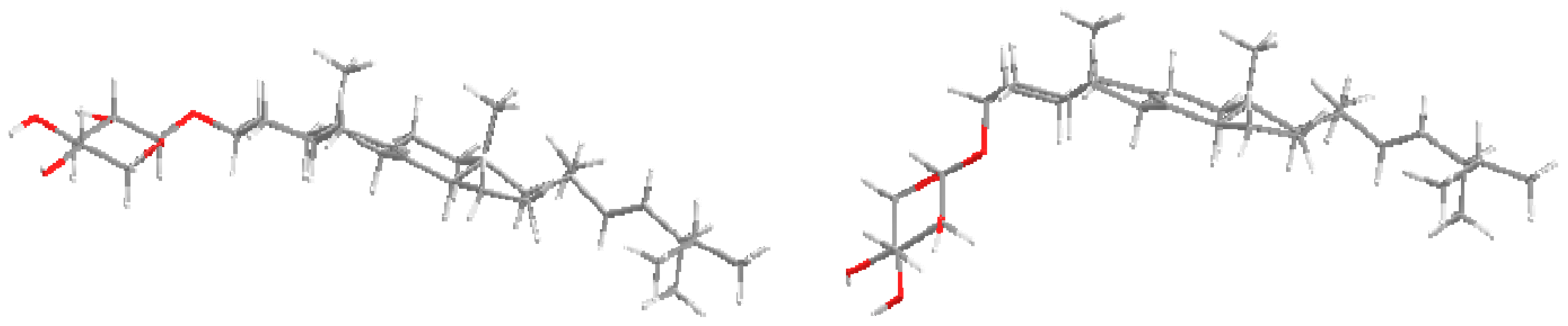
2. Results and Discussion
3. Materials and Methods
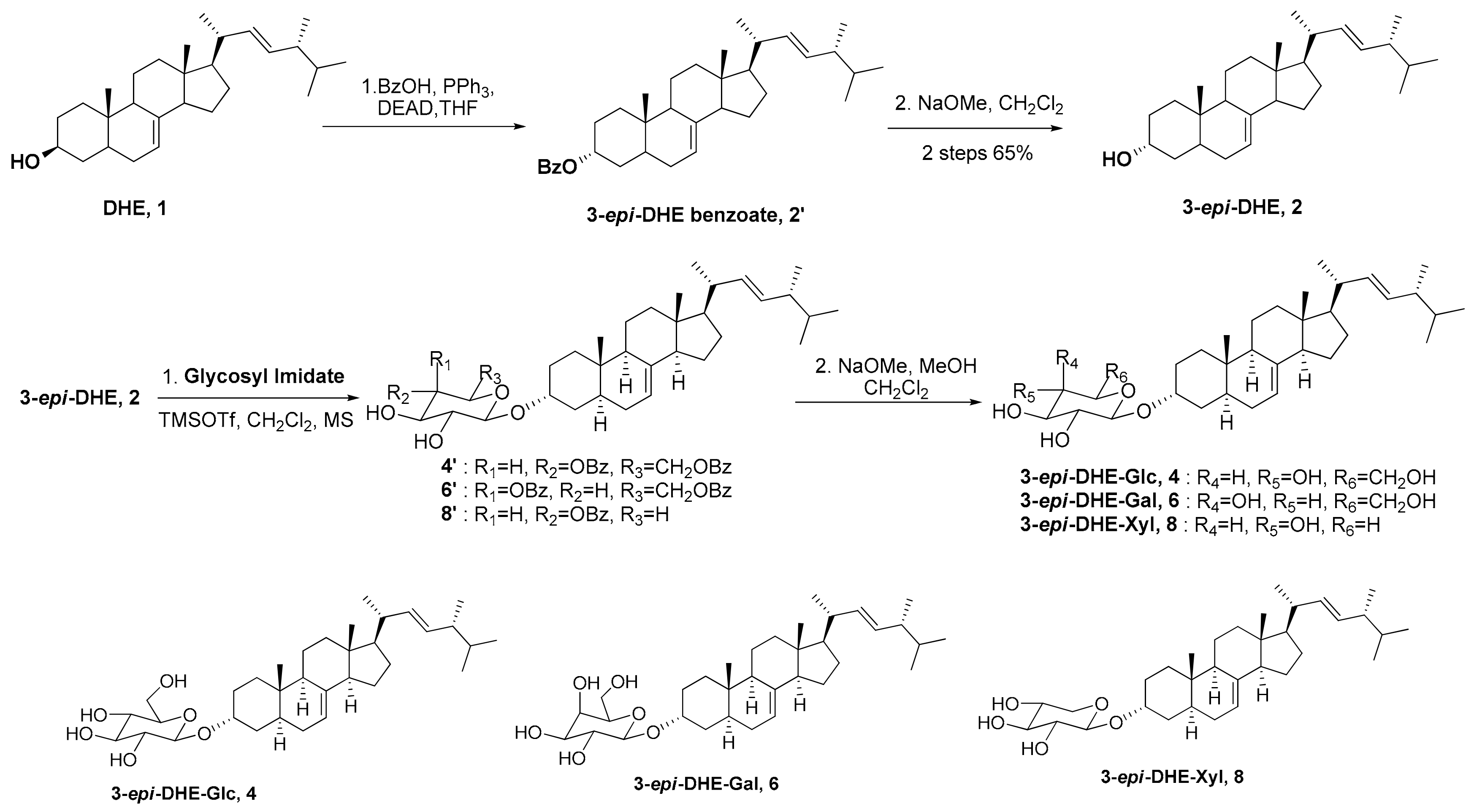
3.1. Synthesis of 3-epi-5,6-dihydroergosterol (3-epi-DHE, 2)
3.2. General Synthetic Procedures of Glycosylation and Hydrolysis
3.2.1. Synthesis of (3α, 22E)-ergosta-7,22-dien-3-yl-β-d-glucopyranoside (3-epi-DHE-β-Glc, 4)
3.2.2. Synthesis of (3α, 22E)-ergosta-7,22-dien-3-yl-β-d-galactopyranoside (3-epi-DHE-β-Gal, 6)
3.2.3. Synthesis of (3α, 22E)-ergosta-7,22-dien-3-yl-β-d-xylopyranoside (3-epi-DHE-β-Xyl, 8)
3.3. Cell Culture
3.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
3.5. Quantitative Real-Time PCR
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khasraw, M.; Bell, R.; Dnag, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Sowa, Y.; Kurobe, M.; Ozono, K.; Caldera, M.; Reddy, G.; Uskokovic, M.; Okano, T. Differential activities of 1alpha,25-dihydroxy-16-ene-vitamin d(3) analogs and their 3-epimers on human promyelocytic leukemia (HL-60) cell differentiation and apoptosis. Steroids 2001, 66, 327–337. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, T.H.; Chang, F.; Kwon, H.J.; Kim, J.Y.; Kim, H.W. Synthesis of ergosterol and 5,6-dihydroergosterol glycosides and their inhibitory activities on lipopolysaccharide-induced nitric oxide production. Bull. Korean Chem. Soc. 2013, 34, 1330–1344. [Google Scholar] [CrossRef]
- Kim, T.K.; Cho, Y.K.; Park, H.G.; Lee, T.H.; Kim, H.W. Comparison of the inhibitory activities of 5,6-dihydroergosterol Glycoside α- and β-anomers on skin inflammation. Molecules 2019, 24, 371. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, T.H.; Oh, H.J.; Kim, H.; Son, Y.; Lee, E.H.; Kim, J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-κB and STAT activation. J. Derm. Sci. 2015, 79, 252–261. [Google Scholar] [CrossRef] [PubMed]
- MacDermott, R.P.; Sanderson, I.R.; Reinecker, H.C. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn’s disease. Inflamm. Bowel. Dis. 1998, 4, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Arimont, M.; Hoffmann, C.; de Graaf, C.; Leurs, R. Chemokine Receptor Crystal Structures: What Can Be Learned from Them? Mol. Pharmacol. 2019, 96, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Matsushita, S.; Tsuchida, T.; Nakamura, K. Inhibitory effect of a histamine 4 receptor antagonist on CCL17 and CCL22 production by monocyte-derived Langerhans cells in patients with atopic dermatitis. J. Derm. 2016, 43, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Nakamura, K.; Oyama, N.; Kaneko, F.; Tsunemi, Y.; Saeki, H.; Tamaki, K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Derm. Sci. 2006, 44, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.S.; Leticia, Q.C.; Jesus, S.R. Regioselective reductions of steroidal conjugated dienes by DIBAH. Tetrahedron Lett. 1995, 36, 8359–8362. [Google Scholar]
- Mitsunobu, O.; Yamada, M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- John, R.W.; Hua, G.; Nathan, H.; Olaoluwa, I.O. Synthesis of the shark repellent pavoninin-4. J. Org. Chem. 2005, 70, 10732–10736. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.S.; Moon, H.J.; Ahn, K.H.; Lee, T.H.; Kim, H. Comparative Study of the Effect of 5,6-Dihydroergosterol and 3-epi-5,6-dihydroergosterol on Chemokine Expression in Human Keratinocytes. Molecules 2020, 25, 522. https://doi.org/10.3390/molecules25030522
Park YS, Moon HJ, Ahn KH, Lee TH, Kim H. Comparative Study of the Effect of 5,6-Dihydroergosterol and 3-epi-5,6-dihydroergosterol on Chemokine Expression in Human Keratinocytes. Molecules. 2020; 25(3):522. https://doi.org/10.3390/molecules25030522
Chicago/Turabian StylePark, Ye Seul, Hye Jin Moon, Kwang Hyun Ahn, Tae Hoon Lee, and Hakwon Kim. 2020. "Comparative Study of the Effect of 5,6-Dihydroergosterol and 3-epi-5,6-dihydroergosterol on Chemokine Expression in Human Keratinocytes" Molecules 25, no. 3: 522. https://doi.org/10.3390/molecules25030522
APA StylePark, Y. S., Moon, H. J., Ahn, K. H., Lee, T. H., & Kim, H. (2020). Comparative Study of the Effect of 5,6-Dihydroergosterol and 3-epi-5,6-dihydroergosterol on Chemokine Expression in Human Keratinocytes. Molecules, 25(3), 522. https://doi.org/10.3390/molecules25030522




