Phase Transition of Ice at High Pressures and Low Temperatures
Abstract
1. Introduction
2. Results and Discussion
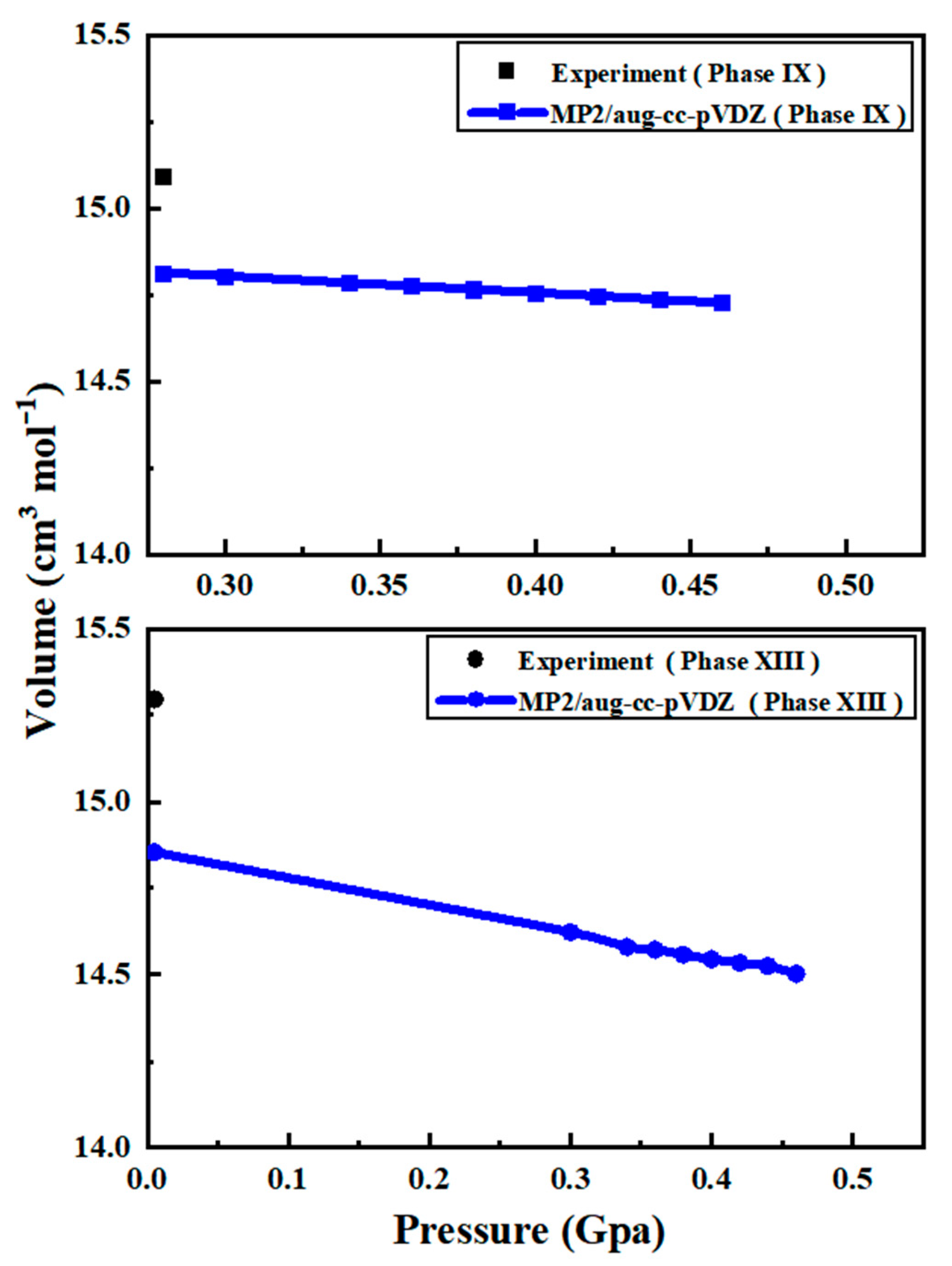
2.1. Crystal Structures
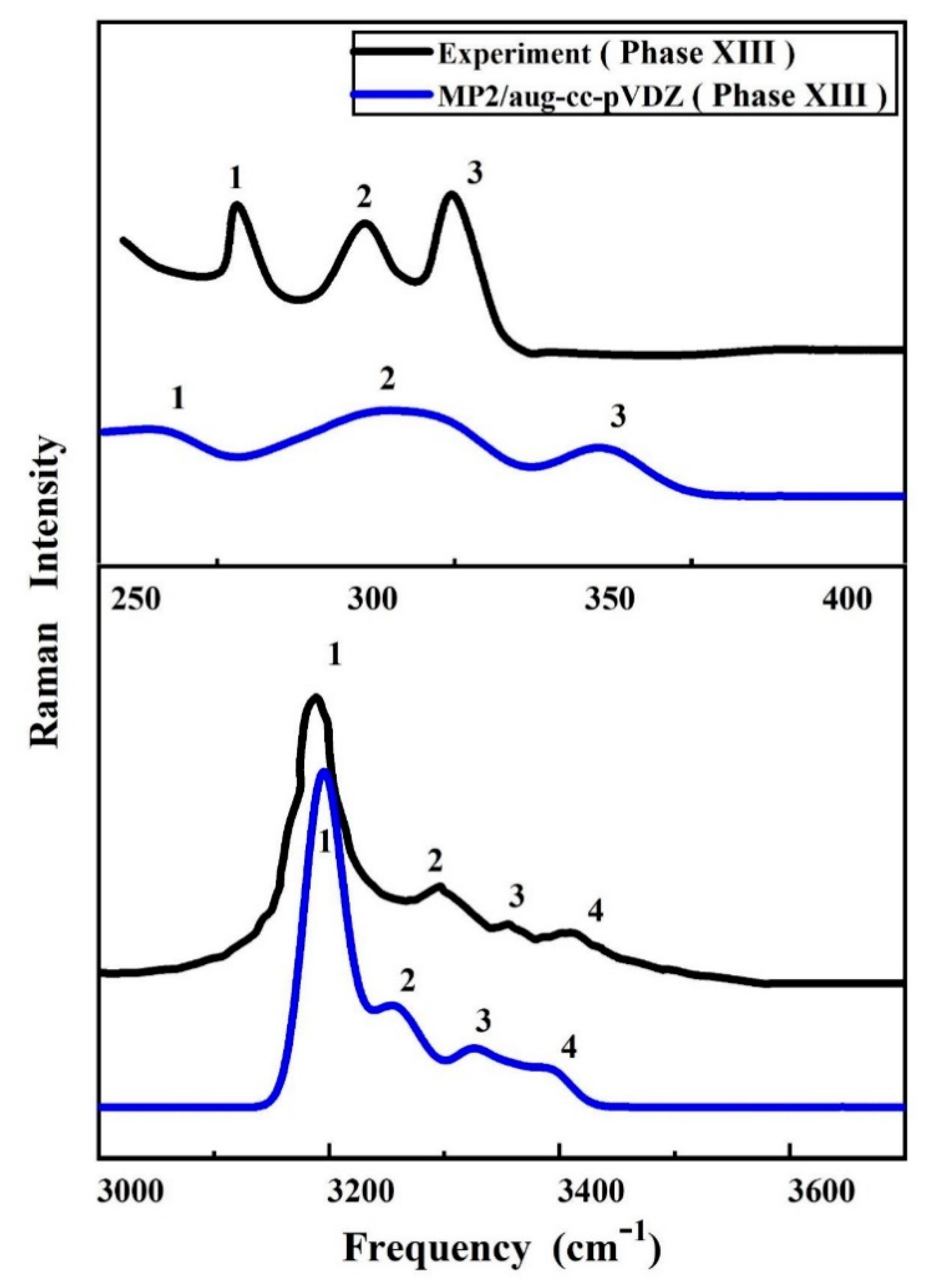
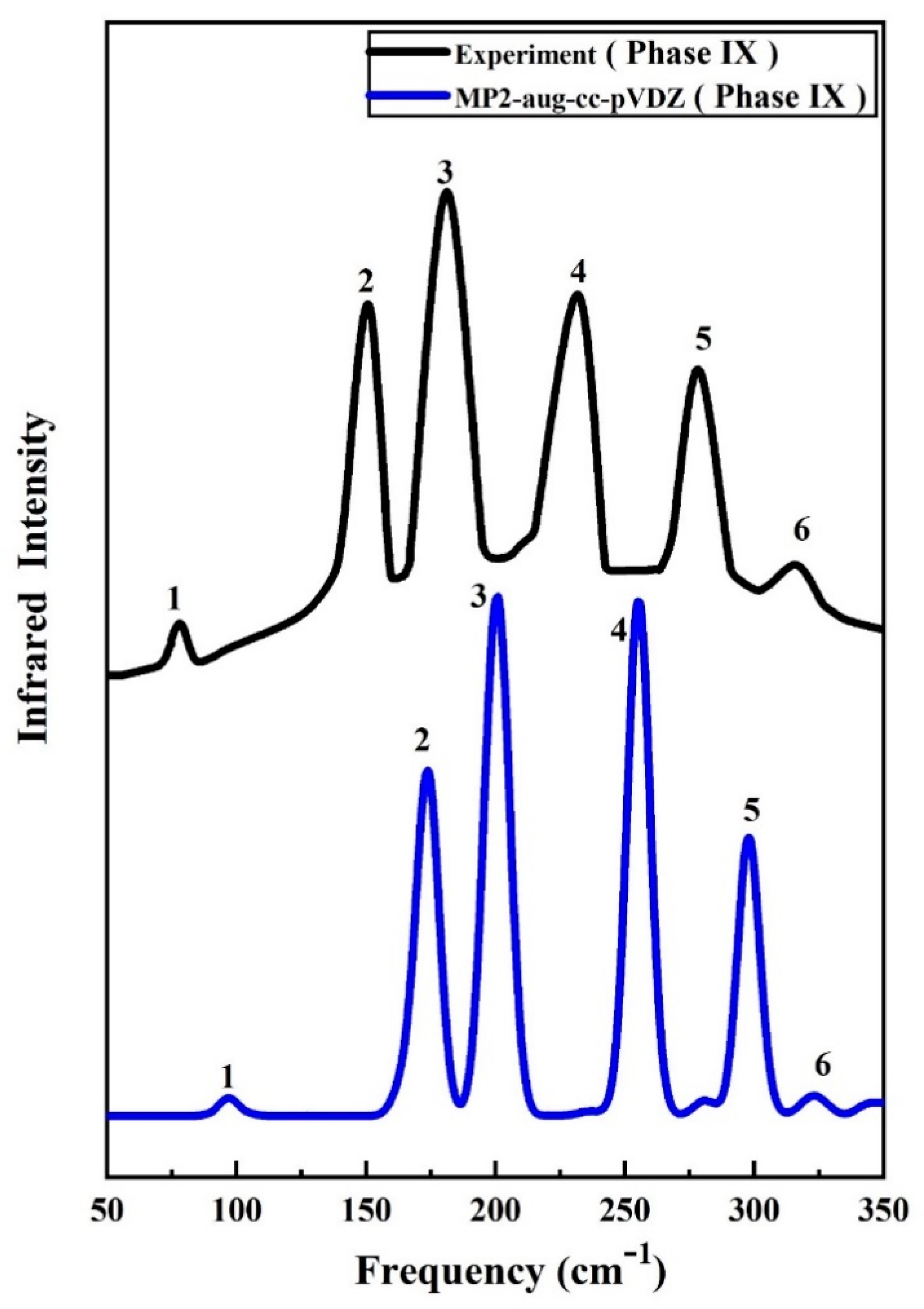
2.2. Vibrational Spectra
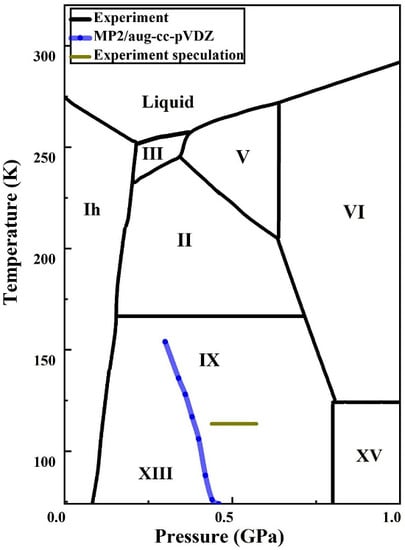
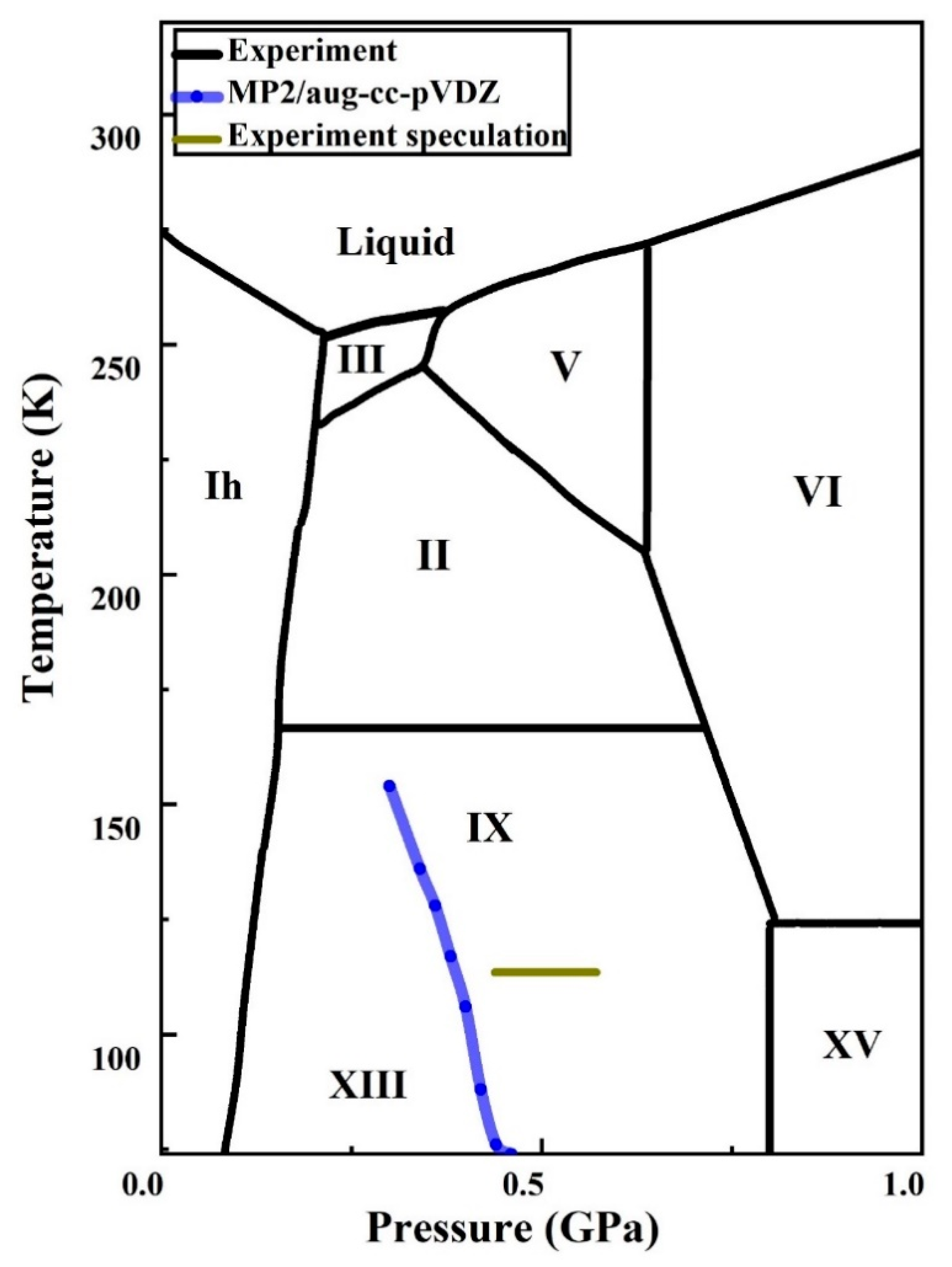
2.3. Phase Transition
2.4. Implementation
2.5. Limitations
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, B.; Engel, E.A.; Behler, J.; Dellago, C.; Ceriotti, M. Ab initio thermodynamics of liquid and solid water. Proc. Natl. Acad. Sci. USA 2019, 116, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.N.; Seidl-Nigsch, M.; Loerting, T. Evidence for high-density liquid water between 0.1 and 0.3 GPa near 150 K. Proc. Natl. Acad. Sci. USA 2019, 116, 9191–9196. [Google Scholar] [CrossRef] [PubMed]
- Millot, M.; Coppari, F.; Rygg, J.R.; Barrios, A.C.; Hamel, S.; Swift, D.C.; Eggert, J.H. Nanosecond X-ray diffraction of shock-compressed superionic water ice. Nature 2019, 569, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Wang, L.; Cao, X.; Su, Y.; Jiang, X.; Meng, S.; Zhao, J.; Zeng, X.C. A new phase diagram of water under negative pressure: The rise of the lowest-density clathrate s-III. Sci. Adv. 2016, 2, e1501010. [Google Scholar] [CrossRef] [PubMed]
- Soper, A.K. Water and ice. Science 2002, 297, 1288–1289. [Google Scholar] [CrossRef]
- Millot, M.; Hamel, S.; Rygg, J.R.; Celliers, P.M.; Collins, G.W.; Coppari, F.; Fratanduono, D.E.; Jeanloz, R.; Swift, D.C.; Eggert, J.H. Experimental evidence for superionic water ice using shock compression. Nat. Phys. 2018, 14, 297–302. [Google Scholar] [CrossRef]
- Londono, J.D.; Kuhs, W.F.; Finney, J.L. Neutron diffraction studies of ices III and IX on under-pressure and recovered samples. J. Chem. Phys. 1993, 98, 4878–4888. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Radaelli, P.G.; Hallbrucker, A.; Mayer, E.; Finney, J.L. The Preparation and Structures of Hydrogen Ordered Phases of Ice. Science 2006, 311, 1758–1761. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Mayer, E.; Hallbrucker, A. Effect of heating rate and pressure on the crystallization kinetics of high-density amorphous ice on isobaric heating between 0.2 and 1.9 GPa. Phys. Chem. Chem. Phys. 2004, 6, 5156–5165. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.Z.H.; He, X. Fragment quantum chemical approach to geometry optimization and vibrational spectrum calculation of proteins. Phys. Chem. Chem. Phys. 2016, 18, 1864–1875. [Google Scholar] [CrossRef]
- Cisneros, G.A.; Wikfeldt, K.T.; Ojamäe, L.; Lu, J.; Xu, Y.; Torabifard, H.; Bartók, A.P.; Csányi, G.; Molinero, V.; Paesani, F. Modeling Molecular Interactions in Water: From Pairwise to Many-Body Potential Energy Functions. Chem. Rev. 2016, 116, 7501–7528. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Biddle, J.W.; DeBenedetti, P.G.; Anisimov, M.A. Two-state thermodynamics and the possibility of a liquid-liquid phase transition in supercooled TIP4P/2005 water. J. Chem. Phys. 2016, 144, 144504. [Google Scholar] [CrossRef] [PubMed]
- Aragones, J.L.; Conde, M.M.; Noya, E.G.; Vega, C. The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: The appearance of a plastic crystal phase. Phys. Chem. Chem. Phys. 2009, 11, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Vega, C.; Abascal, J.; MacDowell, L.G. Phase Diagram of Water from Computer Simulation. Phys. Rev. Lett. 2004, 92, 255701. [Google Scholar] [CrossRef] [PubMed]
- Adler, T.B.; Knizia, G.; Werner, H.-J. A simple and efficient CCSD(T)-F12 approximation. J. Chem. Phys. 2007, 127, 221106. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Sanz, E.; Fernández, R.G.; Vega, C. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Radaelli, P.G.; Slater, B.; Finney, J.L. The polymorphism of ice: Five unresolved questions. Phys. Chem. Chem. Phys. 2011, 13, 18468–18480. [Google Scholar] [CrossRef]
- Kambara, O.; Takahashi, K.; Hayashi, M.; Kuo, J.-L. Assessment of density functional theory to calculate the phase transition pressure of ice. Phys. Chem. Chem. Phys. 2012, 14, 11484–11490. [Google Scholar] [CrossRef]
- Schönherr, M.; Slater, B.; Hutter, J.; Vandevondele, J. Dielectric Properties of Water Ice, the Ice Ih/XI Phase Transition, and an Assessment of Density Functional Theory. J. Phys. Chem. B 2014, 118, 590–596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sode, O.; Keçeli, M.; Hirata, S.; Yagi, K. Coupled-cluster and many-body perturbation study of energies, structures, and phonon dispersions of solid hydrogen fluoride. Int. J. Quantum Chem. 2009, 109, 1928–1939. [Google Scholar] [CrossRef]
- Strain, M.C.; Scuseria, G.E.; Frisch, M.J. Achieving Linear Scaling for the Electronic Quantum Coulomb Problem. Science 1996, 271, 51–53. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Zhang, J.Z.; Qi, L.W. Hydrogen-bond structure dynamics in bulk water: Insights from ab initio simulations with coupled cluster theory. Chem. Sci. 2018, 9, 2065–2073. [Google Scholar] [CrossRef]
- Li, J.; Sode, O.; Voth, G.A.; Hirata, S. A solid–solid phase transition in carbon dioxide at high pressures and intermediate temperatures. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Santra, B.; Klimes, J.; Alfè, D.; Tkatchenko, A.; Slater, B.; Michaelides, A.; Car, R.; Scheffler, M. Hydrogen Bonds and van der Waals Forces in Ice at Ambient and High Pressures. Phys. Rev. Lett. 2011, 107, 185701. [Google Scholar] [CrossRef]
- Knight, C.; Singer, S.J. Prediction of a Phase Transition to a Hydrogen Bond Ordered Form of Ice VI. J. Phys. Chem. B 2005, 109, 21040–21046. [Google Scholar] [CrossRef]
- Fan, X.; Bing, D.; Zhang, J.; Shen, Z.; Kuo, J.-L. Predicting the hydrogen bond ordered structures of ice Ih, II, III, VI and ice VII: DFT methods with localized based set. Comput. Mater. Sci. 2010, 49, S170–S175. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Radaelli, P.G.; Mayer, E.; Finney, J.L. Ice XV: A New Thermodynamically Stable Phase of Ice. Phys. Rev. Lett. 2009, 103, 105701. [Google Scholar] [CrossRef]
- Nanda, K.D.; Beran, G.J.O. What Governs the Proton Ordering in Ice XV? J. Phys. Chem. Lett. 2013, 4, 3165–3169. [Google Scholar] [CrossRef]
- Del Ben, M.; Vandevondele, J.; Slater, B. Periodic MP2, RPA, and Boundary Condition Assessment of Hydrogen Ordering in Ice XV. J. Phys. Chem. Lett. 2014, 5, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhang, J.Z.H.; He, X. Electrostatically Embedded Generalized Molecular Fractionation with Conjugate Caps Method for Full Quantum Mechanical Calculation of Protein Energy. J. Phys. Chem. A 2013, 117, 7149–7161. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, J.; Huang, L.; He, X.; Li, J. Predicting the phase diagram of solid carbon dioxide at high pressure from first principles. Npj Quant. Mater. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Luo, H.; Hao, X.; Gong, Y.; Zhou, J.; He, X.; Li, J. Rational Crystal Polymorph Design of Olanzapine. Cryst. Growth Des. 2019, 19, 2388–2395. [Google Scholar] [CrossRef]
- He, X.; Zhu, T.; Wang, X.; Liu, J.; Zhang, J.Z. Fragment Quantum Mechanical Calculation of Proteins and Its Applications. Acc. Chem. Res. 2014, 47, 2748–2757. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qi, L.W.; Zhang, J.Z.; He, X. Fragment Quantum Mechanical Method for Large-Sized Ion–Water Clusters. J. Chem. Theory Comput. 2017, 13, 2021–2034. [Google Scholar] [CrossRef]
- Liu, J.; He, X. Accurate prediction of energetic properties of ionic liquid clusters using a fragment-based quantum mechanical method. Phys. Chem. Chem. Phys. 2017, 19, 20657–20666. [Google Scholar] [CrossRef]
- Gordon, M.S.; Fedorov, D.G.; Pruitt, S.R.; Slipchenko, L.V. Fragmentation Methods: A Route to Accurate Calculations on Large Systems. Chem. Rev. 2012, 112, 632–672. [Google Scholar] [CrossRef]
- Knight, C.; Singer, S. A reexamination of the ice III/IX hydrogen bond ordering phase transition. J. Chem. Phys. 2006, 125, 064506. [Google Scholar] [CrossRef]
- La Placa, S.J.; Hamilton, W.C.; Kamb, B.; Prakash, A. On a nearly proton-ordered structure for ice IX. J. Chem. Phys. 1973, 58, 567–580. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Radaelli, P.G.; Finney, J.L.; Mayer, E. A calorimetric study on the low temperature dynamics of doped ice V and its reversible phase transition to hydrogen ordered ice XIII. Phys. Chem. Chem. Phys. 2008, 10, 6313–6324. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Houjou, H.; Nagawa, Y.; Goto, M.; Hiratani, K. Cooperative enhancement of water binding to crownophane by multiple hydrogen bonds: Analysis by high level ab initio calculations. J. Am. Chem. Soc. 2001, 123, 4255–4258. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Gilliard, K.; He, X.; Li, J.; Sode, O. Ab Initio Molecular Crystal Structures, Spectra, and Phase Diagrams. Acc. Chem. Res. 2014, 47, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Planas, M.; Sosa, C.; Novoa, J.J. A theoretical study of the ionic dissociation of HF, HCl, and H2S in water clusters. J. Chem. Phys. 1996, 104, 7081–7085. [Google Scholar] [CrossRef]
- Liu, J.F.; He, X.; Zhang, J.Z.H. Structure of liquid water—A dynamical mixture of tetrahedral and ‘ring-and-chain’ like structures. Phys. Chem. Chem. Phys. 2017, 19, 11931–11936. [Google Scholar] [CrossRef]
- Minceva-Sukarova, B.; Sherman, W.F.; Wilkinson, G.R. The Raman spectra of ice (I h, II, III, V, VI and IX) as functions of pressure and temperature. J. Phys. C Solid State Phys. 1984, 17, 5833. [Google Scholar] [CrossRef]
- Bertie, J.E.; Francis, B.F. Raman spectra of ices II and IX above 35 K at atmospheric pressure: Translational and rotational vibrations. J. Chem. Phys. 1982, 77, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Yang, X.; Jiang, S.; Goncharov, A.F. Kinetic boundaries and phase transformations of ice i at high pressure. J. Chem. Phys. 2018, 148, 044508. [Google Scholar] [CrossRef]
- Bertie, J.E.; Francis, B.F. Raman spectra of the O–H and O–D stretching vibrations of ices II and IX to 25 °K at atmospheric pressure. J. Chem. Phys. 1980, 72, 2213–2221. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Hallbrucker, A.; Finney, J.L.; Mayer, E. Raman spectroscopic study of hydrogen ordered ice XIII and of its reversible phase transition to disordered ice V. Phys. Chem. Chem. Phys. 2006, 8, 3088–3093. [Google Scholar] [CrossRef]
- Ramírez, R.; Neuerburg, N.; Herrero, C.P. The phase diagram of ice: A quasi-harmonic study based on a flexible water model. J. Chem. Phys. 2013, 139, 084503. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.; Singer, S.J. Hydrogen bond ordering in ice V and the transition to ice XIII. J. Chem. Phys. 2008, 129, 164513. [Google Scholar] [CrossRef] [PubMed]
- Köster, K.W.; Raidt, A.; Landete, V.F.; Gainaru, C.; Loerting, T.; Böhmer, R. Doping-enhanced dipolar dynamics in ice V as a precursor of hydrogen ordering in ice XIII. Phys. Rev. B 2016, 94, 184306. [Google Scholar] [CrossRef]
- Tajima, Y.; Matsuo, T.; Suga, H. Phase transition in KOH-doped hexagonal ice. Nature 1982, 299, 810–812. [Google Scholar] [CrossRef]
- Lobban, C.; Finney, J.L.; Kuhs, W.F. The structure of a new phase of ice. Nature 1998, 391, 268–270. [Google Scholar] [CrossRef]
- Mercury, L.; Vieillard, P.; Tardy, Y. Thermodynamics of ice polymorphs and ‘ice-like’ water in hydrates and hydroxides. Appl. Geochem. 2001, 16, 161–181. [Google Scholar] [CrossRef]
- Yen, F.; Chi, Z. Proton ordering dynamics of H 2 O ice. Phys. Chem. Chem. Phys. 2015, 17, 12458–12461. [Google Scholar] [CrossRef]
- Fletcher, N.H. The Chemical Physics of Ice; Cambridge University Press (CUP): Cambridge, UK, 1970. [Google Scholar]
- Petrenko, V.F.; Whitworth, R.W. Physics of Ice; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Beran, G.J.O.; Nanda, K. Predicting Organic Crystal Lattice Energies with Chemical Accuracy. J. Phys. Chem. Lett. 2010, 1, 3480–3487. [Google Scholar] [CrossRef]
- Helgaker, T.; Klopper, W.; Tew, D.P. Quantitative quantum chemistry. Mol. Phys. 2008, 106, 2107–2143. [Google Scholar] [CrossRef]
- He, X.; Sode, O.; Xantheas, S.S.; Hirata, S. Second-order many-body perturbation study of ice Ih. J. Chem. Phys. 2012, 137, 204505. [Google Scholar] [CrossRef]
- Hirata, S. Fast electron-correlation methods for molecular crystals: An application to the α, β1, and β2 modifications of solid formic acid. J. Chem. Phys. 2008, 129, 204104. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, J.; Liu, J.; Hu, W.; He, X.; Li, J. Phase Transition of Ice at High Pressures and Low Temperatures. Molecules 2020, 25, 486. https://doi.org/10.3390/molecules25030486
Xu J, Liu J, Liu J, Hu W, He X, Li J. Phase Transition of Ice at High Pressures and Low Temperatures. Molecules. 2020; 25(3):486. https://doi.org/10.3390/molecules25030486
Chicago/Turabian StyleXu, Jinjin, Jinfeng Liu, Jinyun Liu, Wenxin Hu, Xiao He, and Jinjin Li. 2020. "Phase Transition of Ice at High Pressures and Low Temperatures" Molecules 25, no. 3: 486. https://doi.org/10.3390/molecules25030486
APA StyleXu, J., Liu, J., Liu, J., Hu, W., He, X., & Li, J. (2020). Phase Transition of Ice at High Pressures and Low Temperatures. Molecules, 25(3), 486. https://doi.org/10.3390/molecules25030486