Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging
Abstract
1. Introduction
2. Results
2.1. Radiolabeling and In Vitro Stability
2.2. Pretargeted SPECT/CT Bone Imaging
2.3. Pretargeted PET/CT Bone Imaging
2.4. Pretargeted SPECT/CT Tumor Imaging
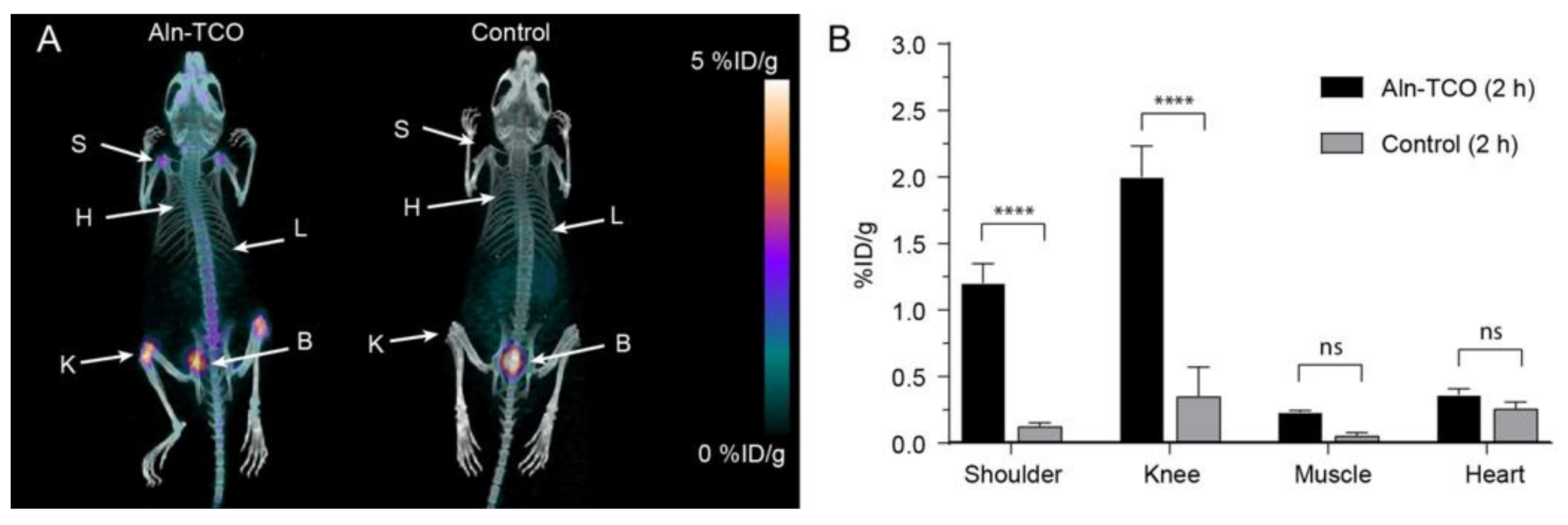
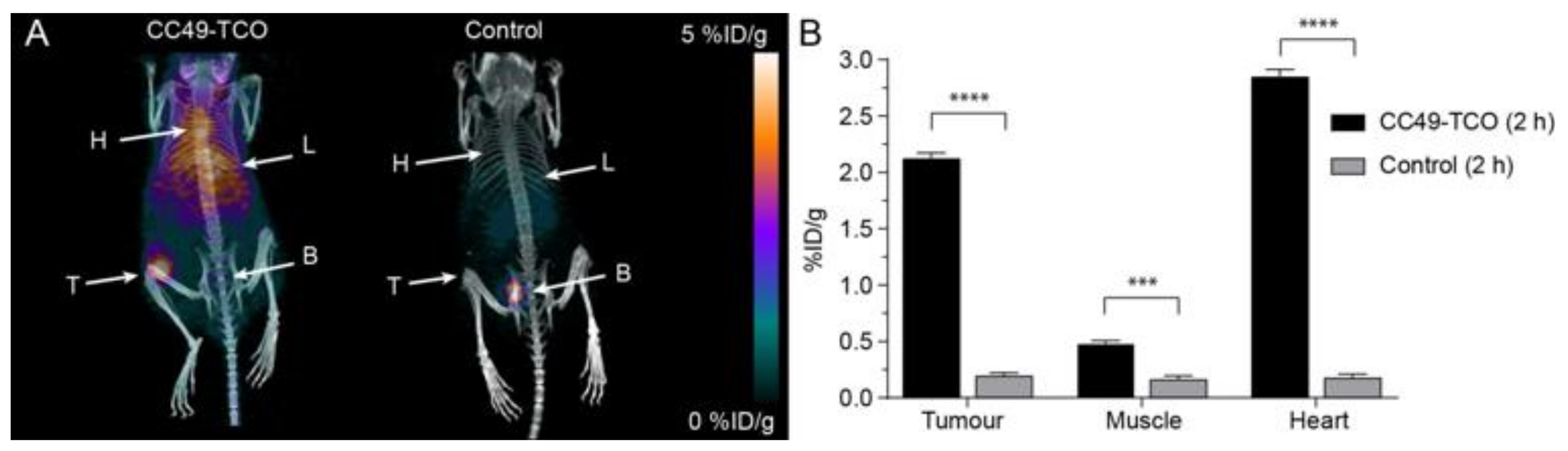
2.5. Pretargeted PET/CT Tumor Imaging
3. Discussion
4. Material and Methods
4.1. General
4.2. Statistics
4.3. Synthesis of Pretargeting Components
4.4. Radiochemistry—Labeling of 1 with Indium-111
4.5. Labeling of 3 with Galium-68
4.6. Determination of Log D (pH 7.4)
4.7. In Vitro Stability
4.8. In Vivo Studies
4.8.1. Evaluation of [111In]2 in Mice Pretreated with 5
4.8.2. Evaluation of [111In]2 in Mice Pretreated with 4
4.8.3. Evaluation of [68Ga]3 in Mice Bearing LS174T Tumor Xenografts
4.8.4. Evaluation of [68Ga]3 in Mice Pre-Treated with 4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraeber-Bodéré, F.; Bodet-Milin, C.; Rousseau, C.; Eugène, T.; Pallardy, A.; Frampas, E.; Carlier, T.; Ferrer, L.; Gaschet, J.; Davodeau, F.; et al. Radioimmunoconjugates for the treatment of cancer. Semin. Oncol. 2014, 41, 613–622. [Google Scholar] [CrossRef]
- Bourgeois MBailly, C.; Frindel, M.; Guerard, F.; Chérel, M.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Bodet-Milin, C. Radioimmunoconjugates for treating cancer: Recent advances and current opportunities. Expert Opin. Biol. Ther. 2017, 17, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Jørgensen, J.T.; Johann, K.; Norregaard, K.; Sohr, B.; Svatunek, D.; Birke, A.; Shalgunov, V.; Edem, P.E.; Rossin, R.; et al. Trans-Cyclooctene-Functionalized PeptoBrushes with Improved Reaction Kinetics of the Tetrazine Ligation for Pretargeted Nuclear Imaging. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Verkerk, P.R.; van den Bosch, S.M.; Vulders, R.C.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.D.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels-Alder Click Chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef]
- Adumeau, P.; Carnazza, K.E.; Brand, C.; Carlin, S.D.; Reiner, T.; Agnew, B.J.; Lewis, J.S.; Zeglis, B.M. A pretargeted approach for the multimodal PET/NIRF imaging of colorectal cancer. Theranostics 2016, 6, 2267–2277. [Google Scholar] [CrossRef]
- García, M.F.; Zhang, X.; Shah, M.; Newton-Northup, J.; Cabral, P.; Cerecetto, H.; Quinn, T. m99mTc-bio-orthogonal click chemistry reagent for in vivo pretargeted imaging. Bioorgan. Med. Chem. 2016, 24, 1209–1215. [Google Scholar]
- Meyer, J.P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef]
- Meyer, J.P.; Kozlowski, P.; Jackson, J.; Cunanan, K.M.; Adumeau, P.; Dilling, T.R.; Zeglis, B.M.; Lewis, J.S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60, 8201–8217. [Google Scholar] [CrossRef]
- Rossin, R.; Lappchen, T.; van den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels-Alder Reaction for Tumor Pretargeting: In Vivo Chemistry Can Boost Tumor Radiation Dose Compared with Directly Labeled Antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; van den Bosch, S.M.; Ten Hoeve, W.; Carvelli, M.; Versteegen, R.M.; Lub, J.; Robillard, M.S. Highly reactive trans-cyclooctene tags with improved stability for diels-alder chemistry in living systems. Bioconjug. Chem. 2013, 24, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Van Duijnhoven, S.M.J.; Läppchen, T.; Van Den Bosch, S.M.; Robillard, M.S. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels-Alder reaction. Mol. Pharm. 2014, 11, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, S.M.J.; Rossin, R.; van den Bosch, S.M.; Wheatcroft, M.P.; Hudson, P.J.; Robillard, M.S. Diabody Pretargeting with Click Chemistry In Vivo. J. Nucl. Med. 2015, 56, 1422–1428. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Brand, C.; Abdel-Atti, D.; Carnazza, K.E.; Cook, B.E.; Carlin, S.; Reiner, T.; Lewis, J.S. Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand pharmacokinetics. Mol. Pharm. 2015, 12, 3575–3587. [Google Scholar] [CrossRef]
- Shah, M.A.; Zhang, X.; Rossin, R.; Robillard, M.S.; Fisher, D.R.; Bueltmann, T.; Hoeben, F.J.M.; Quinn, T.P. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using α-Particle Radiation. Bioconjug. Chem. 2017, 28, 3007–3015. [Google Scholar] [CrossRef]
- Läppchen, T.; Rossin, R.; van Mourik, T.R.; Gruntz, G.; Hoeben, F.J.M.; Versteegen, R.M.; Janssen, H.M.; Lub, J.; Robillard, M.S. DOTA-tetrazine probes with modified linkers for tumor pretargeting. Nucl. Med. Biol. 2017, 55, 19–26. [Google Scholar] [CrossRef]
- Altai, M.; Perols, A.; Tsourma, M.; Mitran, B.; Honarvar, H.; Robillard, M.; Rossin, R.; ten Hoeve, W.; Lubberink, M.; Orlova, A.; et al. Feasibility of Affibody-Based Bio-orthogonal Chemistry-Mediated Radionuclide Pretargeting. J. Nucl. Med. 2016, 57, 431–436. [Google Scholar] [CrossRef]
- Rossin, R.; Versteegen, R.M.; Wu, J.; Khasanov, A.; Wessels, H.J.; Steenbergen, E.J.; Ten Hoeve, W.; Janssen, H.M.; van Onzen, A.H.A.M.; Hudson, P.J.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumor activity in mice. Nat. Commun. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Yazdani, A.; Bilton, H.; Vito, A.; Genady, A.R.; Rathmann, S.M.; Ahmad, Z.; Janzen, N.; Czorny, S.; Zeglis, B.M.; Francesconi, L.C.; et al. A Bone-Seeking trans-Cyclooctene for Pretargeting and Bio-orthogonal Chemistry: A Proof of Concept Study Using 99mTc- and177Lu-Labeled Tetrazines. J. Med. Chem. 2016, 59, 9381–9389. [Google Scholar] [CrossRef] [PubMed]
- Edem, P.E.; Sinnes, J.P.; Pektor, S.; Bausbacher, N.; Rossin, R.; Yazdani, A.; Miederer, M.; Kjær, A.; Valliant, J.F.; Robillard, M.S.; et al. Evaluation of the inverse electron demand Diels-Alder reaction in rats using a scandium-44-labeled tetrazine for pretargeted PET imaging. EJNMMI Res. 2019, 9, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Grassser, W.; Endi, W.; Atkins, R.; Simmonis HThompsona, D.D.; Golub, E.; Rodan, G.A. Bisphosphonate action: Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Investig. 1991, 88, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Isabel, M.; Prata, M. Gallium-68: A New Trend in PET Radiopharmacy. Curr. Radiopharm. 2012, 5, 142–149. [Google Scholar] [CrossRef]
- Vito, A.; Alarabi, H.; Czorny, S.; Beiraghi, O.; Kent, J.; Janzen, N.; Genady, A.R.; Al-Karmi, S.A.; Rathmann, S.; Naperstkow, Z.; et al. A 99mTc-labeled tetrazine for bio-orthogonal chemistry. Synthesis and biodistribution studies with small molecule trans-cyclooctene derivatives. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef]
- Yazdani, A.; Janzen, N.; Czorny, S.; Ungard, R.G.; Miladinovic, T.; Singh, G.; Valliant, J.F. Preparation of tetrazine-containing [2 + 1] complexes of 99mTc and: In vivo targeting using bioorthogonal inverse electron demand Diels-Alder chemistry. Dalt. Trans. 2017, 46, 14691–14699. [Google Scholar] [CrossRef]
- Evans, H.L.; Carroll, L.; Aboagye, E.O.; Spivey, A.C. Bioorthogonal chemistry for 68Ga radiolabeling of DOTA-containing compounds. J. Label. Compd. Radiopharm. 2014, 57, 291–297. [Google Scholar] [CrossRef]
- Albu, S.A.; Vito, A.; Dzandzi, J.P.; Zlitni, A.; Beckford-Vera, D.; Blacker, M.; Janzen, N.; Patel, R.M.; Capretta, A.; Valliant, J.F. 125I-Tetrazines and Inverse-Electron-Demand Diels-Alder Chemistry: A Convenient Radioiodination Strategy for Biomolecule Labeling, Screening, and Biodistribution Studies. Bioconjug. Chem. 2016, 27, 207–216. [Google Scholar] [CrossRef]
- Fujiki, K.; Yano, S.; Ito, T.; Kumagai, Y.; Murakami, Y.; Kamigaito, O.; Haba, H.; Tanaka, K. A One-Pot Three-Component Double-Click Method for Synthesis of [67Cu]-Labeled Biomolecular Radiotherapeutics. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Denk, C.; Svatunek, D.; Filip, T.; Wanek, T.; Lumpi, D.; Fröhlich, J.; Kuntner, C.; Mikula, H. Development of a 18F-labeled tetrazine with favorable pharmacokinetics for bioorthogonal PET imaging. Angew. Chem. Int. Ed. 2014, 53, 9655–9659. [Google Scholar] [CrossRef]
- Herth, M.M.; Andersen, V.L.; Lehel, S.; Madsen, J.; Knudsen, G.M.; Kristensen, J.L. Development of a 11C-labeled tetrazine for rapid tetrazine-trans-cyclooctene ligation. Chem. Commun. 2013, 49, 3805–3807. [Google Scholar] [CrossRef]
- Rashidian, M.; Wang, L.; Edens, J.G.; Jacobsen, J.T.; Hossain, I.; Wang, Q.; Victora, G.D.; Vasdev, N.; Ploegh, H.; Liang, S.H. Enzyme-Mediated Modification of Single-Domain Antibodies for Imaging Modalities with Different Characteristics. Angew. Chem. Int. Ed. 2016, 55, 528–533. [Google Scholar] [CrossRef]
- Denk, C.; Svatunek, D.; Mairinger, S.; Stanek, J.; Filip TMatscheko, D.; Kuntner, C.; Wanek, T.; Mikula, H. Design, Synthesis, and Evaluation of a Low-Molecular-Weight 11C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bio-orthogonal in Vivo Click Chemistry. Bioconjug. Chem. 2016, 27, 1707–1712. [Google Scholar] [CrossRef]
- Keinänen, O.; Mäkilä, E.M.; Lindgren, R.; Virtanen, H.; Liljenbäck, H.; Oikonen, V.; Sarparanta, M.; Molthoff, C.; Windhorst, A.D.; Roivainen, A.; et al. Pretargeted PET Imaging of trans-Cyclooctene-Modified Porous Silicon Nanoparticles. ACS Omega 2017, 2, 62–69. [Google Scholar] [CrossRef]
- Keinänen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of internalizing trastuzumab and cetuximab with a 18F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7, 95. [Google Scholar] [CrossRef]
- Liu, S.; Pietryka, J.; Ellars, C.E.; Edwards, D.S. Comparison of Yttrium and indium complexes of DOTA-BA and DOTA-MBA: Models for 90Y- and 111in-labeled DOTA-biomolecule conjugates. Bioconjug. Chem. 2002, 13, 902–913. [Google Scholar] [CrossRef]
- Broan, C.J.; Cox, J.P.L.; Craig, A.S.; Kataky, R.; Parker, D.; Harrison, A.; Randall, A.M.; Ferguson, G. Structure and solution stability of indium and gallium complexes of 1,4,7-triazacyclononanetriacetate and of yttrium complexes of 1,4,7,10-tetraazacyclododecanetetraacetate and related ligands: kinetically stable complexes for use in imaging and radioimmunotherapy. J. Chem. Soc. Perkin Trans. 1991, 2, 87–99. [Google Scholar] [CrossRef]
- Heppeler, A.; Froidevaux, S.; Mäcke, H.R.; Jermann, E.; Béhé, M.; Powell, P.; Hennig, M. Radiometal-Labeled Macrocyclic Chelator-Derivatised Somatostatin Analogue with Superb Tumor-Targeting Properties and Potential for Receptor-Mediated Internal Radiotherapy. Chem. A Eur. J. 1999, 5, 1974–1981. [Google Scholar] [CrossRef]
- Antunes, P.; Ginj, M.; Zhang, H.; Waser, B.; Baum, R.P.; Reubi, J.C.; Maecke, H. Are radiogallium-labeled DOTA-conjugated somatostatin analogues superior to those labeled with other radiometals? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 982–993. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Cal-Gonzalez, J.; Vaquero, J.J.; Herraiz, J.L.; Pérez-Liva, M.; Soto-Montenegro, M.L.; Peña-Zalbidea, S.; Desco, M.; Udías, J.M. Improving PET Quantification of Small Animal [68Ga]DOTA-Labeled PET/CT Studies by Using a CT-Based Positron Range Correction. Mol. Imaging Biol. 2018, 20, 584Y593. [Google Scholar] [CrossRef] [PubMed]
- Edem, P.E.; Czorny, S.; Valliant, J.F. Synthesis and evaluation of radioiodinated acyloxymethyl ketones as activity-based probes for cathepsin B. J. Med. Chem. 2014, 57, 9564–9577. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |

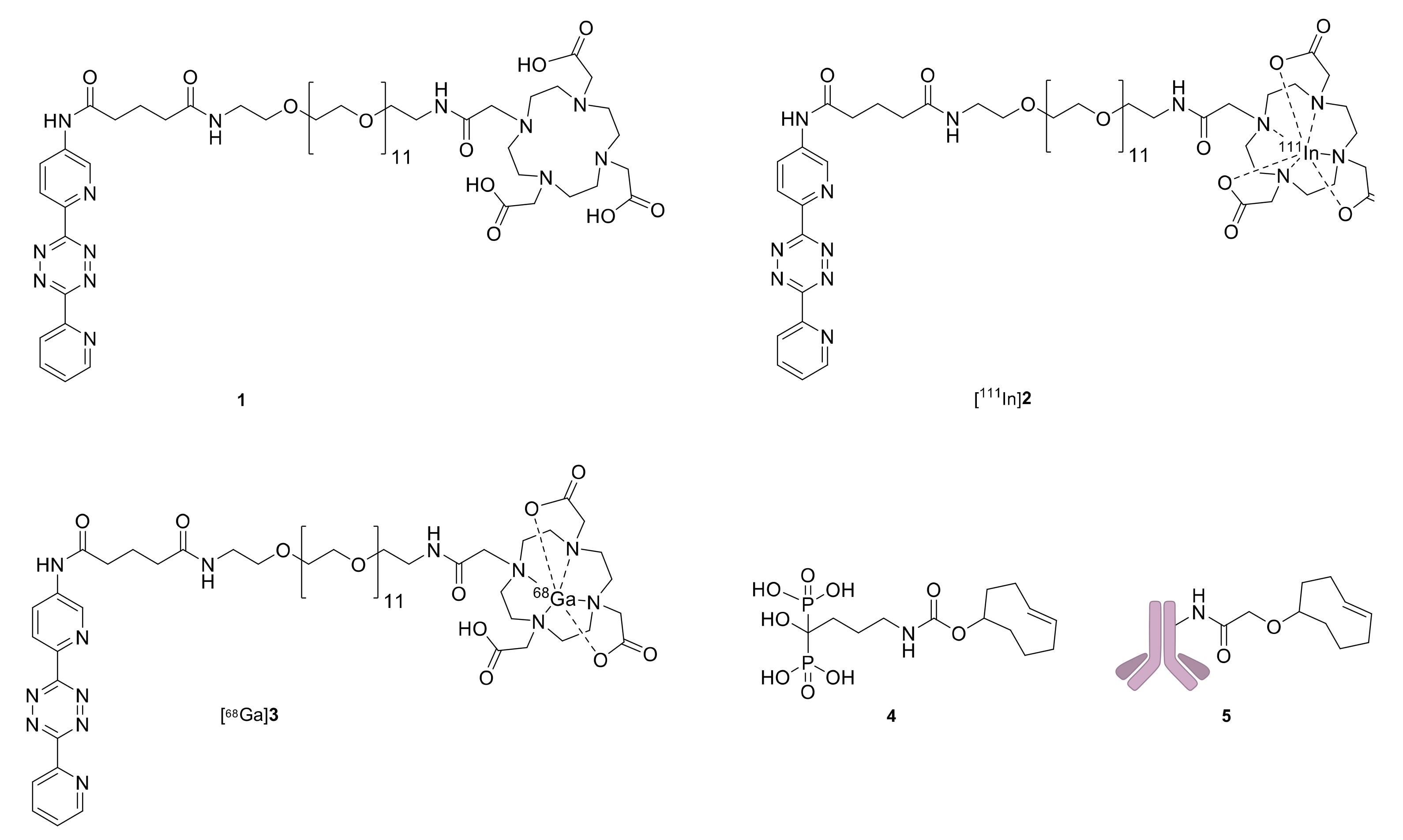
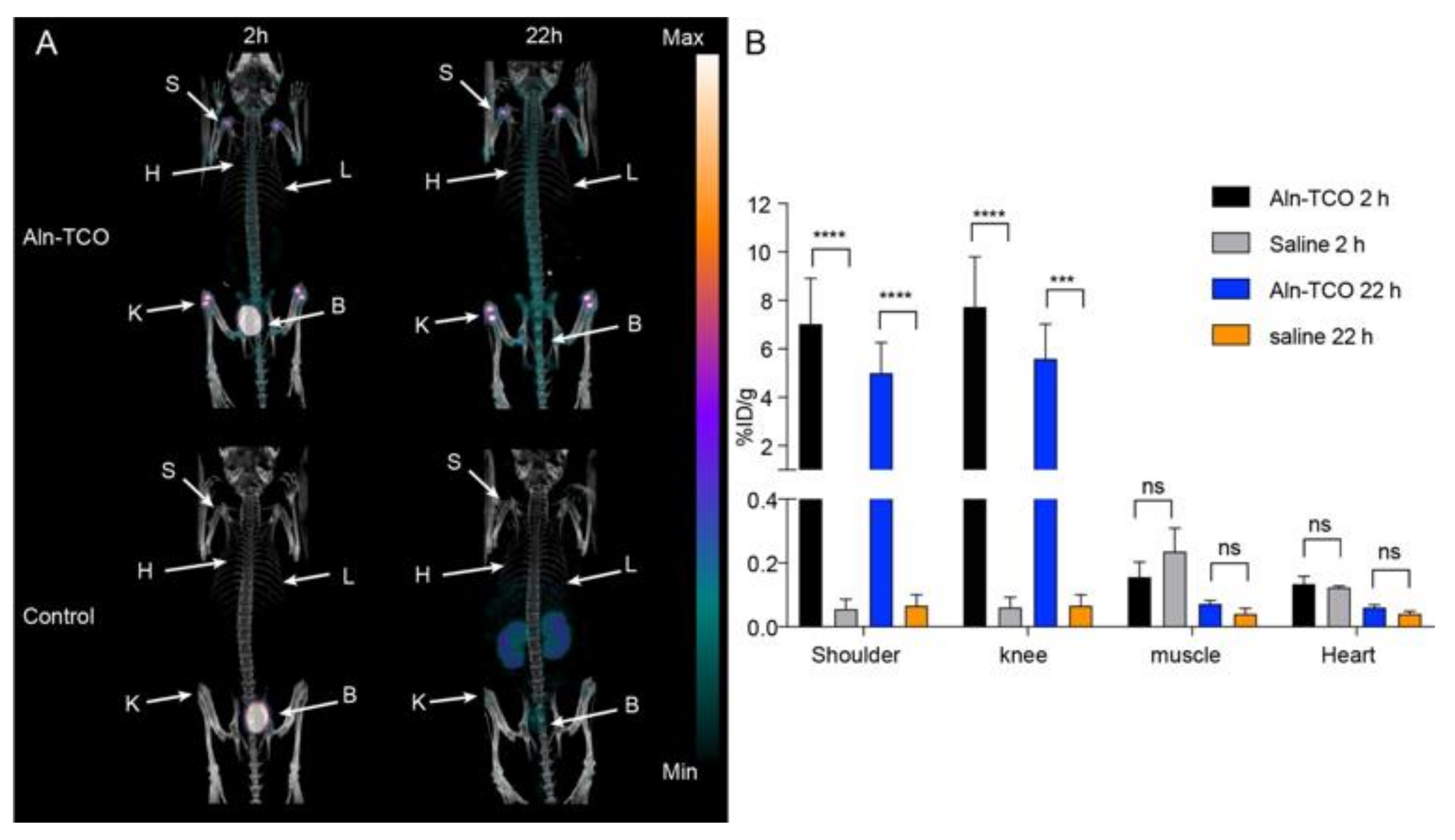
| Pretargeted [111In]2 (%ID/g) | Pretargeted [68Ga]3 (%ID/g) | |||
|---|---|---|---|---|
| SPECT 2 h | SPECT 22 h | PET 2 h | Ex Vivo 2 h | |
| Shoulder | 7.0 ± 1.9 | 5.0 ± 1.2 | 1.20 ± 0.01 | 2.3 ± 0.5 |
| Knee | 7.7 ± 2.1 | 5.6 ± 1.4 | 2.0 ± 0.2 | 3.7 ± 0.6 |
| Muscle | 0.16 ± 0.05 | 0.07 ± 0.01 | 0.23 ± 0.01 | 0.10 ± 0.04 |
| Heart * | 0.13 ± 0.02 | 0.06 ± 0.01 | 0.36 ± 0.05 | -** |
| Pretargeted [111In]2 (%ID/g) | Pretargeted [68Ga]3 | |||
|---|---|---|---|---|
| SPECT 2 h | SPECT 22 h | PET 2 h | Ex Vivo 2 h | |
| Tumor | 4.8 ± 0.4 | 12.3 ± 0.6 | 2.1 ± 0.1 | 5.8 ± 0.3 |
| Heart (blood) | 8.7 ± 0.4 * | 5.2 ± 0.2 * | 2.9 ± 0.1 * | 5.5 ± 0.1 ** |
| Muscle | 1.5 ± 0.1 | 1.3 ± 0.1 | 0.48 ± 0.02 | 0.6 ± 0.1 |
| T/M ratio | 3.2 | 9.2 | 4.4 | 9.8 |
| T/B ratio | 0.6 *** | 2.4 *** | 0.8 *** | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edem, P.E.; Jørgensen, J.T.; Nørregaard, K.; Rossin, R.; Yazdani, A.; Valliant, J.F.; Robillard, M.; Herth, M.M.; Kjaer, A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 2020, 25, 463. https://doi.org/10.3390/molecules25030463
Edem PE, Jørgensen JT, Nørregaard K, Rossin R, Yazdani A, Valliant JF, Robillard M, Herth MM, Kjaer A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules. 2020; 25(3):463. https://doi.org/10.3390/molecules25030463
Chicago/Turabian StyleEdem, Patricia E., Jesper T. Jørgensen, Kamilla Nørregaard, Rafaella Rossin, Abdolreza Yazdani, John F. Valliant, Marc Robillard, Matthias M. Herth, and Andreas Kjaer. 2020. "Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging" Molecules 25, no. 3: 463. https://doi.org/10.3390/molecules25030463
APA StyleEdem, P. E., Jørgensen, J. T., Nørregaard, K., Rossin, R., Yazdani, A., Valliant, J. F., Robillard, M., Herth, M. M., & Kjaer, A. (2020). Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules, 25(3), 463. https://doi.org/10.3390/molecules25030463








