Abstract
Near-infrared (NIR) fluorescent probes are attractive tools for bioimaging applications because of their low auto-fluorescence interference, minimal damage to living samples, and deep tissue penetration. H2S is a gaseous signaling molecule that is involved in redox homeostasis and numerous biological processes in vivo. To this end, we have developed a new red shifted fluorescent probe 1 to detect physiological H2S in live cells. The probe 1 is based on a rhodamine derivative as the red shifted fluorophore and the thiolysis of 7-nitro 1,2,3-benzoxadiazole (NBD) amine as the H2S receptor. The probe 1 displays fast fluorescent enhancement at 660 nm (about 10-fold turn-ons, k2 = 29.8 M−1s−1) after reacting with H2S in buffer (pH 7.4), and the fluorescence quantum yield of the activated red shifted product can reach 0.29. The probe 1 also exhibits high selectivity and sensitivity towards H2S. Moreover, 1 is cell-membrane-permeable and mitochondria-targeting, and can be used for imaging of endogenous H2S in living cells. We believe that this red shifted fluorescent probe can be a useful tool for studies of H2S biology.
1. Introduction
Recently biological reports have demonstrated the important role of H2S, and the results suggested that endogenously-produced hydrogen sulfide (H2S) has been marked as gasotransmitter, allowing the regulation of numerous important physiological functions including; cardiovascular, gastrointestinal, endocrine, nervous, and immune systems [1,2,3]. Generally, endogenous H2S can be produced enzymatically from L-cysteine (Cys) by means of three distinctive enzymatic pathways; cystathionine γ-lyase (CSE), cystathionine β-synthetase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) [4]. The interest in the molecular mechanisms of H2S associated with physiology and pathology was sparked out of its recognition as a vital signaling molecule. However, the abnormal levels of H2S production lead to number of different human diseases including; diabetes [5], Alzheimer’s disease [6] liver cirrhosis [7], and the symptoms of Down’s syndrome [8,9,10]. As an important role played by H2S in tumor biology, it is proposed that the both production and inhibition of H2S concentration beyond a threshold level could exert anticancer effects [10,11]. While in plants, the growth and development, seed germination, and stress tolerance including cross-adaptation are regulated by H2S [12,13,14]. H2S also plays important role in microorganisms [15]. Due to its wide distribution in all organisms, the physiological characters of H2S and the precise mechanisms by which H2S may involve in vivo still remain largely unexplored. Therefore, adequate tools (H2S probes or donors) are necessary to further explore H2S biology [16,17,18,19,20,21]. For cellular H2S detection, various fluorescence probes have been successfully developed [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. However, H2S fluorescence probes for in vivo bioimaging are still rare [16,17,18], especially for the imaging of H2S-related diseases including cancers [22]. Due to the potential limitations of fluorescent probes in deep tissue penetration, photodamage to biological samples, and background auto-fluorescence in living systems, it is crucial to develop probes associated with long wavelength emission especially in the red shifted region [39,40,41,42].
Recently, numbers of red shifted and NIR fluorescent dyes have been developed [43,44,45,46,47,48,49,50,51]. Consequently, various NIR fluorescent probes have been designed on the basis of different organic reaction scenario [8,52,53,54,55,56,57,58]. Among them, we as well as others discovered a reaction of H2S-specifc thiolysis of 7-nitro 1,2,3-benzoxadiazole (NBD) amines [33,58,59] to detect millimolar H2S in a long range wavelength. Herein, this reaction strategy is further employed for the development of a new red shifted fluorescent probe 1 (Scheme 1).
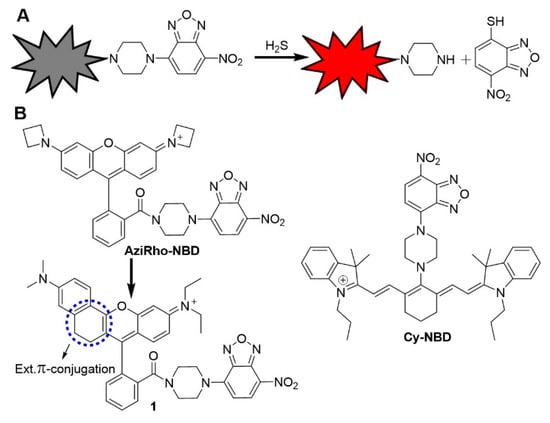
Scheme 1.
(A) The thiolysis of 7-nitro 1,2,3-benzoxadiazole (NBD) amine reaction for H2S fluorescence probe. (B) Chemical structures of selected NBD-based H2S probes.
NIR dyes, including cyanine (Cy), are considered the classic NIR fluorescent dyes [60]. However, due to their flexible molecular structure, some of NIR fluorescent dyes accompanied some short comings for example, small Stokes shift, limited fluorescence quantum yield, low photo-stability lying, and high occupied molecular orbital (HOMO) energy levels [61,62]. Such photo-physical properties strongly affect the fluorescence signals due to the high background signal, which in turn result in low contrast for bioimaging [63,64,65,66]. In 2017, we developed a Cy-NBD probe (Scheme 1) which has the limitation of low quantum yield after H2S activation [58]. On the other side, classical rhodamine dyes contributed much in the field of biomolecular detection and biomedical imaging because of its magnificent photophysical and chemical properties [30,67,68,69,70,71]. Due to limited π-conjugated system of xanthene core derivatives, such as rhodamine B, rhodamine 6G, and rhodamine 123 have their emission wavelengths in the visible region (<600 nm). Recently, significant advancements have been made in the improvement of rhodamines-based fluorescent dyes with extended the π-conjugated system possessing long emission wavelength, high fluorescence quantum yield, and outstanding photostability [70,71,72,73,74,75]. Herein, we report the development of an extended π-conjugated rhodamine-NBD based probe 1 for the highly selective imaging of endogenous H2S in a red shifted region [70,71]. The probe is high selectivity towards H2S among other biothiols, with fluorescence emission in the red shifted region (>660 nm) and high fluorescence quantum yield (0.29) after H2S activation. The probe is successfully used for bioimaging of endogenous H2S in living cells.
2. Results and Discussion
2.1. Synthesis of 1
Probe 1 was constructed using a three-step route with a good yield (Scheme 2). By using the procedure described in the literature [76], 6-(dimethylamino)-3,4-dihydronaphthalen-1 (2H)-one 2 was first synthesized from commercially available 6-amino-3,4-dihydronaphthalen-1(2H)-one, which was then transformed to 3. Finally, the probe 1 in 79% yield, was prepared by the coupling of compound 3 with NBD-piperazine. The facile and economic synthesis is important for the wide use of the probe. The structure of compound 1 was confirmed by 1H NMR, 13C NMR, and high resolution mass spectrum (HRMS). The spectra (Figure S8) are included in the Supplemental Information.
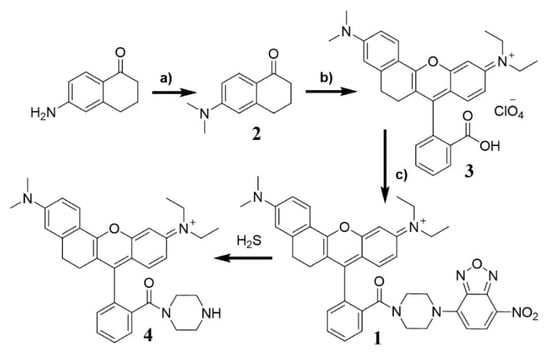
Scheme 2.
Synthetic route for probe 1 and its reaction with H2S. Reagents and conditions: a) 6-aminotetralone, CH3I, K2CO3, DMF, 0 °C, 24 h, 53%. b) 2-(4-diethylamino-2-hydroxybenzoyl)-benzoic acid, N-substituent ketone, H2SO4 0–100 °C, 2 h, 80%. c) NBD-piperazine, HATU, DIPEA, DMF, 79%.
2.2. UV-Vis and Fluorescence Response of 1 towards H2S
With the probe in hand, we first tested the solubility of 1 in buffer solution. The linearity of 1 verified its good solubility up to 20 µM (Figure S1). Further, we tested the optical properties of 1, and the absorbance and emission profiles are illustrated in Figure 1. As shown in Figure 1A, 1 displayed UV absorbance maxima at 620 nm and 500 nm, which are assigned to the rhodamine and NBD absorbance respectively. Since Na2S is a well-known inorganic H2S donor that is widely employed in the study of H2S effects on physiology, we used it as a H2S equivalent [77]. When reacted with H2S, the increase in intensity of absorbance peaks appeared between 600 nm and 520 nm, which could be assigned to the yielding of 4 and NBD-SH. The reaction between 1 and 500 µM H2S in PBS buffer (50 mM, pH 7.4) finished within 5 min. Furthermore, such thiolysis reaction was characterized by NMR with the formation of NBD-SH peaks (Figure S7) and HRMS with the production of peak at 535.3070 (calculated value for [4]+: 535.3068) (Figure S2).
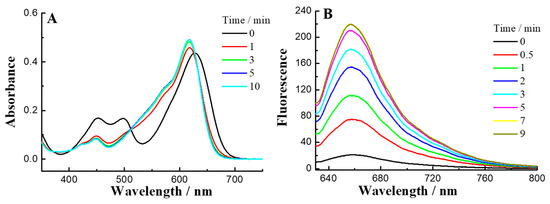
Figure 1.
(A) Time-dependent absorbance spectra of 1 (5 µM) and H2S (500 µM) in PBS (50 mM, pH 7.4, containing 10% DMSO). (B) Time-dependent fluorescence spectra (excitation = 620 nm) of 1 (2 µM) and H2S (200 µM).
The probe 1 showed weak fluorescence (quantum yield ɸ, 0.021) upon excitation at 620 nm, indicating that fluorescence in 1 could be mainly quenched by the photoinduced electron transfer process (PET) effect from the NBD moiety [58]. When 1 reacted with H2S, an excellent fluorescence change in a red shifted range with high brightness was observed (Figure 1B) with 10-fold turn-ons at 660 nm, and the quantum yield of the red shifted product was 0.29. The absorbance and emission data suggested the stokes shift up to 40 nm in PBS. A large Stokes shift could reduce the risk of background fluorescence and thus avoid self-quenching and backscattering effect upon excitation. These preliminary studies suggest the extended π-conjugated system of rhodamines provides excellent red shifted fluorescence probes for detection in long range with high brightness.
2.3. Kinetics Studies
Reaction kinetics, as an important parameter, was investigated for the probe 1 with H2S on account of its biological applicability under physiological conditions. To this end, the time-dependent fluorescence at 660 nm was recorded for data analysis (Figure 2A). The pseudo-first-order rate, kobs, was found by fitting the data with a single exponential function. Plotting log[H2S] versus log[kobs] confirmed a first-order dependence in H2S (Figure 2B). The reaction rate k2 (29.8 M−1s−1) was obtained by linear fitting of the kobs versus H2S concentration (Figure 2C). The H2S-reaction rate of 1 is faster than our previous Rh-NBD-based probe [30], implying that such NBD-based probes can be employed for fast detection of H2S. On the other hand, HPLC was further employed to identify the fast reaction of 1 with H2S (Figure S4). Furthermore, fluorescent titration (Figure 3) was performed to determine the limit of detection (LOD) of 1 for H2S as 0.27 μM by using the 3σ/k method [58].
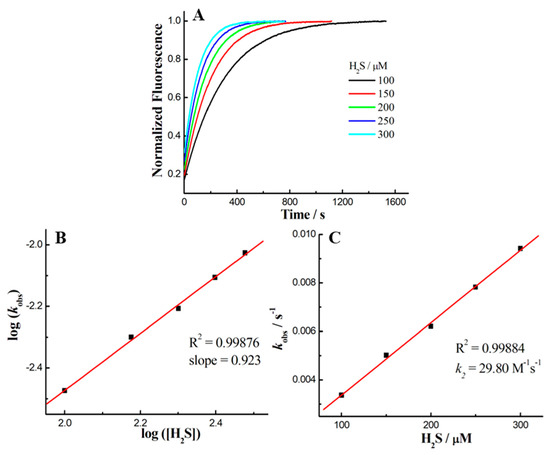
Figure 2.
(A) The time-dependent fluorescence intensities at 660 nm for 1 (2 µM) in the presence of different concentrations of H2S in PBS (50 mM, pH 7.4, containing 10% DMSO). (B) The linear relationship of log(kobs) versus log([H2S]). (C) The linear relationship of kobs versus H2S concentrations.
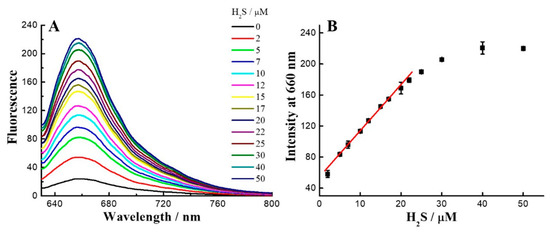
Figure 3.
(A) The fluorescence changes of probe 1 (2 μM) responses to various concentrations of H2S (0–50 μM) in PBS (50 mM, pH 7.4, containing 10% DMSO). (B) The linear relationship between concentrations of H2S and emission at 660 nm. The detection limit was calculated to be 0.27 μM.
2.4. Selectivity and Co-Interference Studies
With above promising outcomes, we further investigated the selectivity and sensitivity of probe 1. The fluorescent ‘‘off–on’’ response of 1 towards biothiols was measured. Probe 1 (2 μM) was treated with Cys, Hcy, and GSH individually (each 1 mM). As shown in Figure 4, the results showed that fluorescence intensity enhancement for analytes was nearly negligible except H2S, suggesting that 1 can selectively sense H2S. In order to check the interference of biothiols with coexistent H2S, we also tested 1 with these analytes in the presence of H2S (Figure 4). These findings suggested that all analytes did not interfere the H2S-specific thiolysis reaction. Furthermore, pH-dependent experiments were carried out to check whether 1 could sense at physiological pH (Figure S5). Obviously, the fluorescence enhancement occurred at pH 7.0–9.0, implying that 1 could work efficiently at physiological conditions.
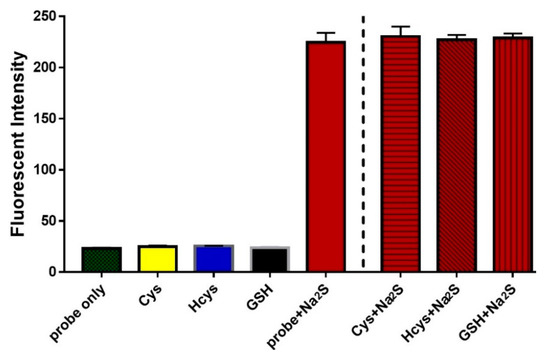
Figure 4.
Fluorescence intensity at 660 nm of 1 (2 μM) upon reacting with biothiols (1 mM) or H2S (200 μM) or biothiols with coexistent H2S species in PBS buffer (pH 7.4) for 30 min.
2.5. Imaging of Probe 1 in Living Cells
Encouraged by the above results, we moved forward to study the biological applications of 1. The cytotoxicity of the 1 was evaluated firstly by using the normal human umbilical vein endothelial cell (HUVEC) line via a standard MTT assay (Figure S6). After 24 h incubation with a varied concentration range of 1 from 5 µM, over 85% of the cells still remained viable, implying the relatively good biocompatibility of 1.
To examine the application potential of 1 for H2S detection in living cells, HeLa cells were chosen as the model biological system. Briefly, the cells were incubated with 1 alone or co-incubated with 1 and Na2S/D-Cys for 30 min. Then, all cells were examined via the confocal microscopy. Cells with probe 1 treatment displayed faint fluorescence (Figure 5E), while cells displayed remarkable red fluorescence in the presence of 1 and Na2S (Figure 5F). These results demonstrated that 1 could be used for selective imaging of exogenous H2S. For detection of endogenous production of H2S, cells were co-incubated with D-Cys and 1, as D-Cys can induce H2S biosynthesis via the 3-MST pathway [4]. Strong fluorescence was observed in cells (Figure 5G), which revealed that the endogenous production of H2S from D-Cys could be detected by 1. To further confirm the detection of endogenous production of H2S from D-Cys by 1, an inhibitor (aminooxyacetic acid, AOAA) was introduced to block the pathway for H2S production from D-Cys [4]. No obvious fluorescence was detected in the AOAA-treated cells (Figure 5H). These preliminary studies suggested that probe 1 could be used for visualization of H2S in cells efficiently and selectively.

Figure 5.
Fluorescence images of probe 1 for H2S detection in living cells. Bright-field and fluorescence images of cells upon incubation with 1 (5 µM) for 30 min (A,E); with 1 and Na2S (100 µM) (B,F); with 1 and D-Cys (100 µM) (C,G); with inhibitor (AOAA), 1 and D-Cys (100 µM) (D,H). Scale bars: 50 μm.
The probe 1 contains a positive charge, which might be mitochondria-targeting [33]. To this end, a fluorescent co-localization assay with Mito-Tracker Green FM (a well-known mitochondria specific dye) and probe 1 with D-Cys was carried out. As shown in Figure 6, the green fluorescence signal produced by Mito-Tracker Green FM and the red fluorescence signal from probe 1 merged well in the cells (Figure 6C). The Pearson’s coefficient is 0.946. These data implied that the probe 1 is a promising tool for imaging of mitochondria H2S.
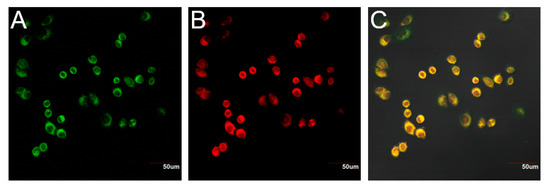
Figure 6.
Confocal microscopy images of mitochondrion by 1 in living cells. Cells were co-stained with 1 (5 μM), D-Cys (100 μM) and commercial MitoTracker® Green FM (2.5 μM). (A) The cell image displayed by green channel (500–530 nm, excitation at 488 nm). (B) The cell image displayed by red channel (620–660 nm, excitation at 594 nm). (C) Merged graph of fluorescence images of 1 and MitoTracker® Green FM. Scale bars: 50 μm.
3. Experimental
3.1. Materials and Methods
All chemicals and solvents used for the synthesis were purchased from commercial suppliers and applied directly in the experiments without further purification. The progress of the reaction was monitored by TLC on pre-coated silica plates (60F-254, 250 μm) in thickness (Merck, Darmstadt, Germany), and spots were visualized by basic KMnO4, UV light or iodine. Merck silica gel 60 (100–200 mesh) was used for general column chromatography purification. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 spectrometer (Karlsruhe, Germany). Chemical shifts are reported in parts per million with respect to the internal standard tetramethylsilane (Si(CH3)4 = 0.00 ppm) or residual solvent peaks (CD2Cl2 = 5.32 ppm; CDCl3 = 7.26 ppm; DMSO-d6 = 2.5 ppm). 1H NMR coupling constants (J) are reported in hertz (Hz), and multiplicity is indicated as the following: s (singlet), d (doublet), t (triplet), dd (doublet of doublets), m (multiple). High-resolution mass spectra (HRMS) were obtained on an Agilent 6540 UHD Accurate-Mass Q-TOF LC/MS or Varian 7.0 T FTICR-MS. The UV-visible spectra were recorded on a UV-3600 UV-VIS-NIR spectrophotometer (Shimadzu, Japan). The fluorescence study was carried out using an F-280 spectrophotometer (Tianjin Gangdong Sci & Tech., Development. Co., Ltd. Tianjin, China).
3.2. Synthesis of 6-(dimethylamino)-3,4-Dihydronaphthalen-1(2H)-One
To a mixture of 6-aminotetralone (0.50 g, 3.1 mmol), CH3I (0.06 g, 4.6 mmol) and K2CO3 (1.3 g, 9.3 mmol) in DMF (5 mL) was stirred for 24 h at 40–45 °C. After completion of reaction, the mixture was cooled to room temperature, water (10 mL) was added and the solution was extracted with EtOAc (3 × 50 mL). The organic layers were combined, dried with anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography using PE (petroleum ether): EtOAc = 6:1 as the eluent to obtain pure compound (Yield: 53.0%, 0.31 g). 1H NMR (400 MHz, CDCl3) δ 7.95 (dd, J = 8.8, 1.6 Hz, 1H), 6.62 (d, J = 8.8 Hz, 1H), 6.43 (s, 1H), 3.06 (s, 6H), 2.88 (t, J = 5.2 Hz, 2H), 2.59–2.55 (m, 2H), 2.10–2.05 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 196.9, 153.5, 146.6, 129.4, 121.9, 110.3, 109.6, 40.2, 38.9, 30.7, 23.7. HRMS [C12H16NO]+: Calcd. for [M+H]+ 190.1232; found: [M+H]+ 190.1228.
3.3. Synthesis of Intermediate 3
2-(4-diethylamino-2-hydroxybenzoyl)-benzoic acid (0.151 g, 0.48 mmol) and 6-(dimethylamino)-3,4-dihydronaphthalen-1(2H)-one (0.091 g, 0.48 mmol) were added to concentrated H2SO4 (10 mL) at 0 °C. The mixture was stirred at 100 °C for 2 h. After completion of reaction the mixture was allowed to cool at room temperature and was poured onto ice (10 g). HClO4 (1 mL) was gently added to the solution and the resulting precipitate was filtered off and washed with cold water. After drying, the residue was purified by silica gel column chromatography using CH2Cl2: methanol = 20:1 as the eluent to obtain pure compound as a purple-green solid (Yield: 80.0%, 0.17 g). 1H NMR (400 MHz, DMSO-d6) δ 13.22 (s, 1H), 8.19 (dd, J = 7.2, 2.4 Hz, 2H), 7.86 (t, J = 7.2 Hz, 1H), 7.75 (t, J = 7.6 Hz, 1H), 7.41 (d, J = 7.2 Hz, 1H), 7.24 (d, J = 1.6 Hz, 1H), 7.09 (d, J = 9.6 Hz, 1H), 6.93 (dd, J = 9.2, 2.0 Hz, 1H), 6.87 (d, J = 9.2 Hz, 1H), 6.75 (s, 1H), 3.58 (q, J = 6.4 Hz, 4H), 3.18 (s, 6H), 2.93–2.80 (m, 2H), 2.49–2.35 (m, 2H), 1.19 (t, J = 6.9 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 166.5, 163.4, 156.1, 155.0, 153.1, 145.1, 134.4, 133.0, 130.9, 130.0, 129.2, 129.1, 128.6, 118.4, 115.2, 114.3, 113.0, 112.0, 110.7, 96.0, 44.8, 40.0, 26.9, 23.6, 12.4. HRMS [C30H31N2O3]+: Calcd. for [M]+ 467.2329; found: [M]+ 467.2331.
3.4. Synthesis of Probe 1
Dissolved compound 2 (0.121 g, 0.2 mmol) in 5 mL DMF, followed by the addition of HATU (0.122 g, 0.32 mmol) and DIPEA (102 μL, 0.75 mmol). Stirred the solution for 5 min, NBD-piperazine (0.064 g, 0.2 mmol) was added to the solution and continue the stirring for 12 h at room temperature. After completion of reaction DMF was removed in vacuo. The residue was purified by silica gel column chromatography to give dark-red solid 1 (0.12 g, 79%). 1H NMR (400 MHz, DMSO-d6) δ 8.50 (d, J = 8.8 Hz, 1H), 8.16 (d, J = 8.8 Hz, 1H), 7.74 (bs, 3H), 7.48 (bs, 1H), 7.20 (bs, 1H), 7.07 (bs, 2H), 6.91 (d, J = 8.0 Hz, 1H), 6.72 (s, 1H), 6.54 (d, J = 9.2 Hz, 1H), 4.22–4.14 (m, 2H), 4.03–3.83 (m, 2H), 3.82–3.46 (m, 8H), 3.19 (s, 6H), 2.98–2.78 (m, 2H), 2.62–2.45 (m, 2H), 1.16 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 167.0, 163.8, 156.0, 155.2, 153.0, 145.5, 145.3, 144.7, 136.2, 134.2, 131.9, 130.2, 129.5, 129.3, 129.3, 129.2, 127.6, 121.5, 119.6, 114.9, 114.3, 113.0, 112.1, 110.7, 103.4, 96.1, 49.0, 48.1, 45.6, 44.8, 40.8, 27.0, 23.9, 12.4. HRMS [C40H40N7O5]+: Calcd. for [M]+ 698.3085; found: [M]+ 698.3090.
3.5. Procedure for Spectroscopic Studies
All spectroscopic measurements were performed in phosphate-buffered saline buffer (PBS, 50 mM, pH 7.4, containing 10% DMSO) at room temperature. Compounds were dissolved into DMSO to prepare the stock solutions with a concentration of 5 mM. 1–500 mM Stock solutions of Na2S in degassed (by bubbling N2 for 30 min) PBS buffer were used as H2S source. Probes were diluted in PBS buffer (50 mM, pH 7.4, containing 10% DMSO) to afford the final concentration of 2–5 µM. For the selectivity experiment, different biologically relevant molecules (100 mM) were prepared as stock solutions in degassed PBS buffer. Appropriate amount of biologically relevant species was added to separate portions of the probe solution and mixed thoroughly. All measurements were performed in a 3 mL corvette with 2 mL solution. The reaction mixture was shaken uniformly before emission spectra were measured.
3.6. Cell Culture and Cytotoxicity Assay
The HUVEC and HeLa cell lines were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). And the cells were cultured in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin/streptomycin under standard cell culture conditions at 37 °C in a humidified CO2 incubator. Before the cytotoxicity assay, the HUVEC cells were transferred to the 96-well plate and cultured for one night. After that, the culture medium was replaced with a fresh one and the HUVEC cells were pre-incubated with probe 1 with a concentration range of 5–25 μM for 24 h. The cell viability was then measured by the standard MTT assay.
3.7. Cell Imaging
Glass bottom dishes were added into a 24-well plate for cell imaging before cells were seeded. Then, the HeLa cells were transferred to the 24-well plate and cultured for one night before the experiments. After that, the culture medium was replaced with the fresh one and the cells were treated with the desired reagents. After incubation, the HeLa cells were quickly washed with PBS three times, and then fixed with 4% paraformaldehyde solution for 10 min. Finally, the HeLa cells were washed with PBS and imaged using a confocal microscope (Olympus FV1000) with a 40× objective lens. Emission was collected at the green channel (500–530 nm, excitation at 488 nm) and the red channel (620–660 nm, excitation at 594 nm).
4. Conclusions
In summary, we have developed a new, extended π-conjugation rhodamine-NBD a red shifted fluorescence probe 1 capable of detection H2S in live cells. The probe shows a relatively large Stokes shift (40 nm), fast response (k = 29.8 M−1s−1), and good quantum yield (ø = 0.29) after H2S activation. Moreover, 1 was water-soluble, cell-membrane-permeable, and had high selectivity and sensitivity for H2S. We believe that this red shifted range probe 1 could be a useful tool for studies of H2S biology in the future.
Supplementary Materials
Supplementary data associated with this article can be found in the online.
Author Contributions
Formal preparation and writing—original draft, (I.I.); writing—review and editing (L.Y., L.S. & Z.C.); Bioimaging (I.I., X.J. & L.S.); Supervision and funding acquisition (L.Y. & Z.X.). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the NFSC (21702111).
Acknowledgments
We thank the prospective project supported by the National Natural Science Foundation of China (21702111).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Investig. 2009, 89, 59–67. [Google Scholar] [CrossRef]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophs. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 2013, 4, 2551–2556. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Wang, P.; Chen, G.; Liu, R.; Lam, Y.W.; Hu, Y.; Zhu, Q.; Sun, H. An iminocoumarin benzothiazole-based fluorescent probe for imaging hydrogen sulfide in living cells. Talanta 2015, 135, 149–154. [Google Scholar] [CrossRef]
- Coletta, C.; Modis, K.; Szczesny, B.; Brunyanszki, A.; Olah, G.; Rios, E.C.; Yanagi, K.; Ahmad, A.; Papapetropoulos, A.; Szabo, C. Regulation of Vascular Tone, Angiogenesis and Cellular Bioenergetics by the 3-Mercaptopyruvate Sulfurtransferase/H2S Pathway: Functional Impairment by Hyperglycemia and Restoration by DL-alpha-Lipoic Acid. Mol. Med. 2015, 21, 1–14. [Google Scholar] [CrossRef]
- Wu, D.; Si, W.; Wang, M.; Lv, S.; Ji, A.; Li, Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide 2015, 50, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide and cell signaling: Team player or referee? Plant Physiol. Biochem. 2014, 78, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide. 2014, 41, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.C.; Garcia, I.; Gotor, C. L-Cysteine Desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal. Behav. 2013, 8, e24007. [Google Scholar] [CrossRef]
- Shukla, P.; Khodade, V.S.; SharathChandra, M.; Chauhan, P.; Mishra, S.; Siddaramappa, S.; Pradeep, B.E.; Singh, A.; Chakrapani, H. “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci. 2017, 8, 4967–4972. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef]
- Hartle, M.D.; Pluth, M.D. A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef]
- Cerda, M.M.; Newton, T.D.; Zhao, Y.; Collins, B.K.; Hendon, C.H.; Pluth, M.D. Dithioesters: Simple, tunable, cysteine-selective H2S donors. Chem. Sci. 2019, 10, 1773–1779. [Google Scholar] [CrossRef]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Wu, W.; Liu, W.; Liang, H.; Gong, D.; Hu, X.; Li, M. Bioluminescence Probe for Detecting Hydrogen Sulfide in Vivo. Anal. Chem. 2016, 88, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Tan, J.; Ding, Z.; Wang, W.; Yuan, D.; Li, G.; Zhang, H.; Zhang, X. Reductive cleavage of C=C bonds as a new strategy for turn-on dual fluorescence in effective sensing of H2S. Chem. Sci. 2018, 9, 8369–8374. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Hu, R.; Liu, X.; Wang, S.; Li, S.; Li, Y.; Yang, G. Molecular Engineering of Aqueous Soluble Triarylboron-Compound-Based Two-Photon Fluorescent Probe for Mitochondria H2S with Analyte-Induced Finite Aggregation and Excellent Membrane Permeability. Anal. Chem. 2016, 88, 1052–1057. [Google Scholar] [CrossRef]
- Mao, Z.; Ye, M.; Hu, W.; Ye, X.; Wang, Y.; Zhang, H.; Li, C.; Liu, Z. Design of a ratiometric two-photon probe for imaging of hypochlorous acid (HClO) in wounded tissues. Chem. Sci. 2018, 9, 6035–6040. [Google Scholar] [CrossRef]
- Zhao, Y.; Cerda, M.M.; Pluth, M.D. Fluorogenic hydrogen sulfide (H2S) donors based on sulfenyl thiocarbonates enable H2S tracking and quantification. Chem. Sci. 2019, 10, 1873–1878. [Google Scholar] [CrossRef]
- Cao, X.; Lin, W.; Zheng, K.; He, L. A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem. Commun. 2012, 48, 10529–10531. [Google Scholar] [CrossRef]
- Shi, B.; Gu, X.; Fei, Q.; Zhao, C. Photoacoustic probes for real-time tracking of endogenous H2S in living mice. Chem. Sci. 2017, 8, 2150–2155. [Google Scholar] [CrossRef]
- Ismail, I.; Wang, D.; Wang, D.; Niu, C.; Huang, H.; Yi, L.; Xi, Z. A mitochondria-targeted red-emitting probe for imaging hydrogen sulfide in living cells and zebrafish. Org. Biomol. Chem. 2019, 17, 3389–3395. [Google Scholar] [CrossRef]
- Ismail, I.; Wang, D.; Wang, Z.; Wang, D.; Zhang, C.; Yi, L.; Xi, Z. A julolidine-fused coumarin-NBD dyad for highly selective and sensitive detection of H2S in biological samples. Dyes Pigment. 2019, 163, 700–706. [Google Scholar] [CrossRef]
- Pak, Y.L.; Li, J.; Ko, K.C.; Kim, G.; Lee, J.Y.; Yoon, J. Mitochondria-Targeted Reaction-Based Fluorescent Probe for Hydrogen Sulfide. Anal. Chem. 2016, 88, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Xi, Z. Thiolysis of NBD-based dyes for colorimetric and fluorescence detection of H2S and biothiols: Design and biological applications. Org. Biomol. Chem. 2017, 15, 3828–3839. [Google Scholar] [CrossRef] [PubMed]
- Karakus, E.; Ucuncu, M.; Emrullahoglu, M. Electrophilic Cyanate As a Recognition Motif for Reactive Sulfur Species: Selective Fluorescence Detection of H2S. Anal. Chem. 2016, 88, 1039–1043. [Google Scholar] [CrossRef]
- He, L.; Yang, X.; Xu, K.; Kong, X.; Lin, W. A multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells. Chem. Sci. 2017, 8, 6257–6265. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.Z.; Zhang, K.; Li, L.Y.; Pluth, M.D.; Yi, L.; Xi, Z. Dual-biomarker-triggered fluorescence probes for differentiating cancer cells and revealing synergistic antioxidant effects under oxidative stress. Chem. Sci. 2019, 10, 1945–1952. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef]
- Slooter, M.D.; Bierau, K.; Chan, A.B.; Lowik, C.W. Near infrared fluorescence imaging for early detection, monitoring and improved intervention of diseases involving the joint. Connect. Tissue Res. 2015, 56, 153–160. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Staudinger, C.; Borisov, S.M. Long-wavelength analyte-sensitive luminescent probes and optical (bio)sensors. Methods Appl. Fluoresc. 2015, 3, 042005. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xiao, Y.; Qian, X.; Zhao, D.; Xu, Y. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: Replacement of oxygen with silicon atom. Chem. Commun. 2008, 15, 1780–1782. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 2011, 6, 600–608. [Google Scholar] [CrossRef]
- Miao, X.; Hu, W.; He, T.; Tao, H.; Wang, Q.; Chen, R.; Jin, L.; Zhao, H.; Lu, X.; Fan, Q.; et al. Deciphering the intersystem crossing in near-infrared BODIPY photosensitizers for highly efficient photodynamic therapy. Chem. Sci. 2019, 10, 3096–3102. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. Development of NIR fluorescent dyes based on Si-rhodamine for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 5029–5031. [Google Scholar] [CrossRef]
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile synthesis of AIEgens with wide color tunability for cellular imaging and therapy. Chem. Sci. 2019, 10, 3494–3501. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A unique class of near-infrared functional fluorescent dyes with carboxylic-acid-modulated fluorescence ON/OFF switching: Rational design, synthesis, optical properties, theoretical calculations, and applications for fluorescence imaging in living animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef]
- Ruan, C.; Liu, C.; Hu, H.; Guo, X.L.; Jiang, B.P.; Liang, H.; Shen, X.C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019, 10, 4699–4706. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A unique approach to development of near-infrared fluorescent sensors for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 13510–13523. [Google Scholar] [CrossRef]
- Chevalier, A.; Renard, P.Y.; Romieu, A. Straightforward access to water-soluble unsymmetrical sulfoxanthene dyes: Application to the preparation of far-red fluorescent dyes with large stokes’ shifts. Chemistry 2014, 20, 8330–8337. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. Int. Ed. 2013, 52, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, L.; Fang, Y.; Shi, W.; Li, X.; Ma, H. In vivo imaging of leucine aminopeptidase activity in drug-induced liver injury and liver cancer via a near-infrared fluorescent probe. Chem. Sci. 2017, 8, 3479–3483. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, J.; Wang, X.; Ding, Q.; Bai, X.; Zhang, Y.; Su, D.; Zhang, W.; Zhang, W.; Tang, B. In situ visualization of ozone in the brains of mice with depression phenotypes by using a new near-infrared fluorescence probe. Chem. Sci. 2019, 10, 2805–2810. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Hu, C.; Cao, J.; Zhang, H.; Xiong, X.; Wang, J.; Cui, S.; Sun, S.; Peng, X. A two-photon fluorescent probe with near-infrared emission for hydrogen sulfide imaging in biosystems. Chem. Commun. 2013, 49, 3890–3892. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, J.; Gao, J.; Chen, J.A.; Xu, G.; Zhu, T.; Gu, X.; Guo, Z.; Zhu, W.H.; Zhao, C. A molecular design strategy toward enzyme-activated probes with near-infrared I and II fluorescence for targeted cancer imaging. Chem. Sci. 2019, 10, 7222–7227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, M.; Qiao, Q.; Liu, H.; Lang, H.; Xu, Z. A near-infrared fluorescent probe for hydrogen sulfide in living cells. Dyes Pigments 2013, 98, 367–371. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Xi, Z.; Li, L.Y.; Gu, X.; Zhang, Q.Z.; Yi, L. A new H2S-specific near-infrared fluorescence-enhanced probe that can visualize the H2S level in colorectal cancer cells in mice. Chem. Sci. 2017, 8, 2776–2781. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, H.; Ismail, I.; Sun, H.; Yi, L.; Xi, Z. A fluorogenic H2S-triggered prodrug based on thiolysis of the NBD amine. Bioorg. Med. Chem. Lett. 2019, 29, 126627. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef]
- Kiyose, K.; Aizawa, S.; Sasaki, E.; Kojima, H.; Hanaoka, K.; Terai, T.; Urano, Y.; Nagano, T. Molecular design strategies for near-infrared ratiometric fluorescent probes based on the unique spectral properties of aminocyanines. Chemistry 2009, 15, 9191–9200. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Hanaoka, K.; Koide, Y.; Ujita, S.; Takahashi, N.; Ikegaya, Y.; Matsuki, N.; Terai, T.; Ueno, T.; Komatsu, T.; et al. Development of a far-red to near-infrared fluorescence probe for calcium ion and its application to multicolor neuronal imaging. J. Am. Chem. Soc. 2011, 133, 14157–14159. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, B.; Akkaya, E.U. Infrared fluorescence sensing of submicromolar calcium: Pushing the limits of photoinduced electron transfer. Tetrahedron Lett. 2000, 41, 9185–9188. [Google Scholar] [CrossRef]
- Sasaki, E.; Kojima, H.; Nishimatsu, H.; Urano, Y.; Kikuchi, K.; Hirata, Y.; Nagano, T. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J. Am. Chem. Soc. 2005, 127, 3684–3685. [Google Scholar] [CrossRef]
- Tang, B.; Yu, F.; Li, P.; Tong, L.; Duan, X.; Xie, T.; Wang, X. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: Imaging in living HepG2 and HL-7702 cells. J. Am. Chem. Soc. 2009, 131, 3016–3023. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Li, G.; Zhao, G.; Chu, T.; Han, K. A near-IR reversible fluorescent probe modulated by selenium for monitoring peroxynitrite and imaging in living cells. J. Am. Chem. Soc. 2011, 133, 11030–11033. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, W.S.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Bhuniya, S.; Kim, J.S. Small conjugate-based theranostic agents: An encouraging approach for cancer therapy. Chem. Soc. Rev. 2015, 44, 6670–6683. [Google Scholar] [CrossRef]
- Dujols, V.; Ford, F.; Czarnik, A.W. A Long-Wavelength Fluorescent Chemodosimeter Selective for Cu(II) Ion in Water. J. Am. Chem. Soc. 1997, 119, 7386–7387. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, P.; Liu, W.; Wang, M.; Zhang, H.; Wu, J.; Zhang, L.; Wang, P. Near-Infrared Probe Based on Rhodamine Derivative for Highly Sensitive and Selective Lysosomal pH Tracking. Anal. Chem. 2017, 89, 1922–1929. [Google Scholar] [CrossRef]
- Wu, D.; Ryu, J.C.; Chung, Y.W.; Lee, D.; Ryu, J.H.; Yoon, J.H.; Yoon, J. A Far-Red-Emitting Fluorescence Probe for Sensitive and Selective Detection of Peroxynitrite in Live Cells and Tissues. Anal. Chem. 2017, 89, 10924–10931. [Google Scholar] [CrossRef] [PubMed]
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Beija, M.; Afonso, C.A.; Martinho, J.M. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef]
- Gong, Y.J.; Zhang, X.B.; Mao, G.J.; Su, L.; Meng, H.M.; Tan, W.; Feng, S.; Zhang, G. A unique approach toward near-infrared fluorescent probes for bioimaging with remarkably enhanced contrast. Chem. Sci. 2016, 7, 2275–2285. [Google Scholar] [CrossRef]
- Song, Z.J.; Ng, M.Y.; Lee, Z.W.; Dai, W.; Hagen, T.; Moore, P.K.; Huang, D.; Deng, L.W.; Tan, C.H. Hydrogen sulfide donors in research and drug development. Med. Chem. Commun. 2014, 5, 557–570. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).