Abstract
For the convenient introduction of simple linear/branched alkyl groups into biologically important azaspirocyclohexadienones, a practical Fe-catalyzed decarbonylative cascade spiro-cyclization of N-aryl cinnamamides with aliphatic aldehydes to provide alkylated 1-azaspiro-cyclohexadienones was developed. Aliphatic aldehydes were oxidative decarbonylated into primary, secondary and tertiary alkyl radicals conveniently and allows for the subsequent cascade construction of dual C(sp3)-C(sp3) and C=O bonds via radical addition, spirocyclization and oxidation sequence.
1. Introduction
The azaspirocyclohexadienone ring represents an important structural motif in natural compounds and pharmaceuticals, such as Annosqualine, Erythratinone and compound I (acting as muscarinic antagonist, Figure 1) [1,2,3,4]. Thus, the development of efficient methods for the synthesis of these azaspirocyclohexadienones has attracted substantial attention. While traditional synthetic routes rely predominantly on the transition-metal-catalyzed intramolecular ipso-carbocyclization [5,6,7,8,9] and electrophilic ipso-cyclization [10,11,12], the radical cascade ipso-cyclization was expanding in recent years, to incorporate various functional groups into the azaspirocyclohexadienone framework [13,14,15]. Among them, the radical difunctionalization and ipso-cyclization of N-arylcinnamamides or N-arylpropiolamides has proven to be an straightforward pathway, where various carbon or heteroatom centered radicals added onto the α,β-unsaturated carbon-carbon multiple bond of substrates, followed by the intramolecular radical ipso-cyclization and dearomatization. In this context, simple ethers [16], alkanes [17,18], ketones [19], acetonitrile [20], acyl chloride [21], aldehydes [22], aryldiazonium salts [23], CF3SO2Na [24,25,26], arylsulfinic acids [27], sulfonylhydrazides [28], AgSCF3 [29], thiophenols [30], disulfides [31], N-sulfanylsuccinimide [32], diselenide [33], tert-butyl nitrite [34], phosphonates [24,35,36] and silanes [37,38] have been demonstrated as the radical precursors for this spirocyclization (Scheme 1a), as independently reported by Li [16,17], Liang [25,29], and Zhu [18,20], etc. The addition of carbon radicals to N-arylcinnamamides and subsequent ipso-cyclization offers a direct synthesis of azaspiro-compounds with the concurrent construction of two C–C single bonds. However, the alkyl radical source for this spirocyclization of alkenes is still confined. When ethers, alkanes, ketones and acetonitrile acted as carbon radical precursors via the homolytic cleavage of sp3 C-H bond, the functional groups (ether, ketone or cyano groups) would inevitably be imported into the products, and the generation of regio-isomers was also troublesome due to the possible existence several different sp3 C-H bonds in these precursors, so readily available alkyl precursors that could introduce ordinary and simple linear/branched alkyl groups into azaspirocyclohexadienones are highly desirable, especially those could realize the convenient radical generation and be compatible with the primary, secondary and tertiary alkyls.
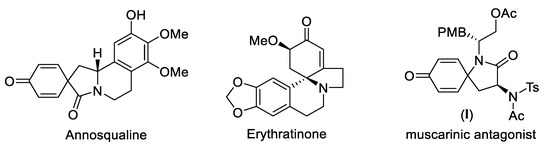
Figure 1.
Representative azaspirocyclohexadienone-based bioactive molecules.
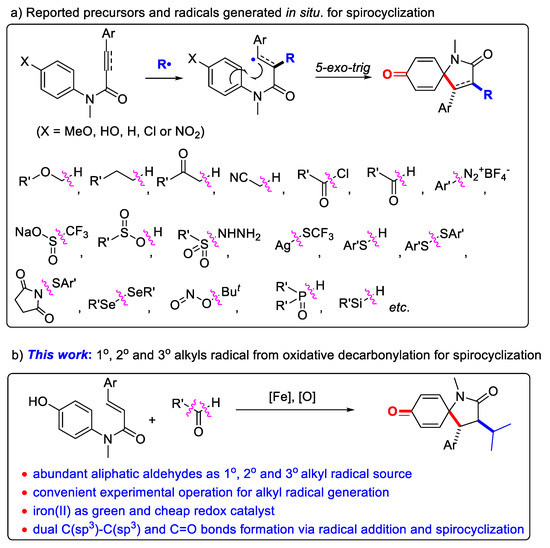
Scheme 1.
Various radicals for the spirocyclization of N-aryl unsaturated amides.
On the other hand, aldehydes are cheap and readily available chemicals and have been directly used for decarbonylative couplings catalyzed by ruthenium or rhodium, as shown by the extensive studies of Li since 2009 [39,40,41,42,43,44]. In contrast, we are interested in the radical-type decarbonylative reactions of aldehydes in the absence of noble metals, with peroxides as radical initiator and oxidant [45,46,47,48,49,50,51,52,53]. The oxidative decarbonylative couplings of aldehydes with (hetero) arenes [45,46], styrenes [47,48,49,50], alkyne and electron-deficient alkenes [51,52,53] were successively developed by our group. These decarbonylative reactions were further updated by other groups, with dioxygen as the radical initiator and oxidant [54,55,56]. Similar radical type decarbonylative alkylations of C=C and C≡C bonds with aldehydes were also separately developed by Li [57,58], Li [59,60,61,62] and Yu [63,64].
The above studies have fully demonstrated that radical-type decarbonylation of aliphatic aldehydes was an economic and convenient way to obtain primary, secondary and tertiary alkyl radicals, thus we postulated that the merging the oxidative decarbonylation of aliphatic aldehydes into the radical difunctionalization of N-arylcinnamamides, would produce a benzyl radical and then facilitate the subsequent radical ipso-cyclization (Scheme 1b). Herein, we report a novel Fe-promoted oxidative decarbonylative alkylative spirocyclization of N-arylcinnamamides with aliphatic aldehydes to provide alkylated 1-azaspirocyclohexadienones.
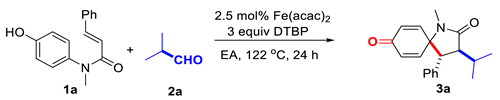
2. Results
Based on the above speculation, N-(4-hydroxyphenyl)-N-methyl cinnamamide (1a) and isobutyraldehyde (2a) were chosen as the model substrates for this oxidative decarbonylative spirocyclization; with di-tert-butyl peroxide (DTBP) as the radical initiator and terminal oxidant, the desired alkylated 1-azaspirocyclohexadienone 3a was isolated and characterized. Detailed optimization was performed focusing on different iron salt and its loading, the dosage of aldehyde and DTBP, the reaction temperature and reaction solvent, which revealed the combination of 2.5 mol% Fe(acac)2/5 equiv aldehyde/3 equiv DTBP proved to be the most effective one, to afford the spirocyclization product 3a in 67% isolated yield (Table 1, entry 1). For the reaction solvent, low polarity solvents including chlorobenzene (PhCl), trifluoromethylbenzene (PhCF3) and much more polar acetonitrile, ethyl acetate (EA) all turned out to be compatible, and among these solvents tested, ethyl acetate provided the best result (entries 12–14). We are glad that this radical cascade reaction favors ethyl acetate, which should be the greenest solvent among the ordinary organic solvents.

Table 1.
Optimization of reaction conditions a.
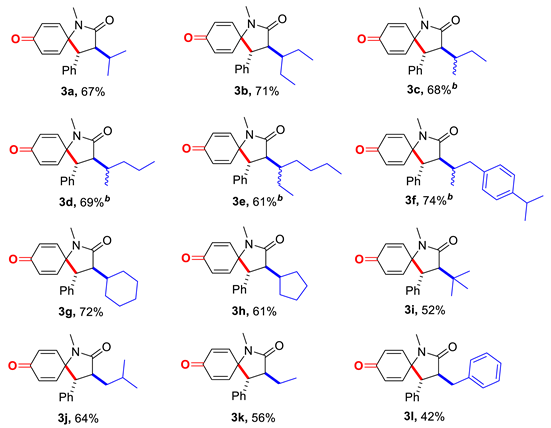
With the optimized reaction conditions in hand, we first examined the generality of this alkylative spirocyclization with different aliphatic aldehydes (2a–2l, Table 2). Various α-mono-substituted aliphatic aldehydes including isobutyraldehyde (2a), 2-ethylbutanal (2b), 2-methyl-butanal (2c), 2-methylpentanal (2d), 2-ethylhexanal (2e), cyclamen aldehyde (2f), cyclohexane-carbaldehyde (2g) and cyclopantanecarbaldehyde (2h) provided the corresponding secondary carbon radicals for the cascade spirocyclization after the oxidative decarbonylation. While the α-di-substituted pivaldehyde (2i) provided a tertiary carbon radical, the α-unsubstituted aliphatic aldehyde 3-methylbutanal (2j), propionaldehyde (2k) and 2-phenylacetaldehyde (2l) would provide primary carbon radicals, similarly. Gratifyingly, all of these aliphatic aldehydes underwent this decarbonylative alkylative spirocyclization witH-N-arylcinnamamide (1a) to produce the targeted alkylated 1-azaspirocyclohexadienone (3a–3l) smoothly. Moreover, the introduced alkyl group and the aryl group exhibit a trans-configuration, determined by the adjacent coupling constant of the proton on C3 and C4 (J = 12.0 Hz), which agreed well with the literature reports [18]. Considering the readily availability of these aliphatic aldehydes, avoidance the possible carbon radical rearrangement (to provide regioisomers as the alkane C-H bonds homolytic cleavage products) and simple operation for the radical generation (overriding pre-functionalization and photoreaction devices), this decarbonylative alkylative spirocyclization demonstrated again that the aliphatic aldehydes were convenient primary, secondary and tertiary alkyl precursors.

Table 2.
Scope of the aliphatic aldehydes a.
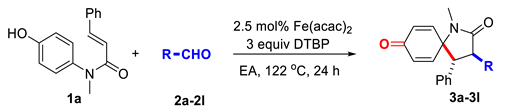
After investigating the scope of aliphatic aldehydes, we next tested the generality of this decarbonylative alkylative spirocyclization on different N-aryl cinnamamides 1b–1k under the optimized conditions. The effect of substituents on the cinnamamide moiety is listed in Figure 2.
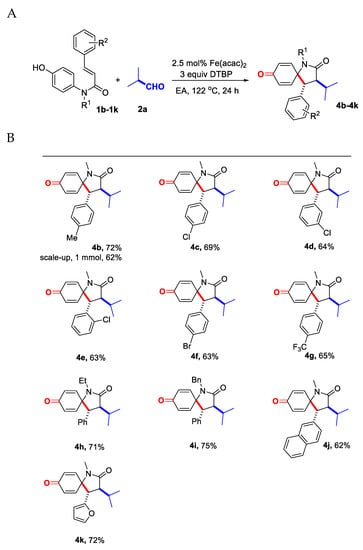
Figure 2.
(A) Scope of the N-aryl cinnamamides. Conditions: cinnamamide 1b–1k (0.1 mmol), aldehyde 2a (5 equiv, 0.5 mmol), Fe(acac)2 (2.5 mol%) in EA (0.5 mL, prepared solution) and DTBP (3 equiv, 0.3 mmol) were reacted at 122 °C (oil bath temperature) for 24 h under air atmosphere. Isolated yield. (B) 4b–4k.
Various electron withdrawing or donating substituents were successfully incorporated into the cinnamamide unit of substrates 1b–1g, such as methyl, halo and trifluoromethyl groups. Among them, the optimized reaction conditions were compatible with the cinnamamides with chloro groups substituted at the para, meta and ortho positions (compounds 1c–1e), and similar yields were obtained, which revealed the substituents didn’t cause obvious steric hindrance for this cascade reaction. For the substituent on the N-linkage, the ethyl and benzyl group-substituted cinnamamides 1h and 1i provided slightly better yields than the model substrate 1a. To our delight, the 2-naphthalenyl and 2-furanyl units (1j and 1k) could also be introduced onto the α,β-unsaturated C=C bond of amide substrates, and the cascade reaction provided the 1-azaspirocyclohexadienone 4j and 4k in moderate yields.
Several control experiments were carried out to understand this decarbonylative alkylative spirocyclization. First, the cascade reaction of cinnamamide 1a and aliphatic aldehyde 2a was inhibited in the presence of di-tert-butylhydroxytoluene (BHT); instead, the decarbonylated alkyl radical was captured as 2,6-di-tert-butyl-4-isopropyl-4-methylcyclohexa-2,5-dien-1-one (5), which confirmed the radical-type decarbonylation mechanism (Scheme 2a). Second, the control experiment using the N-(4-methoxyphenyl)-N-methyl cinnamamide (1l) to replace the N-(4-hydroxyphenyl)-N-methyl cinnamamide (1a) was conducted under the optimized conditions, however no desired 5-exo-trig spirocyclization product (3a) could be detected; in contrast, the C3-alkylated 3,4-dihydroquinolin-2(1H)-one 6 was formed in 74% yield, via 6-endo-trig cyclization pathway (Scheme 2b). The sharp difference on reactivity demonstrated the importance of the para-hydroxyl substituent for this spirocyclization, maybe due to its ability to stabilize the radical intermediate (B, Scheme 3) obtained from the 5-exo-trig spirocyclization and accelerating the subsequent cyclohexadienone formation.
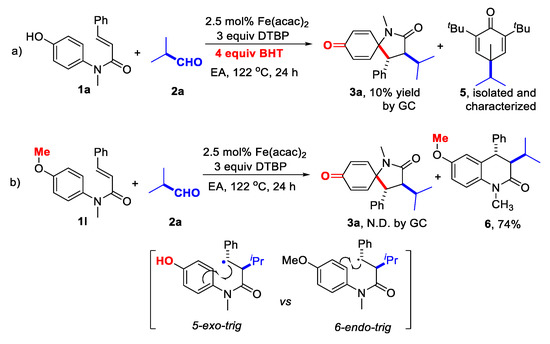
Scheme 2.
Control experiments.
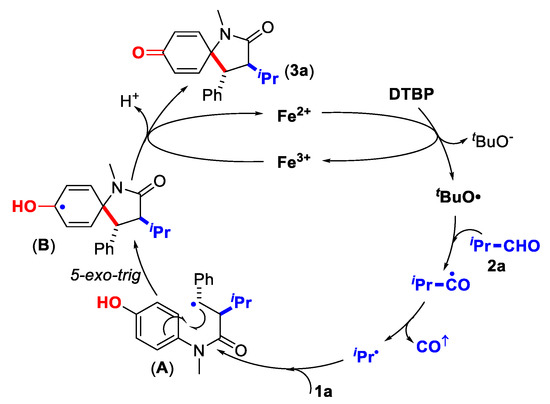
Scheme 3.
Proposed mechanism.
Based on the mechanistic experiments and the previous studies [46,47,48,49,50,51,52,53], a possible reaction pathway is depicted in Scheme 3, with the reaction of N-(4-hydroxyphenyl)-N-methyl cinnamamide (1a) and isobutyraldehyde (2a) as an example.
First, promoted by the iron-catalyst, the homolytic cleavage of DTBP at elevated temperature forms tert-butoxy radical. Subsequent intermolecular hydrogen atom abstraction of the aldehyde (2a), spontaneous decarbonylation and insertion into the C=C bond of the cinnamamide (1a) affords a metastable benzyl radical A, which then adds onto the ispo-carbon to give the spiro-cyclohexadienyl radical B. The radical B is preferably oxidized by Fe3+ and deprotonated by a tert-butoxide anion to afford the 1-azaspirocyclohexadienone (3a).
3. Experimental
3.1. General Information
Unless otherwise noted, all commercially available compounds were used as provided without further purification. The substrates (various N-aryl cinnamamides) were synthesized from cinnamic acid and para-anisidine according to literature reports [18]. Dry solvents (toluene, ethyl acetate, dichloroethane, acetonitrile, chlorobenzene, fluorobenzene) were used as commercially available. Thin-layer chromatography (TLC) was performed using silica gel 60 F254 precoated plates (0.25 mm) or Sorbent Silica Gel 60 F254 plates (E. Merck). The developed chromatography was analyzed by UV lamp (254 nm). Unless other noted, high-resolution mass spectra (HRMS) were obtained from a JMS-700 instrument (ESI; JEOL). Melting points are uncorrected. Nuclear magnetic resonance (NMR) spectra were recorded on an Avance 400 spectrometer (Bruker) at ambient temperature. Chemical shifts for 1H-NMR spectra are reported in parts per million (ppm) from tetramethylsilane with the solvent resonance as the internal standard (chloroform: δ 7.26 ppm). Chemical shifts for 13C-NMR spectra are reported in parts per million (ppm) from tetramethylsilane with the solvent as the internal standard (CDCl3: δ 77.16 ppm). Data are reported as following: chemical shift, multiplicity (s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet, br = broad signal), coupling constant (Hz), and integration.
3.2. General Experimental Procedures
An oven-dried microwave reaction vessel was charged with FeCl2 (2.5 mol%) in EA (0.5 mL, pre-prepared solution), N-(4-hydroxyphenyl)-N-methylcinnamamide (1a, 0.1 mmol, 1.0 equiv), isobutyraldehyde (2a, 0.5 mmol, 5.0 equiv) and DTBP (0.3 mmol, 3.0 equiv). The vessel was sealed and heated at 122 °C (oil bath temperature) for 24 h. Afterwards the resulting mixture was cooled to room temperature, the solvent was removed in vacuo. The residue was purified by column chromatography on silica gel with a mixture of ethyl acetate/petroleum ether (1:3) as eluent to give the product 3a.
3.3. Spectra Data of Products 3a–3l, 4b–4k, 5, 6 (see “Supplementary Materials” for details)
3-Isopropyl-1-methyl-4-phenyl-1-azaspiro [4.5]deca-6,9-diene-2,8-dione (3a). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (19.8 mg, 67%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24 (m, 3H), 7.10 (dd, J = 7.6, 2.4 Hz, 2H), 6.78 (dd, J = 10.0, 3.2 Hz, 1H), 6.55 (dd, J = 10.2, 3.0 Hz, 1H), 6.39 (dd, J = 10.2, 2.0 Hz, 1H), 6.00 (dd, J = 10.2, 2.0 Hz, 1H), 3.43 (d, J = 12.0 Hz, 1H), 3.14 (dd, J = 11.8, 3.6 Hz, 1H), 2.73 (s, 3H), 2.38–2.34 (m, 1H), 1.01 (d, J = 6.8 Hz, 3H), 0.83 (d, J = 7.2 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.46, 175.22, 149.40, 147.05, 135.04, 132.33, 131.51, 128.63, 128.21, 64.95, 50.84, 49.05, 28.07, 27.14, 20.14, 18.78. IR (cm−1): 3032, 2965, 2932, 2875, 1672, 1630, 1606, 1498, 1446, 1454, 1418, 1393, 1374, 1260, 1141, 1119, 1065, 991, 865, 794, 724, 700. HRMS: calcd. for C19H21NO2 Na+ [M + Na]+: 318.1465; Found: 318.1442.
1-Methyl-3-(pentan-3-yl)-4-phenyl-1-azaspiro[4 .5]deca-6,9-diene-2,8-dione (3b). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 2-ethylbutanal (2b), and purified by flash column chromatography as yellow oil (23.0 mg, 71%). 1H-NMR (400 MHz, CDCl3) δ 7.26–7.24 (m, 3H), 7.09 (dd, J = 7.6, 2.8 Hz, 2H), 6.77 (dd, J = 10.0, 3.2 Hz, 1H), 6.60 (dd, J = 10.4, 3.2 Hz, 1H), 6.37 (dd, J = 10.0, 2.0 Hz, 1H), 6.00 (dd, J = 10.0, 2.0 Hz, 1H), 3.45 (d, J = 11.8 Hz, 1H), 3.31 (dd, J = 11.8, 2.6 Hz, 1H), 2.74 (s, 3H), 1.78–1.74 (m, 2H), 1.53–1.46 (m, 1H), 1.39–1.32 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H), 0.80 (t, J = 7.3 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.46, 175.15, 149.45, 146.94, 134.72, 132.36, 131.58, 128.68, 128.59, 128.28, 65.02, 51.48, 48.30, 34.68, 27.12, 26.47, 16.47, 12.44. IR (cm−1): 3032, 2961, 2931, 2875, 1692, 1672, 1630, 1454, 1419, 1392, 1376, 1260, 1173, 1141, 1119, 1066, 992, 865, 723,700, 662, 563. HRMS: calcd. for C21H25NO2 Na+ [M + Na]+: 346.1778; Found: 346.1753.
3-(sec-Butyl)-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3c). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 2-methylbutanal (2c), and purified by flash column chromatography as yellow oil (21.0 mg, 68%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24 (m, 3H), 7.09 (d, J = 9.6, 2.4 Hz, 2H), 6.78 (dd, J = 10.0, 2.8 Hz, 1H), 6.54 (dd, J = 10.0, 3.2 Hz, 1H), 6.39 (dd, J = 10.4, 2.0 Hz, 1H), 6.00 (dd, J = 10.0, 2.0 Hz, 1H), 3.45 (d, J = 12.0 Hz, 1H), 3.20 (dd, J = 12.0, 2.8 Hz, 1H), 2.73 (s, 3H), 1.93–1.85 (m, 1H), 1.60–1.56 (m, 1H), 1.45–1.37 (m, 1H), 0.93 (t, J = 7.4 Hz, 3H), 0.85 (d, J = 7.0 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.46, 175.15, 149.45, 146.94, 134.72, 132.36, 131.58, 128.68, 128.59, 128.28, 65.02, 51.48, 48.30, 34.68, 27.12, 26.47, 16.47, 12.44. IR (cm−1): 3059, 3032, 2962, 2932, 2875, 1672, 1630, 1498, 1454, 1419, 1392, 1375, 1260, 1172, 1143, 1065, 990, 865, 767, 700, 621. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1605.
1-Methyl-3-(pentan-2-yl)-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3d). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 2-methylpentanal (2d), and purified by flash column chromatography as yellow oil (22.3 mg, 69%). 1H-NMR (400 MHz, CDCl3) δ 7.26–7.23 (m, 3H), 7.09 (dd, J = 7.6, 2.4 Hz, 2H), 6.76 (dd, J = 10.0, 2.8 Hz, 1H), 6.57 (dd, J = 10.0, 2.8 Hz, 1H), 6.38 (dd, J = 10.0, 2.0 Hz, 1H), 6.00 (dd, J = 10.0, 2.0 Hz, 1H), 3.44 (d, J = 10.8 Hz, 1H), 3.21 (dd, J = 11.6, 3.2 Hz, 1H), 2.74 (s, 3H), 2.30–2.23 (m, 1H), 1.37–1.23 (m, 2H), 1.16–1.01 (m, 2H), 0.96 (d, J = 6.9 Hz, 3H), 0.70 (t, J = 7.4 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.48, 175.64, 149.44, 147.26, 146.96, 135.09, 132.34, 132.24, 131.58, 131.47, 128.66, 128.58, 128.33, 128.29, 128.23, 65.05, 50.43, 48.48, 48.25, 36.50, 35.77, 32.70, 27.24, 20.94, 20.67, 15.97, 14.31, 13.98. IR (cm−1): 3059, 3032, 2958, 2928, 2872, 1672, 1630, 1498, 1454, 1421, 1377, 1261, 1172, 1119, 1067, 990, 865, 794, 724, 700, 621. HRMS: calcd. for C21H25NO2 Na+ [M + Na]+: 346.1778; Found: 346.1752.
3-(Heptan-3-yl)-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3e) [18]. The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 2-ethylhexanal (2e), and purified by flash column chromatography as yellow oil (21.4 mg, 61%). 1H-NMR (400 MHz, CDCl3) δ 7.25–7.22 (m, 3H), 7.09 (dd, J = 7.6, 2.4 Hz, 2H), 6.77 (dt, J = 10.0, 2.8 Hz, 1H), 6.60 (dt, J = 10.2, 3.2 Hz, 1H), 6.37 (dd, J = 10.0, 2.0 Hz, 1H), 6.00 (dt, J = 10.2, 2.2 Hz, 1H), 3.44 (dd, J = 11.8, 3.2 Hz, 1H), 3.30 (dd, J = 12.0, 3.0 Hz, 1H), 2.74 (s, 3H), 1.84–1.79 (m, 1H), 1.53–1.37 (m, 1H), 1.37–1.26 (m, 4H), 1.23–1.11 (m, 3H), 0.95 (t, J = 8.6 Hz, 3H), 0.81–0.72 (m, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.48, 175.79, 149.53, 147.23, 134.93, 132.23, 131.54, 128.61, 128.44, 128.29, 65.08, 51.27, 46.04, 45.96, 40.21, 40.10, 31.12, 30.60, 30.31, 30.01, 27.22, 24.47, 24.24, 23.10, 22.80, 14.24, 14.02, 12.69, 12.24. IR (cm−1): 3060, 3032, 2958, 2929, 2872, 1691, 1672, 1630, 1499, 1455, 1393, 1376, 1259, 1173, 1119, 1066, 991, 865, 766,722, 700, 662.
(3f) 3-(1-(4-isopropylphenyl)propan-2-yl)-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione. The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 3-(4-isopropylphenyl)-2-methylpropanal (2f), and purified by flash column chromatography as yellow oil (30.6 mg, 74%). 1H-NMR (400 MHz, CDCl3) δ 7.24–7.23 (m, 1H), 7.17–7.13 (m, 2.5H), 7.20–7.07 (m, 2.5H), 7.00 (dd, J = 6.0, 2.0 Hz, 1H), 6.89 (dd, J = 6.0, 1.6 Hz, 1H), 6.80–6.73 (m, 1.5H), 6.65 (dd, J = 10.0, 3.2 Hz, 0.5H), 6.53 (dd, J = 11.4, 3.2 Hz, 0.5H), 6.43–6.32 (m, 1.5H), 5.96 (td, J = 10.1, 2.0 Hz, 1H), 3.44–3.38 (m, 1H), 3.22–3.14 (m, 1H), 3.08–3.02 (m, 1H), 2.91–2.84 (m, 1H), 2.72 (d, J = 8 Hz, 3H), 2.67 (t, J = 6.8 Hz, 0.5H), 2.49 (q, J = 6.8 Hz, 0.5H), 2.28 (d, 5.6 Hz, 0.5H), 2.06–2.00(m, 1H), 1.28–1.21 (m, 6H), 0.99 (dd, J = 6.8, 4.4 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 174.77, 149.42, 149.36, 147.09, 146.84, 146.76, 146.58, 137.97, 137.48, 134.68, 133.85, 132.44, 132.18, 131.53, 131.45, 129.49, 129.06, 128.61, 128.58, 128.54, 128.30, 128.20, 128.00, 126.41, 126.33, 64.95, 64.89, 51.61, 50.78, 47.81, 45.16, 40.13, 40.02, 34.93, 34.56, 33.86, 33.79, 27.19, 26.98, 24.33, 24.19, 16.74, 15.80. IR (cm−1): 3049, 3030, 2960, 2928, 2873, 1689, 1631, 1499, 1454, 1392, 1378, 1265, 1172, 1114, 1058, 990, 864, 735, 700, 570. HRMS: calcd. for C28H31NO2 Na+ [M + Na]+: 436.2247; Found: 436.2217.
3-Cyclohexyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3g) [18]. The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with cyclohexanecarbaldehyde (2g), and purified by flash column chromatography as yellow oil (24.1 mg, 72%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24(m, 3H), 7.09 (dd, J = 7.6, 2.4 Hz, 2H), 6.76 (dd, J = 10.0, 3.2 Hz, 1H), 6.54 (dd, J = 10.2, 3.0 Hz, 1H), 6.39 (dd, J = 10.4, 2.0 Hz, 1H), 5.98 (dd, J = 10.2, 2.0 Hz, 1H), 3.48 (d, J = 12.0 Hz, 1H), 3.11 (dd, J = 11.8, 3.6 Hz, 1H), 2.73 (s, 3H), 2.01–1.94 (m, 1H), 1.73 (d, J = 13.2, 1H), 1.63–1.56 (m, 3H), 1.38–1.13 (m, 4H), 1.08–0.99 (m, 1H), 0.87–0.81 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 184.50, 175.30, 149.45, 147.09, 135.06, 132.33, 131.45, 128.63, 128.22, 128.19, 65.00, 51.02, 48.81, 38.27, 30.98, 29.14, 27.19, 26.71, 26.56, 26.23. IR (cm−1): 3057, 3032, 2925, 2852, 1690, 1672, 1499, 1450, 1419, 1393, 1377, 1260, 1172, 1134, 1096, 1069, 991, 864, 796, 732, 657, 569.
3-Cyclopentyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3h) [18]. The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with cyclopentanecarbaldehyde (2h), and purified by flash column chromatography as yellow oil (19.6 mg, 61%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24 (m, 3H), 7.09 (dd, J = 7.6, 2.4 Hz, 2H), 6.77 (dd, J = 10.2, 3.0 Hz, 1H), 6.54 (dd, J = 10.2, 3.1 Hz, 1H), 6.39 (dd, J = 10.2, 2.0 Hz, 1H), 5.99 (dd, J = 10.2, 2.0 Hz, 1H), 3.38 (d, J = 11.6 Hz, 1H), 3.21 (dd, J = 11.8, 6.2 Hz, 1H), 2.73 (s, 3H), 2.24–2.18 (m, 1H), 2.05–1.96 (m, 1H), 1.87–1.76 (m, 3H), 1.50–1.38 (m, 3H), 1.25–1.21 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 184.50, 175.30, 149.45, 147.09, 135.06, 132.33, 131.45, 128.63, 128.22, 128.19, 65.06, 53.45, 46.83, 41.20, 29.85, 29.56, 27.16, 25.18, 24.99. IR (cm−1): 3059, 3031, 2923, 2869, 1730, 1692, 1671, 1630, 1453, 1442, 1393, 1375, 1260, 1172, 1075, 991, 865, 794, 732,700, 645, 569.
3-(tert-Butyl)-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3i). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with pivalaldehyde (2i), and purified by flash column chromatography as yellow oil (16.7 mg, 52%). 1H-NMR (400 MHz, CDCl3) δ 7.27 (s, 3H), 7.21 (s, 2H), 6.77 (dd, J = 10.0, 3.2 Hz, 1H), 6.46 (dd, J = 10.2, 3.2 Hz, 1H), 6.38 (dd, J = 10.0, 2.0 Hz, 1H), 5.92 (dd, J = 10.2, 2.0 Hz, 1H), 3.40 (d, J = 11.2 Hz, 1H), 3.01 (d, J = 11.6 Hz, 1H), 2.70 (s, 3H), 1.00 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ 184.48, 175.09, 149.54, 147.98, 136.60, 132.11, 131.04, 128.02, 64.35, 52.50, 51.33, 33.87, 28.08, 27.22. IR (cm−1): 3031, 2958, 2870, 1688, 1672, 1630, 1605, 1468, 1420, 1392, 1370, 1260, 1244, 1171, 1119, 1093, 864, 794, 720, 735,700, 610, 563. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1597.
3-Isobutyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3j). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 3-methylbutanal (2j), and purified by flash column chromatography as yellow oil (19.8 mg, 64%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24 (m, 3H), 7.09 (dd, J = 7.6, 2.4 Hz, 2H), 6.76 (dd, J = 10.0, 3.2 Hz, 1H), 6.59 (dd, J = 10.4, 3.0 Hz, 1H), 6.39 (dd, J = 10.0, 2.0 Hz, 1H), 6.01 (dd, J = 10.2, 2.0 Hz, 1H), 3.25 (d, J = 11.6 Hz, 1H), 3.15 – 3.09 (m, 1H), 2.74 (s, 3H), 1.89–1.83 (m, 1H), 1.74–1.67 (m, 1H), 1.38–1.31 (m, 1H), 0.86 (d, J = 7.2 Hz, 3H), 0.80 (d, J = 6.4 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.45, 176.38, 149.36, 146.81, 134.34, 132.40, 131.59, 128.66, 128.36, 128.25, 65.23, 56.35, 41.66, 41.10, 27.23, 25.20, 22.93, 22.37. IR (cm−1): 3056, 3033, 2956, 2927, 2869, 1692, 1672, 1630, 1467, 1454, 1393, 1376, 1262, 1172, 1137, 1080, 1060, 866, 790, 721, 700. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1597.
3-Ethyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3k). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with propionaldehyde (2k), and purified by flash column chromatography as yellow oil (16.1 mg, 56%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.24 (m, 3H), 7.10 (dd, J = 8.0, 2.4 Hz 2H), 6.79 (dd, J = 10.0, 3.2 Hz, 1H), 6.57 (dd, J = 10.2, 3.2 Hz, 1H), 6.41 (dd, J = 10.0, 2.0 Hz, 1H), 6.02 (dd, J = 10.2, 2.0 Hz, 1H), 3.34 (d, J = 12.0 Hz, 1H), 3.13–3.06 (m, 1H), 2.75 (s, 3H), 1.96–1.86 (m, 1H), 1.77–1.68 (m, 1H), 0.89 (t, J = 7.4 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.46, 175.72, 149.32, 146.57, 134.25, 132.53, 131.70, 128.70, 128.34, 128.17, 65.14, 54.39, 44.64, 27.17, 23.01, 11.18. IR (cm−1): 3056, 3032, 2965, 2931, 2877, 1692, 1672, 1629, 1454, 1420, 1394, 1376, 1262, 1174, 1125, 1060, 988, 865, 719, 699, 659. HRMS: calcd. for C18H19NO2 Na+ [M + Na]+: 304.1308; Found: 304.1286.
3-Benzyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (3l). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methylcinnamamide (1a) with 2-phenylacetaldehyde (2l), and purified by flash column chromatography as yellow oil (14.4 mg, 42%). 1H-NMR (400 MHz, CDCl3) δ 7.24–7.16 (m, 6H), 7.07–7.04 (m, 2H), 6.99–6.97 (m, 2H), 6.56 (dd, J = 10.2, 3.0 Hz, 1H), 6.47 (dd, J = 10.0, 3.0 Hz, 1H), 6.30 (dd, J = 10.2, 2.0 Hz, 1H), 6.00 (dd, J = 10.2, 2.0 Hz, 1H), 3.42 (dt, J = 12.2, 5.4 Hz, 1H), 3.30–3.20 (m, 2H), 2.93 (dd, J = 13.6, 5.8 Hz, 1H), 2.71 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.41, 174.77, 149.24, 146.36, 137.36, 133.43, 132.39, 131.76, 129.98, 128.69, 128.47, 128.28, 128.27, 126.71, 65.01, 52.74, 45.16, 34.06, 27.23. IR (cm−1): 3060, 3029, 2922, 2853, 1693, 1672, 1630, 1496, 1453, 1393, 1377, 1261, 1172, 1130, 1092, 1075, 865, 788, 721, 699. HRMS: calcd. for C23H21NO2 Na+ [M + Na]+: 366.1465; Found: 366.1443.
3-Isopropyl-1-methyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4b). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methyl-3-(p-tolyl)acrylamide (1b) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (22.2 mg, 72%). 1H-NMR (400 MHz, CDCl3) δ 7.05 (d, J = 7.6 Hz, 2H), 6.53 (d, J = 7.6 Hz, 2H), 6.77 (dd, J = 10.0, 3.2 Hz, 1H), 6.56 (dd, J = 10.2, 3.0 Hz, 1H), 6.38 (dd, J = 10.0, 2.0 Hz, 1H), 6.01 (dd, J = 10.2, 1.8 Hz, 1H), 3.40 (d, J = 11.6 Hz, 1H), 3.11 (dd, J = 12.0, 3.6 Hz, 1H), 2.73 (s, 3H), 2.36–2.31 (m, 1H), 2.28 (s, 3H), 1.01 (d, J = 6.8 Hz, 3H), 0.83 (d, J = 7.2 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.60, 175.34, 149.56, 147.20, 137.93, 132.28, 131.95, 131.50, 129.33, 128.09, 65.05, 50.58, 49.13, 28.07, 27.14, 21.16, 20.13, 18.83. IR (cm−1): 3046, 3030, 2960, 2929, 2876, 1690, 1672, 1629, 1516, 1465, 1419, 1393, 1375, 1263, 1065, 992, 864, 736, 639, 575. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1602.
4-(4-Chlorophenyl)-3-isopropyl-1-methyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4c). The title compound was prepared according to the general procedure described above by the reaction between 3-(4-chlorophenyl)-N-(4-hydroxyphenyl)-N-methylacrylamide (1c) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (22.7 mg, 69%). 1H-NMR (400 MHz, CDCl3) δ 7.24 (dd, J = 6.4, 2.0 Hz, 2H), 7.05 (dd J = 6.4, 2.0 Hz, 2H), 6.76 (dd, J = 10.2, 3.0 Hz, 1H), 6.54 (dd, J = 10.2, 3.2 Hz, 1H), 6.40 (dd, J = 10.0, 2.0 Hz, 1H), 6.05 (dd, J = 10.2, 2.0 Hz, 1H), 3.40 (d, J = 12.0 Hz, 1H), 3.08 (dd, J = 12.0, 3.6 Hz, 1H), 2.73 (s, 3H), 2.38–2.30 (m, 1H), 1.00 (d, J = 6.8 Hz, 3H), 0.82 (d, J = 6.8 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.23, 174.90, 149.09, 146.66, 134.10, 133.70, 132.53, 131.86, 129.53, 128.91, 64.83, 50.23, 49.23, 28.06, 27.19, 20.29, 18.68. IR (cm−1): 3050, 2961, 2931, 2874, 1692, 1672, 1630, 1494, 1466, 1417, 1392, 1373, 1259, 1173, 1121, 1092, 1014, 866, 830, 736. HRMS: calcd. for C19H20ClNO2 Na+ [M + Na]+: 352.1075; Found: 352.1059.
4-(3-Chlorophenyl)-3-isopropyl-1-methyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4d). The title compound was prepared according to the general procedure described above by the reaction between 3-(3-chlorophenyl)-N-(4-hydroxyphenyl)-N-methylacrylamide (1d) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (21.1 mg, 64%). 1H-NMR (400 MHz, CDCl3) δ 7.25–7.20 (m, 2H), 7.10 (s 1H), 7.00 (dt, J = 7.0, 1.8 Hz, 1H), 6.76 (dd, J = 10.2, 3.2 Hz, 1H), 6.56 (dd, J = 10.2, 3.0 Hz, 1H), 6.42 (dd, J = 10.0, 2.0 Hz, 1H), 6.06 (dd, J = 10.2, 2.0 Hz, 1H), 3.40 (d, J = 12.0 Hz, 1H), 3.08 (dd, J = 12.0, 3.6 Hz, 1H), 2.73 (s, 3H), 2.38–2.30 (m, 1H), 1.01 (d, J = 6.8 Hz, 3H), 0.83 (d, J = 6.8 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.22, 174.78, 148.99, 146.52, 137.35, 134.58, 132.62, 131.87, 129.96, 128.55, 128.35, 126.53, 64.76, 50.52, 49.20, 28.09, 27.17, 20.23, 18.75. IR (cm−1): 3056, 2961, 2931, 2874, 1691, 1672, 1630, 1468, 1422, 1392, 1375, 1261, 1173, 1119, 1082, 880, 852, 736, 690, 623. HRMS: calcd. for C19H20ClNO2 Na+ [M + Na]+: 352.1075; Found: 352.1053.
4-(2-Chlorophenyl)-3-isopopyl-1-methyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4e). The title compound was prepared according to the general procedure described above by the reaction between 3-(2-chlorophenyl)-N-(4-hydroxyphenyl)-N-methylacrylamide (1e) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (21.0 mg, 63%). 1H-NMR (400 MHz, CDCl3) δ 7.34–7.24 (m, 3H), 7.22–7.16 (m, 2H), 6.88 (dd, J = 10.2, 3.0 Hz, 1H), 6.68 (dd, J = 10.2, 3.2 Hz, 1H), 6.33 (dd, J = 10.2, 2.0 Hz, 1H), 6.12 (dd, J = 10.2, 2.0 Hz, 1H), 4.25 (d, J = 11.8 Hz, 1H), 3.04 (dd, J = 11.8, 3.6 Hz, 1H), 2.74 (s, 3H), 2.39–2.31 (m, 1H), 1.00 (d, J = 6.8 Hz, 3H), 0.76 (d, J = 7.0 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.41, 174.88, 149.76, 146.58, 133.44, 131.83, 131.81, 130.45, 135.01, 129.37, 129.22, 126.68, 64.64, 51.18, 46.06, 28.23, 26.94, 20.50, 18.43. IR (cm−1): 3059, 2960, 2931, 2873, 1692, 1672, 1631, 1468, 1422, 1392, 1374, 1260, 1174, 1116, 1065, 1037, 864, 750, 736, 698. HRMS: calcd. for C19H20ClNO2 Na+ [M + Na]+: 352.1075; Found: 352.1054.
4-(4-Bromophenyl)-3-isopropyl-1-methyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4f). The title compound was prepared according to the general procedure described above by the reaction between 3-(4-bromophenyl)-N-(4-hydroxyphenyl)-N-methylacrylamide (1f) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (23.5 mg, 63%). 1H-NMR (400 MHz, CDCl3) δ 7.41–7.38 (m, 2H), 6.99 (dd, J = 6.4, 2.0 Hz, 2H), 6.75 (dd, J = 10.0, 3.2 Hz, 1H), 6.53 (dd, J = 10.2, 3.0 Hz, 1H), 6.40 (dd, J = 10.2, 2.0 Hz, 1H), 6.05 (dd, J = 10.2, 2.0 Hz, 1H), 3.39 (d, J = 12.0 Hz, 1H), 3.07 (dd, J = 12.0, 3.6 Hz, 1H), 2.73 (s, 3H), 2.38–2.30 (m, 1H), 0.99 (d, J = 7.0 Hz, 3H), 0.82 (d, J = 7.0 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.22, 174.83, 149.09, 146.60, 134.26, 132.56, 131.90, 131.88, 129.86, 122.24, 64.73, 50.31, 49.19, 28.09, 27.19, 20.31, 18.70. IR (cm−1): 3048, 2960, 2929, 2873, 1693, 1671, 1630, 1491, 1466, 1392, 1374, 1260, 1173, 1120, 1010, 866, 827, 755, 629, 571. HRMS: calcd. for C19H20BrNO2 Na+[M + Na]+: 396.0570; Found: 396.0550.
3-Isopropyl-1-methyl-4-(4-(trifluoromethyl)phenyl)-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4g). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methyl-3-(4-(trifluoromethyl)phenyl)acrylamide (1g) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (23.6 mg, 65%). 1H-NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.8 Hz, 2H), 7.25 (t, J = 7.4 Hz, 2H), 6.79 (dd, J = 10.2, 3.2 Hz, 1H), 6.55 (dd, J = 10.2, 3.2 Hz, 1H), 6.42 (dd, J = 10.0, 2.0 Hz, 1H), 6.04 (dd, J = 10.2, 2.0 Hz, 1H), 3.49 (d, J = 11.8 Hz, 1H), 3.15 (dd, J = 12.0, 3.8 Hz, 1H), 2.74 (s, 3H), 2.39–2.31 (m, 1H), 1.01 (d, J = 7.0 Hz, 3H), 0.82 (d, J = 7.0 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.05, 174.67, 148.90, 146.28, 139.43, 132.68, 131.97, 128.69, 125.73, 125.69, 64.68, 50.57, 49.24, 28.13, 27.17, 20.30, 18.72. 19F-NMR (376 MHz, CDCl3) δ 62.65 (s, 1F). IR (cm−1): 3050, 2962, 2933, 2876, 1692, 1672, 1631, 1468, 1422, 1392, 1375, 1327, 1166, 1124, 1069, 1017, 869, 853, 737, 602. HRMS: calcd. for C20H20F3NO2 Na+ [M + Na]+: 386.1338; Found: 386.1319.
(1-Ethyl-3-isopropyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4h). The title compound was prepared according to the general procedure described above by the reaction between N-ethyl-N-(4-hydroxyphenyl)cinnamamide (1h) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (22.0 mg, 71%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 3H), 7.09 (dd, J = 8.0, 2.0 Hz, 2H), 6.82 (dd, J = 10.1, 3.1 Hz, 1H), 6.62 (dd, J = 10.2, 3.0 Hz, 1H), 6.37 (dd, J = 10.1, 2.0 Hz, 1H), 5.95 (dd, J = 10.2, 2.0 Hz, 1H), 3.42 (d, J = 12.0 Hz, 1H), 3.36–3.27 (m, 1H), 3.14 (dd, J = 12.0, 3.6 Hz, 1H), 3.10–3.01 (m, 1H), 2.38–2.30 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.01 (d, J = 7.0 Hz, 3H), 0.82 (d, J = 7.2 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.71, 175.02, 149.52, 148.09, 135.02, 131.86, 130.60, 128.61, 128.28, 128.23, 65.24, 51.25, 49.04, 36.86, 28.11, 20.17, 18.71, 15.29. IR (cm−1): 3057, 3033, 2962, 2934, 2874, 1678, 1629, 1498, 1454, 1402, 1376, 1310, 1262, 1140, 1125, 1064, 940, 866, 724, 700. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1597.
1-Benzyl-3-isopropyl-4-phenyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4i). The title compound was prepared according to the general procedure described above by the reaction between N-benzyl-N-(4-hydroxyphenyl)cinnamamide (1i) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (27.8 mg, 75%). 1H-NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 3H), 7.22–7.18 (m, 5H), 7.04 (dd, J = 6.8, 3.0 Hz, 2H), 6.54 (dd, J = 10.4, 3.2 Hz, 1H), 6.47 (dd, J = 10.2, 3.0 Hz, 1H), 6.18 (dd, J = 10.0, 2.0 Hz, 1H), 5.81 (dd, J = 10.2, 2.0 Hz, 1H), 4.66 (d, J = 15.0 Hz, 1H), 4.07 (d, J = 14.8 Hz, 1H), 3.42 (d, J = 12.0 Hz, 1H), 3.22 (dd, J = 12.0, 3.8 Hz, 1H), 2.43–2.35 (m, 1H), 1.07 (d, J = 6.8 Hz, 3H), 0.85 (d, J = 6.8 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.70, 175.31, 149.35, 147.30, 137.95, 134.66, 131.35, 130.71, 128.64, 128.60, 128.53, 128.30, 128.24, 127.78, 65.26, 51.27, 48.88, 45.36, 28.14, 20.11, 18.86. IR (cm−1): 3061, 3031, 2961, 2930, 2873, 1670, 1629,1496, 1454, 1399, 1264, 1179, 1065, 1011, 958, 930, 863, 792, 734, 700. HRMS: calcd. for C25H25NO2 Na+ [M + Na]+: 394.1778; Found: 394.1761.
3-Isopropyl-1-methyl-4-(naphthalen-2-yl)-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4j). The title compound was prepared according to the general procedure described above by the reaction between N-(4-hydroxyphenyl)-N-methyl-3-(naphthalen-2-yl)acrylamide (1j) with isobutyraldehyde (2a), and purified by flash column chromatography as white solid (21.7 mg, 62%). m.p. 84.4–86.8 °C. 1H-NMR (400 MHz, CDCl3) δ 7.80–7.73 (m, 3H), 7.58–7.54 (m, 1H), 7.50–7.45 (m, 2H), 7.22 (dd, J = 8.4, 2.0 Hz, 1H), 6.84 (dd, J = 10.2, 3.2 Hz, 1H), 6.63 (dd, J = 10.2, 3.0 Hz, 1H), 6.41 (dd, J = 10.2, 2.0 Hz, 1H), 5.94 (dd, J = 10.2, 2.0 Hz, 1H), 3.61 (d, J = 11.8 Hz, 1H), 3.27 (dd, J = 11.8, 3.8 Hz, 1H), 2.76 (s, 3H), 2.42–2.34(m, 1H), 1.04 (d, J = 6.8 Hz, 3H), 0.83 (d, J = 7.0 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.38, 175.20, 149.49, 146.96, 133.11, 132.76, 132.40, 131.58, 133.02, 128.46, 127.88, 127.80, 127.46, 126.63, 126.46, 125.83, 65.04, 50.97, 49.34, 28.21, 27.15, 20.25, 18.80. IR (cm−1): 3052, 2960, 2931, 2874, 1691, 1672, 1629, 1466, 1418, 1392, 1374, 1261, 1173, 1141, 1064, 992, 854, 822, 735, 607. HRMS: calcd. for C23H23NO2 Na+ [M + Na]+: 368.1621; Found: 368.1605.
4-(Furan-2-yl)-3-isopropyl-1-methyl-1-azaspiro[4.5]deca-6,9-diene-2,8-dione (4k). The title compound was prepared according to the general procedure described above by the reaction between 3-(furan-2-yl)-N-(4-hydroxyphenyl)-N-methylacrylamide (1k) with isobutyraldehyde (2a), and purified by flash column chromatography as yellow oil (20.5 mg, 72%). 1H-NMR (400 MHz, CDCl3) δ 7.29–7.28 (m, 1H), 6.70 (dd, J = 10.0, 3.1 Hz, 1H), 6.63 (dd, J = 10.2, 3.0 Hz, 1H), 6.43 (dd, J = 10.0, 2.0 Hz, 1H), 6.24 (dd, J = 3.2, 2.0 Hz, 1H), 6.10–6.07 (m, 2H), 3.48 (d, J = 11.8 Hz, 1H), 3.17 (dd, J = 12.0, 4.0 Hz, 1H), 2.72 (s, 3H), 2.42–2.34 (m, 1H), 0.98 (d, J = 6.8 Hz, 3H), 0.86 (d, J = 7.2 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 184.51, 174.69, 149.14, 149.04, 146.70, 142.45, 132.12, 131.24, 110.67, 108.59, 64.25, 48.72, 44.06, 27.76, 27.04, 19.70, 18.14. IR (cm−1): 3049, 2962, 2933, 2876, 1697, 1674, 1631, 1505, 1420, 1390, 1373, 1261, 1173, 1149, 1118, 1065, 1021, 859, 736, 599. HRMS: calcd. for C17H19NO3 Na+ [M + Na]+: 308.1257; Found: 308.1248.
2,6-di-tert-Butyl-4-isopropyl-4-methylcyclohexa-2,5-dienone (5) [52]. The title compound was prepared from mechanistic experiment. 1H-NMR (400 MHz, CDCl3) δ 6.44 (s, 2H), 1.80–174 (m, 1H), 1.24 (s, 18H), 1.17 (s, 3H), 0.84 (d, J = 6.8 Hz, 6H). 13 C-NMR (100 MHz, CDCl3) δ 186.90, 146.95, 145.80, 42.43, 37.55, 34.89, 29.70, 24.72, 18.09. IR (cm−1): 3001, 2961, 2876, 1658, 1643, 1460, 1374, 1267, 1061, 867, 751.
3-Isopropyl-6-methoxy-1-methyl-4-phenyl-3,4-dihydroquinolin-2(1H)-one (6). The title compound was prepared according to the general procedure described above by the reaction between N-(4-methoxyphenyl)-N-methylcinnamamide with isobutyraldehyde (2a), and purified by flash column chromatography as colorless oil (22.9 mg, 74%). 1H-NMR (400 MHz, CDCl3) δ 7.24–7.13 (m, 3H), 7.00–6.97 (m, 3H), 6.85 (dd, J = 8.8, 3.2 Hz, 1H), 6.75 (d, J = 2.8 Hz, 1H), 4.13 (s, 1H), 3.78 (s, 3H), 3.33 (s, 3H), 2.57 (dd, J = 9.2, 2.0 Hz, 1H), 1.70–1.63 (m, 1H), 1.04 (d, J = 6.6 Hz, 3H), 0.98 (d, J = 6.8 Hz, 3H). 13C-NMR (100 MHz, CDCl3) δ 170.31, 155.69, 141.99, 133.86, 128.82, 128.23, 127.19, 126.84, 115.88, 115.52, 112.61, 56.64, 55.56, 45.37, 29.73, 28.55, 21.19, 21.05. IR (cm−1): 3060, 3025, 2960, 2933, 2872, 2835, 1731, 1666, 1590, 1503, 1469, 1432, 1387, 1341, 1249, 1034, 910, 810, 699, 621. HRMS: calcd. for C20H23NO2 Na+ [M + Na]+: 332.1621; Found: 332.1610.
4. Conclusions
We have developed a convenient Fe-catalyzed decarbonylative alkylative spirocyclization of N-aryl cinnamamide with aliphatic aldehydes to provide 1-azaspirocyclohexadienones. Readily available aliphatic aldehydes were decarbonylated into primary, secondary and tertiary alkyl radicals readily for the cascade construction of dual C(sp3)-C(sp3) and C=O bonds. The application of cheap and readily available aliphatic aldehydes as alkyl source, convenient experimental operation for the alkyl radical generation, green ferrous catalyst and reaction solvent, C=C bond difunctionalization strategy and versatile synthetic utilities of the 1-azaspirocyclohexadienone, would render this decarbonylative alkylative spirocyclization attractive for organic synthesis and medicinal chemistry.
Supplementary Materials
The following are available online, experimental details, characterisation of products and copies of NMR spectrums.
Author Contributions
X.P. and R.-X.L. contributed equally to this work on data collection, data analysis, data interpretation; data collection, X.-Y.X.; conception and supervision, L.Y.; All X.P., R.-X.L., X.-Y.X. and L.Y. have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21772168), Opening Fund of Beijing National Laboratory for Molecular Sciences, Key Foundation of Education Bureau of Hunan Province (17A208), Hunan 2011 Collaborative Innovation Centre of Chemical Engineering & Technology with Environmental Benignity and Effective Resource Utilization, the project of innovation team of the ministry of education (IRT_17R90).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kotha, S.; Deb, A.C.; Lahiri, K.; Manivannan, E. Selected Synthetic Strategies to Spirocyclics. Synthesis 2009, 2009, 165–193. [Google Scholar] [CrossRef]
- Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef]
- Sannigrahi, M. Stereocontrolled synthesis of spirocyclics. Tetrahedron 1999, 55, 9007–9071. [Google Scholar] [CrossRef]
- Rosenberg, S.; Leino, R. Synthesis of Spirocyclic Ethers. Synthesis 2009, 2009, 2651–2673. [Google Scholar]
- Nemoto, T.; Zhao, Z.-D.; Yokosaka, T.; Suzuki, Y.; Wu, R.; Hamada, Y. Palladium-Catalyzed Intramolecular ipso-Friedel-Crafts Alkylation of Phenols and Indoles: Rearomatization-Assisted Oxidative Addition. Angew. Chem. Int. Ed. 2013, 52, 2217–2220. [Google Scholar] [CrossRef]
- Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Iridium-Catalyzed Intramolecular Asymmetric Allylic Dearomatization of Phenol. Angew. Chem. Int. Ed. 2011, 50, 4455–4458. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, B.S.; Condie, A.G.; Buff, R.C.; Karahalis, G.J.; Stephenson, C.R.J. Intercepting Wacker Intermediates with Arenes: C-H Functionalization and Dearomatization. Org. Lett. 2011, 13, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Zhang, L.; Lee, J.-Y. Copper-Catalyzed Synthesis of Azaspirocyclohexadienones from α-Azido-N-arylamides under an Oxygen Atmosphere. J. Am. Chem. Soc. 2010, 132, 7266–7267. [Google Scholar] [CrossRef]
- Pigge, F.C.; Dhanya, R.; Hoefgen, E.R. Morita-Baylis-Hillman Cyclizations of Arene-Ruthenium-Functionalized Acrylamides. Angew. Chem. Int. Ed. 2007, 46, 2887–2890. [Google Scholar] [CrossRef]
- Tang, B.-X.; Tang, D.-J.; Tang, S.; Yu, Q.-F.; Zhang, Y.-H.; Liang, Y.; Zhong, P.; Li, J.-H. Intramolecular ipso-Halocyclization of 4-(p-Unsubstituted-aryl)-1-alkynes Leading to Spiro[4,5]trienones: Scope, Application, and Mechanistic Investigations. J. Org. Chem. 2012, 77, 2837–2849. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Larock, R.C. Synthesis of Spiro[4.5]trienones by Intramolecular ipso-Halocyclization of 4-(p-Methoxyaryl)-1-alkynes. J. Am. Chem. Soc. 2005, 127, 12230–12231. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.-X.; Tang, D.-J.; Tang, S.; Yu, Q.-F.; Zhang, Y.-H.; Liang, Y.; Zhong, P.; Li, J.-H. Selective Synthesis of Spiro[4,5]trienyl Acetates via an Intramolecular Electrophilic ipso-Iodocyclization Process. Org. Lett. 2008, 10, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-J.; Xie, Y.-X. Recent Advances in Oxidative ipso-Annulation of N-Arylpropiolamides. Chin. J. Chem. 2017, 35, 280–288. [Google Scholar] [CrossRef]
- Yang, X.-H.; Song, R.-J.; Xie, Y.-X.; Li, J.-H. Iron Catalyzed Oxidative Coupling, Addition, and Functionalization. ChemCatChem. 2016, 8, 2429–2445. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, G.-T.; Wang, H.-M.; Huang, Z.-Y.; Wang, J.; Singh, A.K.; Lei, A.-W. Recent Advances in Radical C-H Activation/Radical Cross-Coupling. Chem. Rev. 2017, 17, 9016–9085. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-T.; Song, R.-J.; Ouyang, X.-H.; Li, Y.; Li, H.-B.; Li, J.-H. Copper-catalyzed oxidative ipso-carboalkylation of activated alkynes with ethers leading to 3-etherified azaspiro[4.5]trienones. Org. Chem. Front. 2014, 1, 484–489. [Google Scholar] [CrossRef]
- Ouyang, X.-H.; Song, R.-J.; Liu, B.; Li, J.-H. Synthesis of 3-Alkyl Spiro[4,5]trienones by Copper-Catalyzed Oxidative ipso-Annulation of Activated Alkynes with Unactivated Alkanes. Chem. Commun. 2016, 52, 2573–2576. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Gu, Z.-X.; Xu, P.; Hu, H.-W.; Cheng, Y.-X.; Zhu, C.-J. Metal-free tandem oxidative C(sp3)-H bond functionalization of alkanes and dearomatization of N-phenyl-cinnam-amides: Access to alkylated 1-azaspiro[4.5]decanes. Chem. Commun. 2016, 52, 477–480. [Google Scholar] [CrossRef]
- Wang, C.-S.; Roisnel, T.; Dixneuf, P.H.; Soulé, J.F. Access to 3-(2-Oxoalkyl)-azaspiro[4.5]trienones via AcidTriggered Oxidative Cascade Reaction through Alkenyl Peroxide Radical Intermediate. Adv. Synth. Catal. 2019, 361, 445–450. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Zhu, C.-J. Iron-Catalyzed Cascade Cyanoalkylation/Radical Dearomatization of N-phenylcinnamamides: Access to Cyanoalkylated 1-Azaspiro[4.5]decanes. Org. Chem. Front. 2017, 4, 1272–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Zhou, C.-S.; Xiong, B.-Q.; Zhang, P.-L.; Yang, C.-A.; Tang, K.-W. Visible-Light-Mediated Ipso-Carboacylation of Alkynes: Synthesis of 3-Acylspiro[4,5]trienones from N-(p-Methoxyaryl)propiolamides and Acyl Chlorides. J. Org. Chem. 2018, 83, 2210–2218. [Google Scholar] [CrossRef]
- Ouyang, X.-H.; Song, R.-J.; Li, Y.; Liu, B.; Li, J.-H. Metal-Free Oxidative Ipso-Carboacylation of Alkynes: Synthesis of 3-Acylspiro[4,5]trienones from N-Arylpropiolamides and Aldehydes. J. Org. Chem. 2014, 79, 4582–4589. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, S.-M.; Li, Z.-Z.; Zhu, Y.; Yu, J.; Li, L.; Li, M.-Z.; Tang, S.; Sheng, R.-R. Photocatalyzed cascade Meerwein addition cyclization of N-benzylacrylamides toward azaspirocycles. Org. Biomol. Chem. 2018, 16, 2406–2410. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, D.-M.; Tang, G.; Zhao, Y.-F. Copper-Catalyzed Phosphonylation/Trifluoromethylation of N-p-NO2-Benzoylacrylamides Coupled with Dearomatization and Denitration. Org. Lett. 2019, 21, 7674–7678. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.-L.; He, Y.-T.; Qiu, Y.-F.; Li, Y.-X.; Song, B.; Gao, P.; Song, X.-R.; Guo, D.-H.; Liu, X.-Y.; Liang, Y.-M. Copper-Catalyzed Difunctionalization of Activated Alkynes by Radical Oxidation-Tandem Cyclization/Dearomatization to Synthesize 3-Trifluoromethyl Spiro[4.5]trienones. Chem. Eur. J. 2015, 21, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-F.; Wang, Q.; Liu, Y.-X.; Wang, Q.-M. Copper-Mediated α-Trifluoromethylation of N-Phenylcinnamamides Coupled with Dearomatization: Access to Trifluoromethylated 1-Azaspiro [4.5]decanes. Org. Lett. 2014, 16, 5914–5917. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, H.-H.; Yang, D.-S.; Yue, H.-L.; He, C.-L.; Zhang, Y.-L.; Wang, H. Visible-light-enabled spirocyclization of alkynes leading to 3-sulfonyl and 3- sulfenyl azaspiro[4,5]trienones. Green Chem. 2017, 19, 5608–5613. [Google Scholar] [CrossRef]
- Wen, J.-W.; Wei, W.; Xue, S.-N.; Yang, D.-S.; Lou, Y.; Gao, C.-Y.; Wang, H. Metal-Free Oxidative Spirocyclization of Alkynes with Sulfonylhydrazides Leading to 3-Sulfonated Azaspiro[4,5]trienones. J. Org. Chem. 2015, 80, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. AgSCF3-Mediated Oxidative Trifluoromethythiolation of Alkynes with Dearomatization to Synthesize SCF3-Substituted Spiro[4,5]trienones. Org. Lett. 2016, 18, 3486–3489. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-H.; Wei, W.; Yang, D.-S.; Zhang, J.-M.; Xu, Z.-H.; Wen, J.-W.; Wang, H. Silver-catalyzed direct spirocyclization of alkynes with thiophenols: A simple and facile approach to 3-thioazaspiro[4,5]trienones. RSC Adv. 2015, 5, 84657–84661. [Google Scholar] [CrossRef]
- Qian, P.-C.; Liu, Y.; Song, R.-J.; Xiang, J.-N.; Li, J.-H. Copper-Catalyzed Oxidative ipso-Cyclization of N-(p-Methoxyaryl)propiolamides with Disulfides and Water Leading to 3-(Arylthio)-1-azaspiro[4.5]deca-3,6,9-triene-2,8-diones. Synlett 2015, 26, 1213–1216. [Google Scholar] [CrossRef]
- Gao, W.-C.; Liu, T.; Cheng, Y.-F.; Chang, H.-H.; Li, X.; Zhou, R.; Wei, W.-L.; Qiao, Y. AlCl3-Catalyzed Intramolecular Cyclization of N-Arylpropynamides witH-N-Sulfanylsuccinimides: Divergent Synthesis of 3-Sulfenyl Quinolin-2-ones and Azaspiro[4,5]trienones. J. Org. Chem. 2017, 82, 13459–13467. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.; Mandal, A.; Dana, S.; Baidya, M. Visible Light-Induced Synthetic Approach for Selenylative Spirocyclization of N-Aryl Alkynamides with Molecular Oxygen as Oxidant. Adv. Synth. Catal. 2018, 360, 1099–1130. [Google Scholar] [CrossRef]
- Yang, X.-H.; Ouyang, X.-H.; Wei, W.-T.; Song, R.-J.; Li, J.-H. Nitrative Spirocyclization Mediated by TEMPO: Synthesis of Nitrated Spirocycles from N-Arylpropiolamides, tert-Butyl Nitrite and Water. Adv. Synth. Catal. 2015, 357, 1161–1166. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wang, A.-Q.; Xia, Y.; Wu, X.-X.; Liu, X.-Y.; Liang, Y.-M. Silver-catalyzed carbon–phosphorus functionalization of N-(p-methoxyaryl)propiolamides coupled with dearomatization: Access to phosphorylated aza-decenones. Chem. Commun. 2014, 50, 13998–14001. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.-H.; Hu, B.-Y.; Ji, M.-M.; Ye, S.-Y.; Zhu, G.-G. Synthesis of Difluoromethylated and Phosphorated Spiro[5.5]trienones via Dearomative Spirocyclization of Biaryl Ynones. Org. Lett. 2018, 20, 2988–2992. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, W.-W.; Zhang, Z.-C. Copper-Catalyzed Oxidative ipso-Annulation of Activated Alkynes with Silanes: An Approach to 3-Silyl Azaspiro[4,5] trienones. Org. Lett. 2016, 18, 5820–5823. [Google Scholar] [CrossRef]
- Wu, L.-J.; Tan, F.-L.; Li, M.; Song, R.-J.; Li, J.-H. Fe-Catalyzed Oxidative Spirocyclization of N-Arylpropiolamides with Silanes and TBHP Involving the Formation of C-Si Bond. Org. Chem. Front. 2017, 4, 350–353. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Li, C.-J. An Olefination via Ruthenium-Catalyzed Decarbonylative Addition of Aldehydes to Terminal Alkynes. J. Am. Chem. Soc. 2009, 131, 15092–15093. [Google Scholar] [CrossRef]
- Shuai, Q.; Yang, L.; Guo, X.; Basle, O.; Li, C.-J. Rhodium-Catalyzed Oxidative C–H Arylation of 2-Arylpyridine Derivatives via Decarbonylation of Aromatic Aldehydes. J. Am. Chem. Soc. 2010, 132, 12212–12213. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Li, C.-J. Ru-Catalyzed Decarbonylative Addition of Aliphatic Aldehydes to Terminal Alkynes. Org. Lett. 2010, 12, 3176–3178. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Correia, C.A.; Guo, X.; Li, C.-J. A novel catalytic decarbonylative Heck-type reaction and conjugate addition of aldehydes to unsaturated carbonyl compounds. Tetrahedron Lett. 2010, 51, 5486–5489. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Li, C.-J. The First Decarbonylative Coupling of Aldehydes and Norbornenes Catalyzed by Rhodium. Adv. Synth. Catal. 2010, 352, 2899–2904. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, T.; Shuai, Q.; Guo, X.; Li, C.-J. Phosphine ligand triggered oxidative decarbonylative homocoupling of aromatic aldehydes: Selectively generating biaryls and diarylketones. Chem. Commun. 2011, 47, 2161–2163. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; He, Q.; Yang, L. Metal-free oxidative decarbonylative coupling of aromatic aldehydes with arenes: Direct access to biaryls. Chem. Commun. 2015, 51, 5925–5928. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; Kang, L.; Yang, L. Metal-Free Oxidative Decarbonylative Coupling of Aliphatic Aldehydes with Azaarenes: Successful Minisci-Type Alkylation of Various Heterocycles. Adv. Synth. Catal. 2015, 357, 2055–2060. [Google Scholar] [CrossRef]
- Wu, C.-S.; Li, R.; Wang, Q.-Q.; Yang, L. Fe-Catalyzed decarbonylative alkylation–peroxidation of alkenes with aliphatic aldehydes and hydroperoxide under mild conditions. Green Chem. 2019, 21, 269–274. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, Y.-Y.; Du, X.-J.; Ma, D.-Y.; Yang, L. Co-Catalyzed decarbonylative alkylative esterification of styrenes with aliphatic aldehydes and hypervalent iodine(III) reagents. Org. Chem. Front. 2019, 6, 3065–3070. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wang, Q.-Q.; Yang, L. Metal-free decarbonylative alkylation-aminoxidation of styrene derivatives with aliphatic aldehydes and N-hydroxyphthalimide. Org. Biomol. Chem. 2017, 15, 1338–1342. [Google Scholar] [CrossRef]
- Li, W.-Y.; Wang, Q.-Q.; Yang, L. Metal-free decarbonylative alkylation–aminoxidation of styrene derivatives with aliphatic aldehydes and N-hydroxyphthalimide. Org. Biomol. Chem. 2017, 15, 9987–9991. [Google Scholar] [CrossRef]
- Yang, L.; Lu, W.; Zhou, W.; Zhang, F. Metal-free cascade oxidative decarbonylative alkylarylation of acrylamides with aliphatic aldehydes: A convenient approach to oxindoles via dual C(sp2)-H bond functionalization. Green Chem. 2016, 18, 2941–2945. [Google Scholar] [CrossRef]
- Gao, R.-X.; Luan, X.-Q.; Xie, Z.-Y.; Yang, L.; Pei, Y. Fe-Catalyzed decarbonylative cascade reaction of N-aryl cinnamamides with aliphatic aldehydes to construct 3,4-dihydroquinolin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Liu, R.-X.; Ma, D.-Y.; Luo, C.-P.; Yang, L. Four-Component Radical Dual Difunctionalization (RDD) of Two Different Alkenes with Aldehydes and tert-Butyl Hydroperoxide (TBHP): An Easy Access to β,δ-Functionalized Ketones. Org. Lett. 2019, 21, 6117–6121. [Google Scholar] [CrossRef]
- Paul, S.; Guin, J. Dioxygen-Mediated Decarbonylative C-H Alkylation of Heteroaromatic Bases with Aldehydes. Chem. Eur. J. 2015, 21, 17618–17622. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Paul, S.; Guin, J. Aerobic Radical-Cascade Alkylation/Cyclization of α,β-Unsaturated Amides: An Efficient Approach to Quaternary Oxindoles. Angew. Chem. Int. Ed. 2016, 55, 7756–7760. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shah, B.A. Synthesis of Biaryls via Benzylic C-C Bond Cleavage of Styrenes and Benzyl Alcohols. Org. Lett. 2015, 17, 5232–5235. [Google Scholar] [CrossRef]
- Zong, Z.; Wang, W.; Bai, X.; Xi, H.; Li, Z.-P. Manganese-Catalyzed Alkyl-Heck-Type Reaction via Oxidative Decarbonylation of Aldehydes. Asian J. Org. Chem. 2015, 4, 622–625. [Google Scholar]
- Lv, L.; Bai, X.; Yan, X.; Li, Z.-P. Iron-catalyzed decarbonylation initiated [2+2+m] annulation of benzene-linked 1,n-enynes with aliphatic aldehydes. Org. Chem. Front. 2016, 3, 1509–1513. [Google Scholar] [CrossRef]
- Ouyang, X.-H.; Song, R.-J.; Liu, B.; Li, J.-H. Metal-Free Oxidative Decarbonylative Hydroalkylation of Alkynes with Secondary and Tertiary Alkyl Aldehydes. Adv. Synth. Catal. 2016, 358, 1903–1909. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.-H.; Hu, M.; Liu, B.; Song, R.-J.; Li, J.-H. Intermolecular oxidative decarbonylative [2 + 2 + 2] carbocyclization of N-(2-ethynylaryl)acrylamides with tertiary and secondary alkyl aldehydes involving C(sp3)-H functionalization. Chem. Sci. 2016, 7, 7050–7054. [Google Scholar] [CrossRef]
- Zou, H.-X.; Li, Y.; Yang, H.-X.; Xiang, J.; Li, J.-H. Metal-Free Oxidative Decarbonylative [3 + 2] Annulation of Terminal Alkynes with Tertiary Alkyl Aldehydes toward Cyclopentenes. J. Org. Chem. 2018, 83, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.-H. Decarbonylative Formation of Homoallyl Radical Capable of Annulation witH-N-Arylpropiolamides via Aldehyde Auto-oxidation. Org. Lett. 2018, 20, 5323–5326. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Chen, Y.; Song, J.; Li, L.; Yu, J.-T. Metal-Free Cascade Oxidative Decarbonylative Alkylation/Arylation of Alkynoates with Alphatic Aldehydes. J. Org. Chem. 2016, 81, 12065–12069. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Huang, B.; Hu, W.; Feng, X.; Yu, J.-T. Metal-Free Radical Oxidative Annulation of Ynones with Alkanes To Access Indenones. J. Org. Chem. 2016, 81, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).