Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas
Abstract
1. Introduction
2. Results
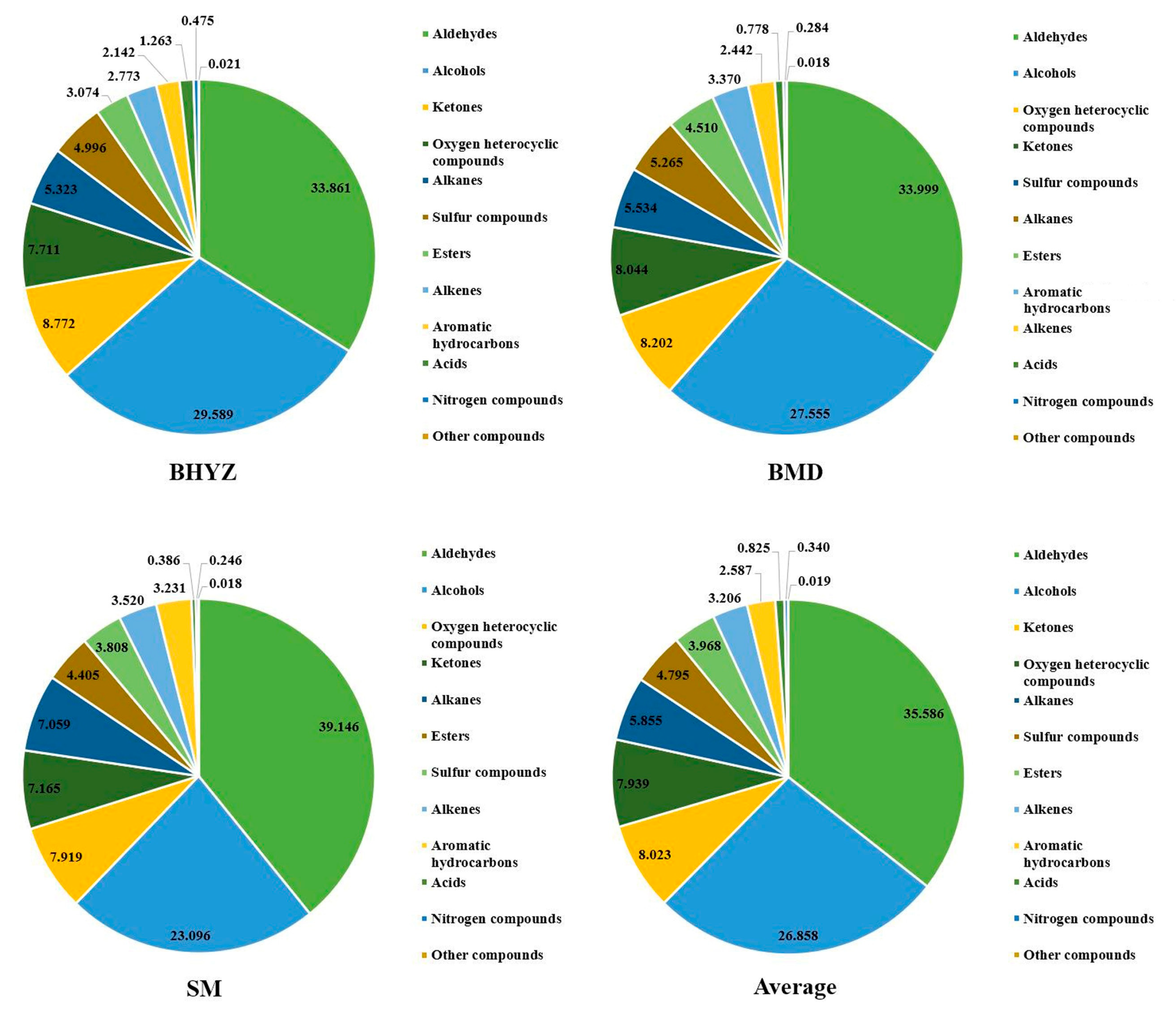
2.1. The Aroma Profile in Different Subtypes of White Teas
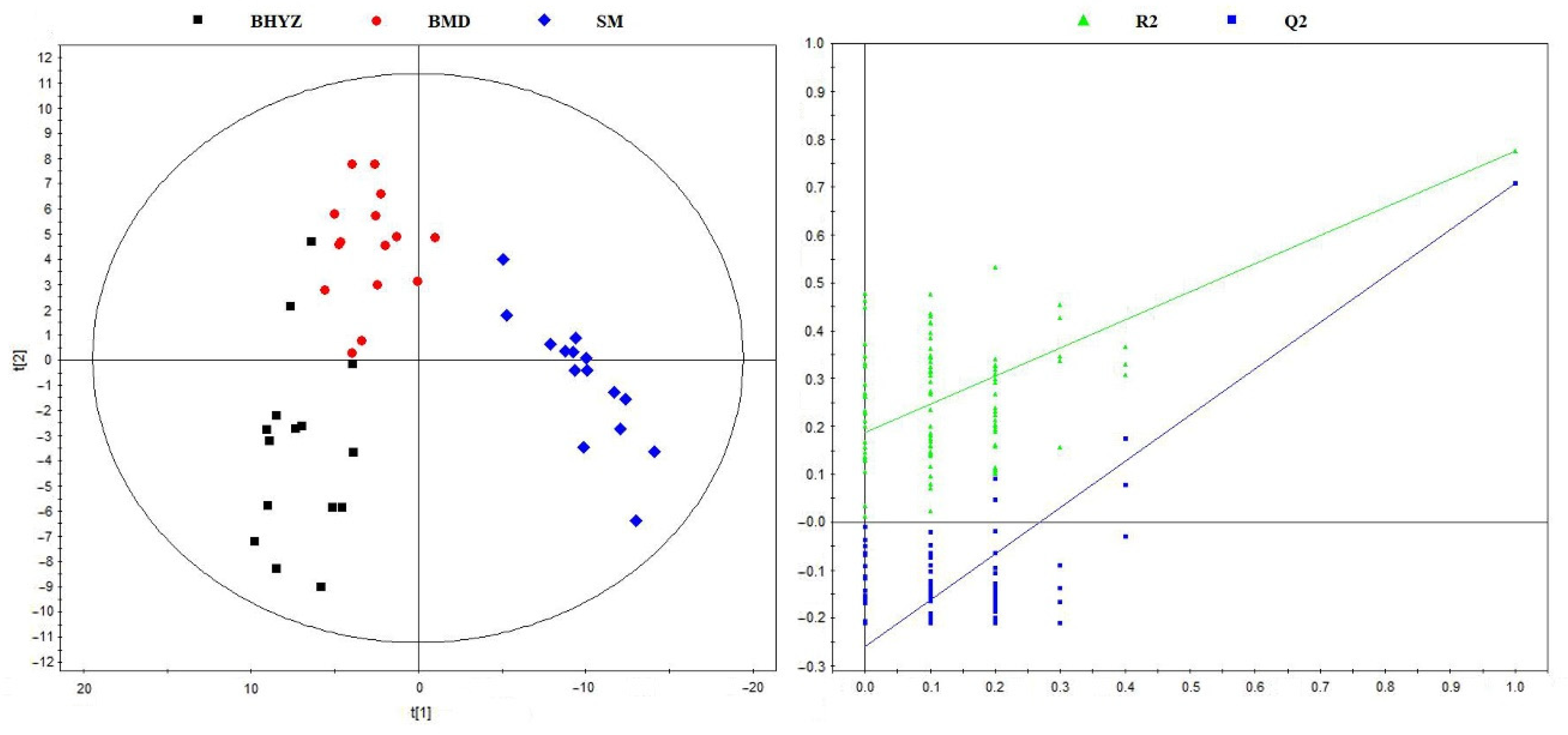
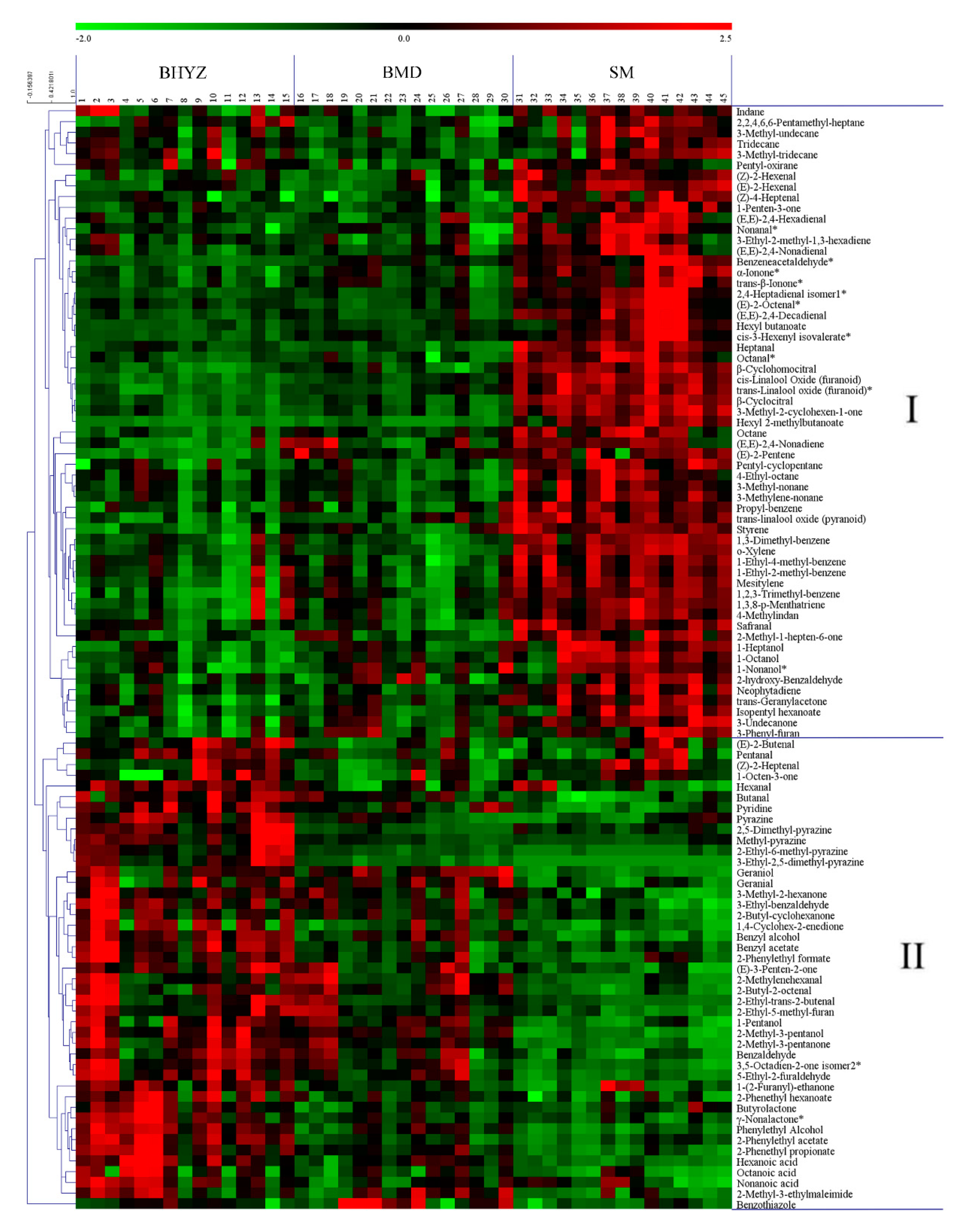
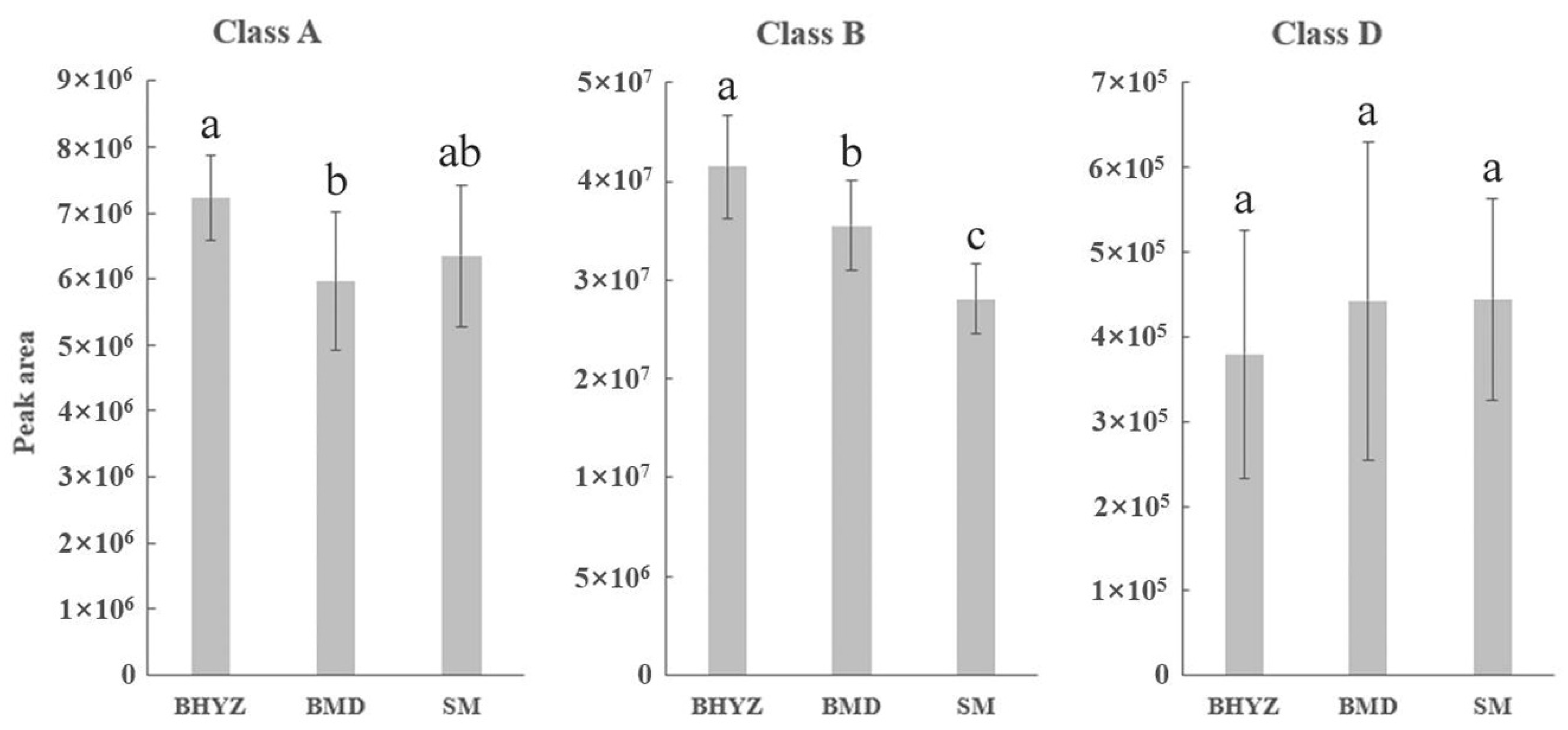
2.2. Screening of Differential Volatile Compounds among the Three Subtypes of White Teas
2.3. Identification of Aroma-Active Compounds in White Teas Using GC–O/MS
3. Discussion
3.1. The Comparison of Obtained and Reported Aroma Profiles of White Teas
3.2. The Comparison of Obtained and Reported Aroma-Active Compounds in Teas
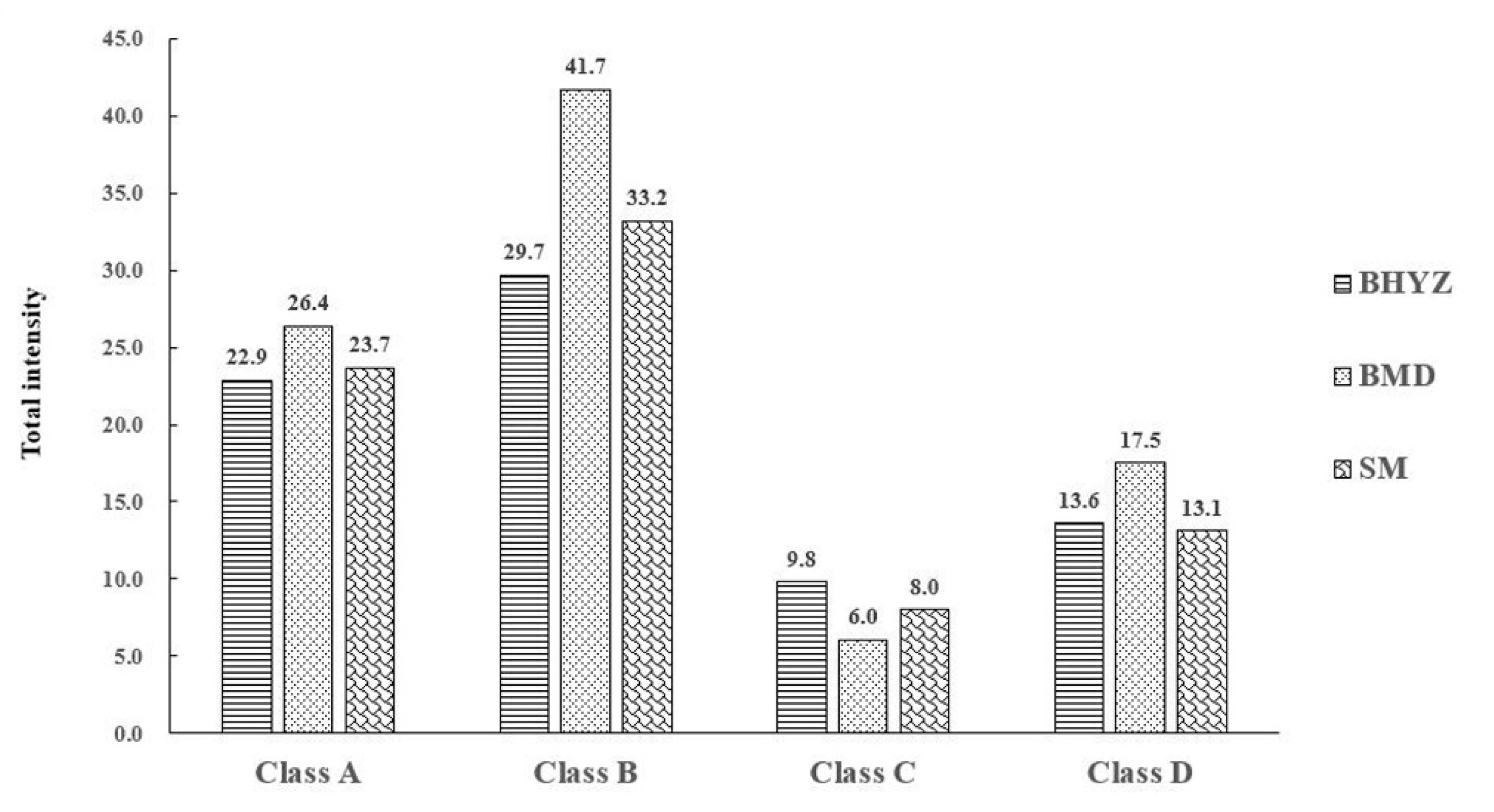
3.3. The Comparison of Total Aroma Intensities of Aroma-Active Compounds among Different Subtypes of White Teas
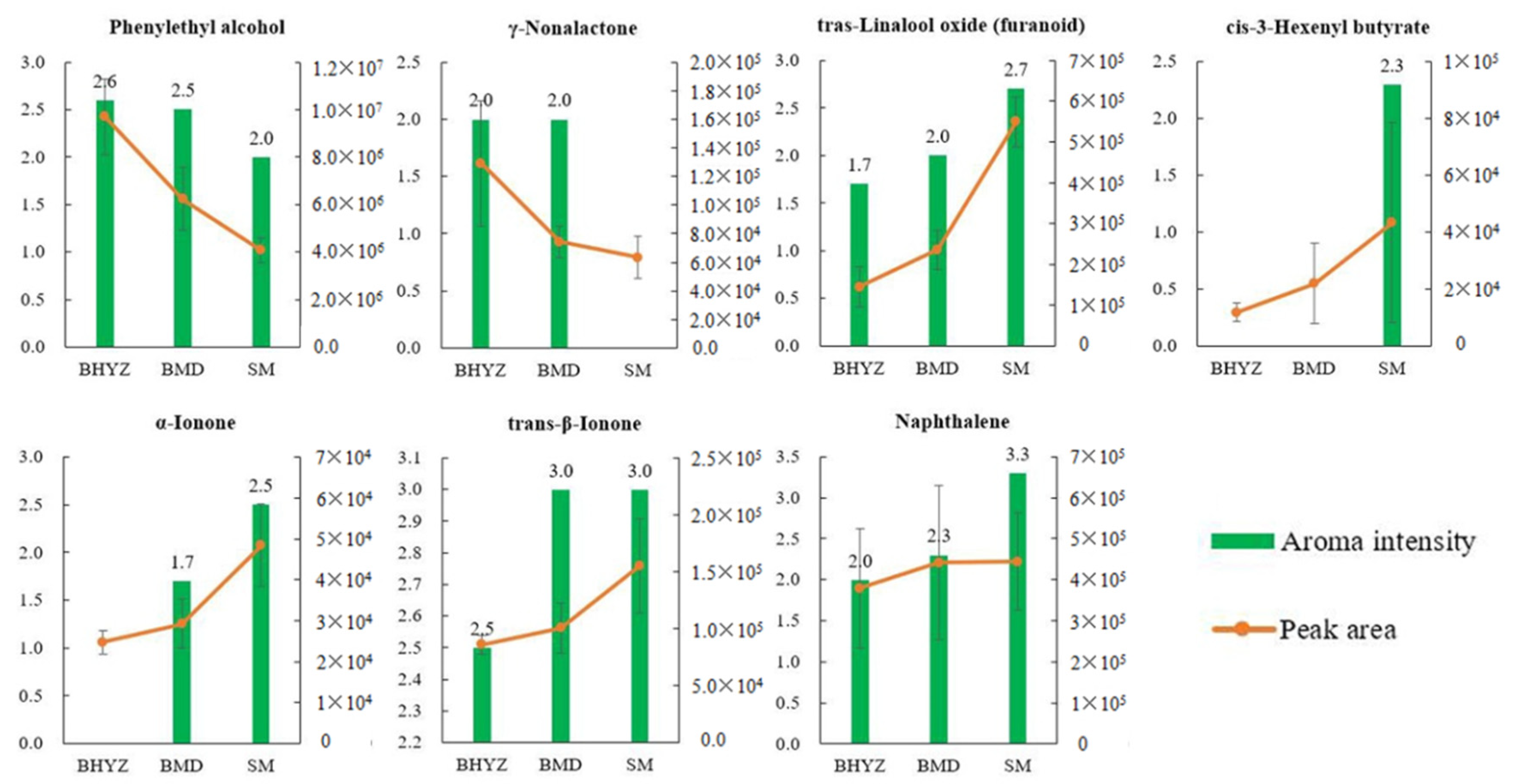
3.4. The Comparison of the Results Obtained from GC × GC–TOFMS and GC–O/MS
3.5. Determination of Key Odorants Accounting for Aroma Characteristics of the Three Subtypes of White Teas
4. Materials and Methods
4.1. Tea Samples
4.2. Chemicals and Reagents
4.3. Headspace Solid-Phase Microextraction (HS-SPME)
4.4. GC × GC–TOFMS Analysis
4.5. GC–O/MS Analysis
4.6. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanlier, N.; Atik, I.; Atik, A. A minireview of effects of white tea consumption on diseases. Trends Food Sci. Tech. 2018, 82, 82–88. [Google Scholar]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [PubMed]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [PubMed]
- Tan, J.; Engelhardt, U.H.; Lin, Z.; Kaiser, N.; Maiwald, B. Flavonoids, phenolic acids, alkaloids and theanine in different types of authentic Chinese white tea samples. J. Food Compos. Anal. 2017, 57, 8–15. [Google Scholar]
- Zhao, F.; Qiu, X.; Ye, N.; Qian, J.; Wang, D.; Zhou, P.; Chen, M. Hydrophilic interaction liquid chromatography coupled with quadrupole-orbitrap ultra high resolution mass spectrometry to quantitate nucleobases, nucleosides, and nucleotides during white tea withering process. Food Chem. 2018, 266, 343–349. [Google Scholar]
- Yue, W.; Sun, W.; Rao, R.S.P.; Ye, N.; Yang, Z.; Chen, M. Non-targeted metabolomics reveals distinct chemical compositions among different grades of Bai Mudan white tea. Food Chem. 2019, 277, 289–297. [Google Scholar]
- Wang, L.; Cai, L.S.; Lin, Z.; Zhong, Q.S.; Lv, H.P.; Tan, J.F.; Guo, L. Analysis of aroma compounds in white tea using headspace solid-phase micro-extraction and GC-MS. J. Tea Sci. 2010, 30, 115–123. (In Chinese) [Google Scholar]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Comparison of catechins and volatile compounds among different types of tea using high performance liquid chromatograph and gas chromatograph mass spectrometer. Int. J. Food Sci. Tech. 2011, 46, 1406–1412. [Google Scholar]
- Zhu, Y.; Shao, C.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; Lin, Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the effects of rapid aging technology on the aroma quality of white tea using GC-MS combined with chemometrics: In comparison with natural aged and fresh white tea. Food Chem. 2018, 265, 189–199. [Google Scholar]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Dai, W.D.; Guo, L.; Tan, J.F.; Zhang, Y.; Yu, F.L.; Shao, C.Y.; Peng, Q.H.; Lin, Z. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Sep. Purif. Technol. 2016, 164, 146–154. [Google Scholar] [CrossRef]
- Lv, H.; Zhong, Q.; Lin, Z.; Wang, L.; Tan, J.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Human Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Baba, R.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of Chinese green tea infusions by aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black yea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Li, C.; Liu, S.; Zhang, C.; Li, L.; Jiang, D. Characterization of aroma-active compounds of Pu-erh tea by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation-extraction (SDE) coupled with GC-olfactometry and GC-MS. Food Anal. Method 2016, 9, 1118–1198. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, C.; Wang, W.; Hu, H.; Chen, H.; Pang, S.; Miao, A. Effects of withering duration on the aroma profile of Yinghong No. 9 white tea. Food Sci. 2017, 38, 138–143. (In Chinese) [Google Scholar]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of matcha by gas chromatography-olfactometry techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef]
- Jumtee, K.; Komura, H.; Bamba, T.; Fukusaki, E. Predication of Japanese green tea (Sen-cha) ranking by volatile profiling using gas chromatography mass spectrometry and multivariate analysis. J. Biosci. Bioeng. 2011, 112, 252–255. [Google Scholar] [CrossRef]
- Tadmor, Y.; Fridman, E.; Gur, A.; Larkov, O.; Lastochkin, E.; Ravid, U.; Zamir, D.; Lewinsohn, E. Identification of malodorous, a wild species allele affecting tomato aroma that was selected against during domestication. J. Agric. Food Chem. 2002, 50, 2005–2009. [Google Scholar] [CrossRef]
| No. | Compounds | BHYZ | BMD | SM | Average |
|---|---|---|---|---|---|
| 1 | Benzaldehyde ** | 160.4 ± 19.4 | 156.8 ± 17.7 | 128.0 ± 7.8 | 148.4 ± 20.9 |
| 2 | Linalool | 67.3 ± 4.6 | 71.7 ± 2.8 | 77.5 ± 9.8 | 72.2 ± 7.4 |
| 3 | Phenylethyl alcohol ** | 101.2 ± 22.9 | 68.9 ± 16.2 | 45.9 ± 5.0 | 72.0 ± 28.0 |
| 4 | (E)-2-Hexenal ** | 54.8 ± 9.3 | 54.8 ± 9.1 | 86.3 ± 12.6 | 65.3 ± 18.2 |
| 5 | Dimethyl sulfide ** | 49.5 ± 9.7 | 54.8 ± 12.5 | 37.8 ± 11.3 | 47.4 ± 12.7 |
| 6 | 2-Pentyl-furan | 42.3 ± 6.7 | 46.4 ± 12.6 | 52.3 ± 6.3 | 47.0 ± 9.3 |
| 7 | Geraniol ** | 47.7 ± 10.1 | 56.1 ± 15.2 | 26.8 ± 3.6 | 43.5 ± 16.2 |
| 8 | Decane | 29.2 ± 7.1 | 30.1 ± 2.4 | 38.3 ± 6.8 | 32.5 ± 6.9 |
| 9 | Methyl salicylate | 23.0 ± 6.9 | 37.3 ± 14.8 | 34.3 ± 13.8 | 31.5 ± 13.1 |
| 10 | Hexanal ** | 31.5 ± 3.6 | 25.4 ± 4.7 | 27.6 ± 7.6 | 28.1 ± 5.8 |
| 11 | Benzyl alcohol ** | 32.4 ± 1.1 | 26.7 ± 4.8 | 18.1 ± 3.0 | 25.7 ± 6.8 |
| 12 | 2-Ethyl-furan ** | 25.1 ± 5.0 | 24.1 ± 10.6 | 16.9 ± 3.3 | 22.0 ± 7.5 |
| 13 | Benzene acetaldehyde ** | 8.9 ± 2.1 | 17.5 ± 4.5 | 33.3 ± 14.2 | 19.9 ± 13.2 |
| 14 | 2-Methyl-butanal | 14.1 ± 3.3 | 14.0 ± 3.7 | 20.4 ± 9.3 | 16.1 ± 6.4 |
| 15 | (Z)-3-Hexen-1-ol | 13.1 ± 3.0 | 13.5 ± 2.0 | 16.8 ± 5.2 | 14.5 ± 3.8 |
| 16 | 3,5-Octadien-2-one isomer 1 * | 15.3 ± 5.0 | 15.4 ± 8.2 | 10.7 ± 3.1 | 13.8 ± 5.8 |
| 17 | Nonanal ** | 16.8 ± 4.2 | 13.3 ± 4.7 | 6.8 ± 1.6 | 12.3 ± 5.5 |
| 18 | 3,5-Octadien-2-one isomer 2 ** | 9.2 ± 2.2 | 9.3 ± 4.3 | 19.4 ± 5.9 | 12.6 ± 6.4 |
| 19 | 6-Methyl-5-hepten-2-one * | 10.4 ± 1.1 | 8.5 ± 1.7 | 10.3 ± 1.4 | 9.7 ± 1.6 |
| 20 | 2-Heptanone ** | 11.0 ± 2.1 | 9.4 ± 1.9 | 7.8 ± 1.5 | 9.4 ± 2.2 |
| 21 | Butanal ** | 12.5 ± 1.8 | 9.8 ± 1.9 | 5.9 ± 2.7 | 9.4 ± 3.5 |
| 22 | 2,4-Heptadienal isomer 2 ** | 4.7 ± 0.8 | 6.1 ± 2.8 | 15.7 ± 9.2 | 8.8 ± 7.2 |
| 23 | β-Myrcene ** | 8.6 ± 1.7 | 9.4 ± 0.9 | 7.5 ± 1.1 | 8.5 ± 1.4 |
| 24 | Toluene ** | 6.4 ± 1.5 | 7.3 ± 1.6 | 9.5 ± 1.4 | 7.8 ± 1.9 |
| 25 | Dodecane | 6.9 ± 0.8 | 7.1 ± 1.5 | 8.6 ± 1.8 | 7.5 ± 1.5 |
| 26 | Hexanoic acid ** | 11.1 ± 6.0 | 6.8 ± 1.8 | 2.8 ± 0.5 | 6.9 ± 4.9 |
| 27 | 1-Octen-3-ol | 7.3 ± 0.7 | 6.1 ± 1.5 | 7.1 ± 1.5 | 6.8 ± 1.3 |
| 28 | 1-Penten-3-ol ** | 6.6 ± 1.4 | 6.1 ± 0.9 | 4.1 ± 0.7 | 5.6 ± 1.5 |
| 29 | 5-Ethyl-6-methyl-3-hepten-2-one | 5.7 ± 1.4 | 6.0 ± 0.4 | 5.0 ± 1.2 | 5.6 ± 1.1 |
| 30 | Limonene | 3.8 ± 0.9 | 5.4 ± 2.2 | 5.0 ± 0.6 | 4.8 ± 1.5 |
| No. | Compounds | Odor Characteristic | Aroma Intensity [1] | Identification Method [2] | Class [3] | ||
|---|---|---|---|---|---|---|---|
| BHYZ | BMD | SM | |||||
| 1 | Hexanal | Green, grassy, fresh | 2.0 | 2.8 | 2.6 | MS, RI | A |
| 2 | 1-Octen-3-ol | Mushroom-like, fresh | 2.0 | 2.5 | 3.0 | MS, RI, STD | A |
| 3 | β-Cymene | Floral, fruity, rice-like | 1.3 | MS, RI | B | ||
| 4 | Benzyl alcohol | Floral, rose-like, pollen-like | 2.0 | 3.0 | 2.3 | MS, RI | B |
| 5 | Benzene acetaldehyde | Green, sweet, green lemon-like | 3.0 | 3.2 | 2.8 | MS, RI, STD | B |
| 6 | (E)-2-Octenal | Stinky, green, sauce-like | 2.0 | 1.7 | MS, RI, STD | A | |
| 7 | trans-Linalool oxide (furanoid) | Floral, sweet, roasted | 1.7 | 2.0 | 2.7 | MS, RI, STD | B |
| 8 | 3,5-Octadien-2-one isomer 2 | Green, fresh, sweet | 2.5 | 3.2 | 2.8 | MS, RI, STD | A |
| 9 | Linalool | Floral, fruity, sweet | 2.8 | 3.4 | 3.2 | MS, RI, STD | B |
| 10 | Nonanal | Floral, fruity, green, fresh | 3.0 | 3.6 | 3.5 | MS, RI, STD | B |
| 11 | Phenylethyl alcohol | Floral, sweet, rose-like | 2.6 | 2.5 | 2.0 | MS, RI | B |
| 12 | 5-Ethyl-6-methyl-3-hepten-2-one | Stinky, grassy, fresh | 2.5 | 3.0 | 2.8 | MS, RI | A |
| 13 | (E)-3-Nonen-1-ol | Green, grassy, stinky | 3.0 | 3.2 | 3.6 | MS, RI, STD | A |
| 14 | (E)-2-Nonenal | Stinky, fresh, cucumber-like | 3.0 | 2.5 | MS, RI, STD | A | |
| 15 | 1-Nonanol | Green, fresh, grassy | 2.0 | 2.0 | MS, RI, STD | A | |
| 16 | Naphthalene | Non-pleasant, irritating | 2.0 | 2.3 | 3.3 | MS, RI, STD | D |
| 17 | cis-3-Hexenyl butyrate | Fresh, cucumber-like | 2.3 | MS, RI, STD | A | ||
| 18 | Terpineol | Floral, sweet, green, fresh | 2.0 | 2.7 | 2.0 | MS, RI | B |
| 19 | Neral | Floral, fruity, hay-like | 1.7 | MS, RI | B | ||
| 20 | cis-3-Hexenyl isovalerate | Green, fresh, sweet, floral | 2.2 | 3.0 | 2.6 | MS, RI, STD | A |
| 21 | Geraniol | Floral, rose-like, fresh | 2.2 | 2.6 | 3.2 | MS, RI | B |
| 22 | γ-Nonalactone | Sweet, cream-like, floral | 2.0 | 2.0 | MS, RI, STD | B | |
| 23 | α-Ionone | Pleasant, cream-like, rose-like | 1.7 | 2.5 | MS, RI, STD | B | |
| 24 | trans-β-Ionone | Sweet, cream-like, floral, fruity | 3.0 | 3.0 | 3.0 | MS, RI, STD | B |
| 25 | cis-Calamenene | Fresh, minty, toothpaste-like | 1.7 | 2.2 | MS, RI | A | |
| 26 | Unknown 1 | Green, fresh, grassy | 2.3 | A | |||
| 27 | Unknown 2 | Floral, coffee-like, sweet | 1.8 | 3.0 | B | ||
| 28 | Unknown 3 | Roasted, milky, sweet | 1.8 | 2.0 | C | ||
| 29 | Unknown 4 | Sweet, roasted, floral, burnt | 2.7 | 3.3 | 3.0 | C | |
| 30 | Unknown 5 | Roasted, sweet, cream-like | 2.8 | 2.7 | 3.0 | C | |
| 31 | Unknown 6 | Capsule-like, powder-like | 1.3 | D | |||
| 32 | Unknown 7 | Non-pleasant, irritating | 2.3 | 2.3 | 1.8 | D | |
| 33 | Unknown 8 | Non-pleasant, irritating | 2.6 | 3.0 | 3.3 | D | |
| 34 | Unknown 9 | Stinky | 2.0 | 2.7 | D | ||
| 35 | Unknown 10 | Roasted, coffee-like, burnt | 2.5 | C | |||
| 36 | Unknown 11 | Non-pleasant, plastic-like | 3.0 | 2.0 | D | ||
| 37 | Unknown 12 | Green, fresh, cucumber-like | 2.0 | A | |||
| 38 | Unknown 13 | Non-pleasant, spicy | 2.7 | D | |||
| 39 | Unknown 14 | Non-pleasant, powder-like | 1.7 | 2.5 | D | ||
| 40 | Unknown 15 | Floral, fruity, sweet | 2.0 | B | |||
| 41 | Unknown 16 | Sweet, fruity, minty, citrus | 2.0 | B | |||
| 42 | Unknown 17 | Non-pleasant, irritating | 1.7 | 1.7 | D | ||
| 43 | Unknown 18 | Sweet, caramel-like, floral | 2.3 | 3.3 | 4.0 | B | |
| 44 | Unknown 19 | Sweet, honey, caramel-like | 2.0 | B | |||
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.-C.; Wang, M.-Q.; Ni, D.-J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. https://doi.org/10.3390/molecules25246050
Chen Q-C, Zhu Y, Yan H, Chen M, Xie D-C, Wang M-Q, Ni D-J, Lin Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules. 2020; 25(24):6050. https://doi.org/10.3390/molecules25246050
Chicago/Turabian StyleChen, Qin-Cao, Yin Zhu, Han Yan, Mei Chen, Dong-Chao Xie, Meng-Qi Wang, De-Jiang Ni, and Zhi Lin. 2020. "Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas" Molecules 25, no. 24: 6050. https://doi.org/10.3390/molecules25246050
APA StyleChen, Q.-C., Zhu, Y., Yan, H., Chen, M., Xie, D.-C., Wang, M.-Q., Ni, D.-J., & Lin, Z. (2020). Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules, 25(24), 6050. https://doi.org/10.3390/molecules25246050