Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation
Abstract
1. Introduction
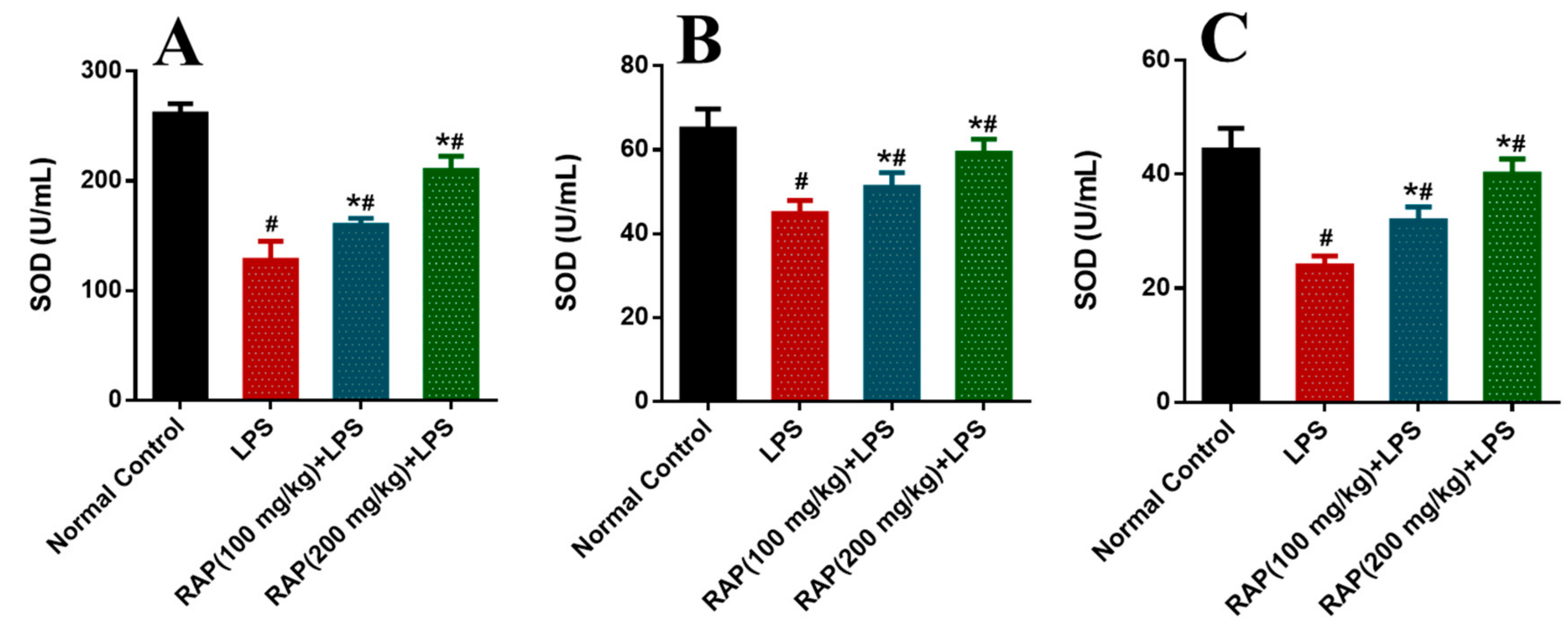
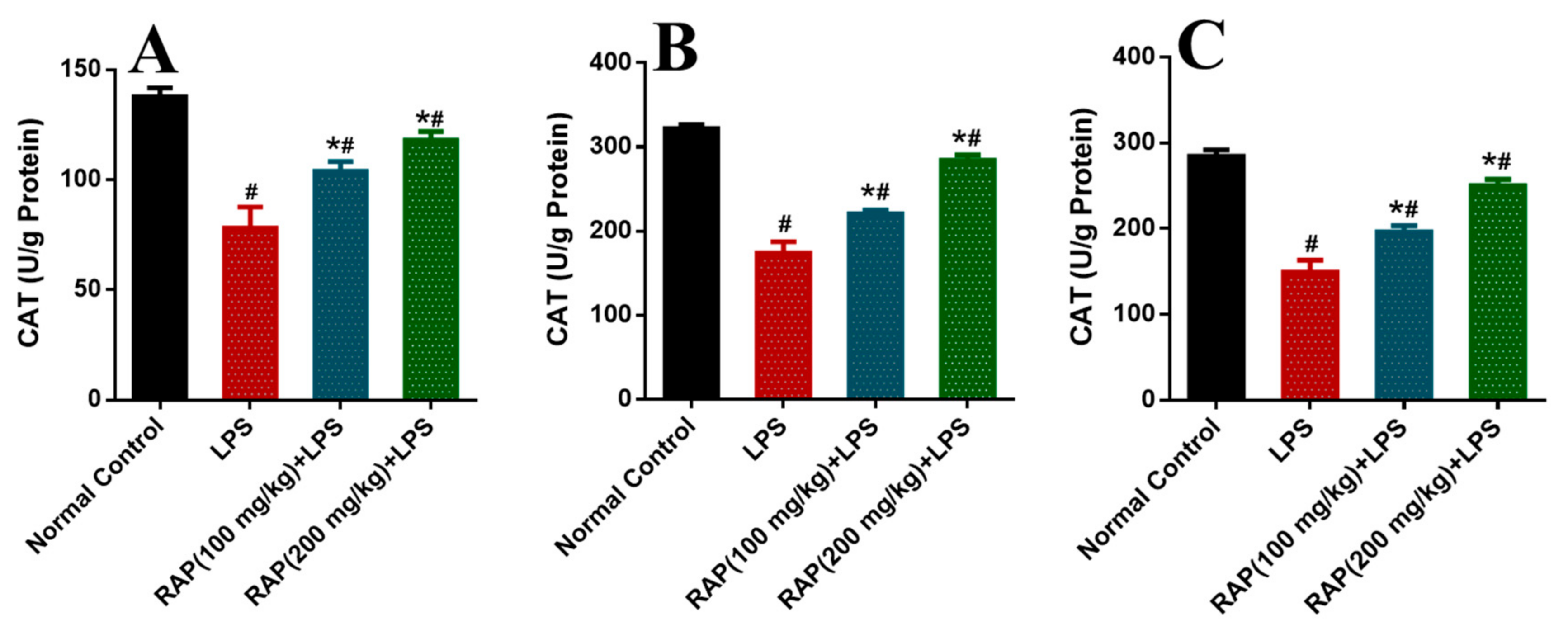
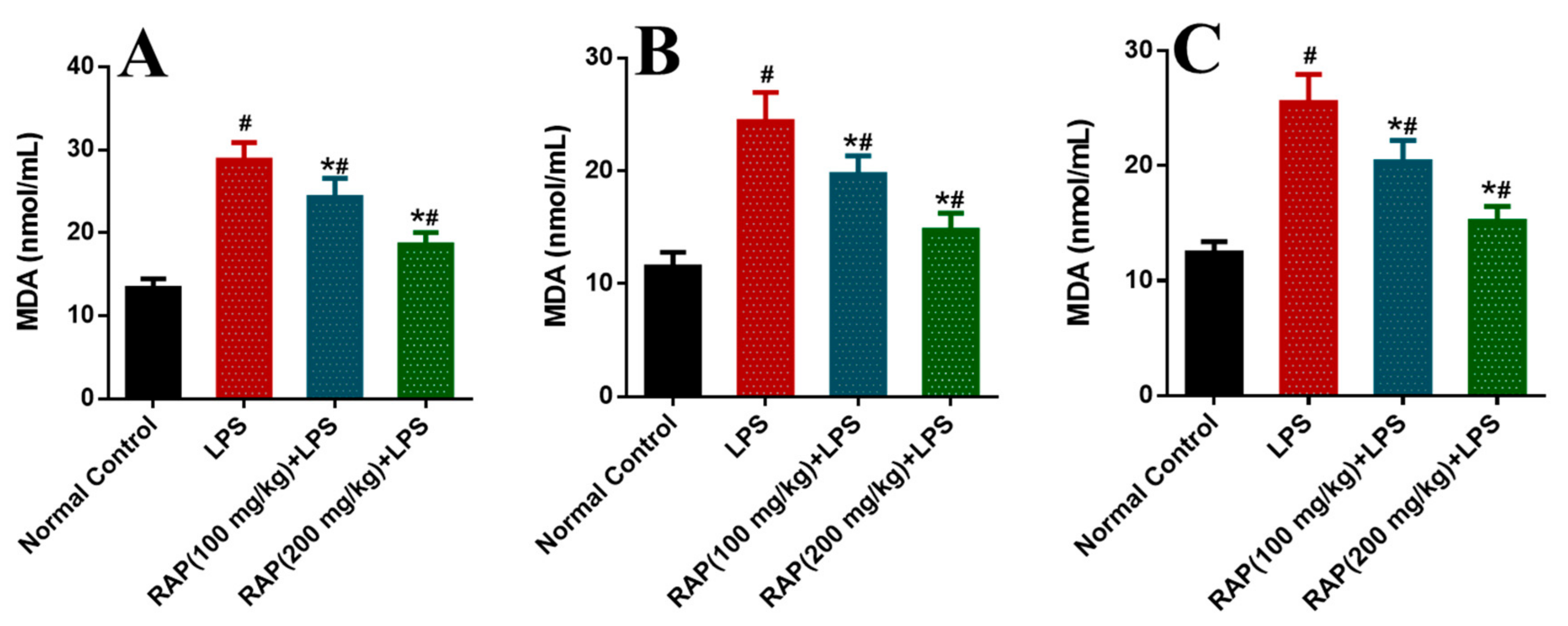
2. Results
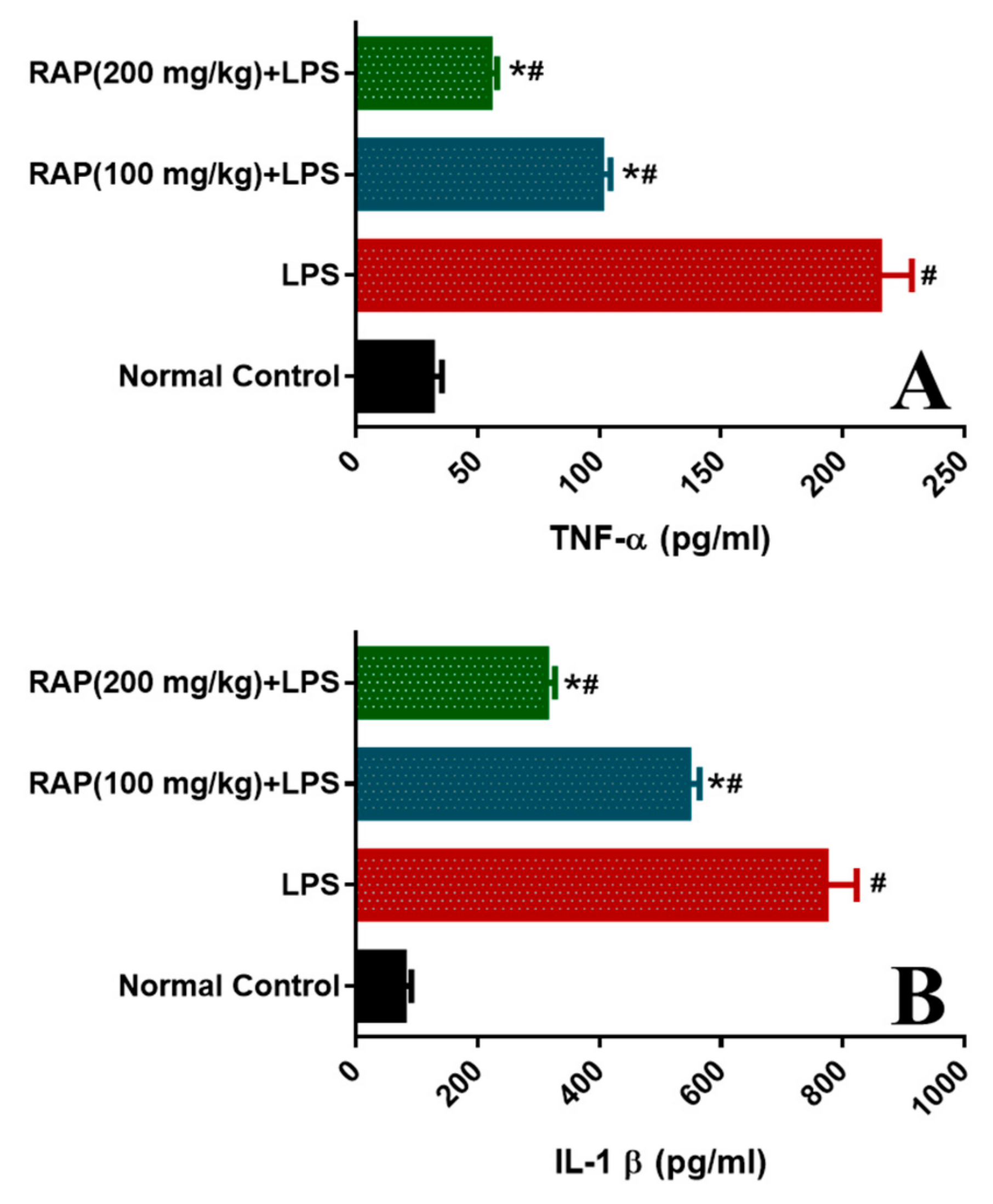
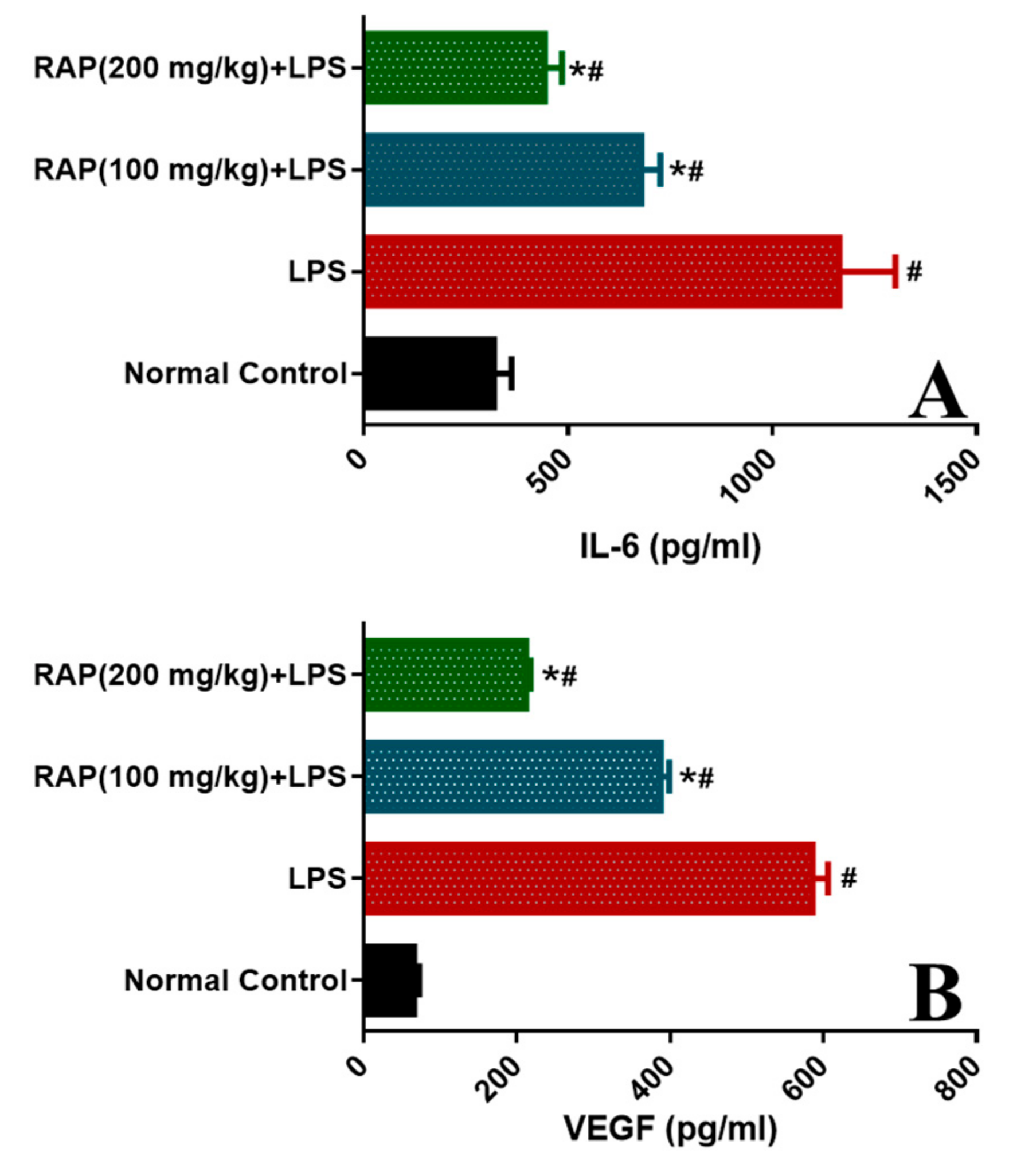
2.1. Effect of RAP on Levels of TNF-α, IL-1β, IL-6, and VEGF
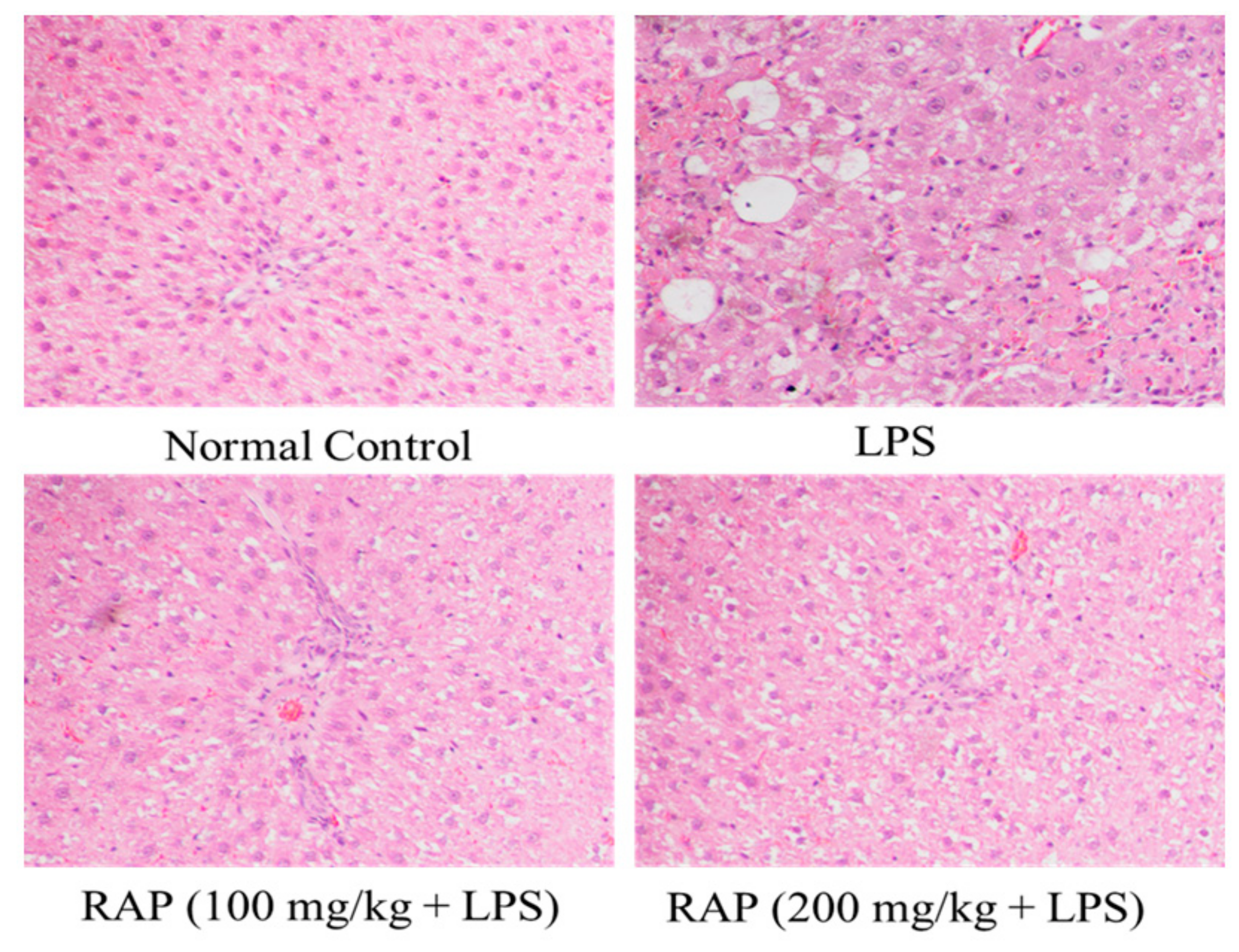
2.2. Histopathology Results
3. Discussion
4. Materials and Methods
4.1. Extraction of Polysaccharides
4.2. Drugs and Chemicals
4.3. Animals and Study Protocol
4.4. Statistical Analysis
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Stearns-Kurosawa, D.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The Pathogenesis of Sepsis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 19–48. [Google Scholar] [CrossRef]
- Marx, G.; Vangerow, B.; Burczyk, C.; Gratz, K.; Maassen, N.; Meyer, M.C.; Leuwer, M.; Kuse, E.; Rueckoldt, H. Evaluation of noninvasive determinants for capillary leakage syndrome in septic shock patients. Intensiv. Care Med. 2000, 26, 1252–1258. [Google Scholar] [CrossRef]
- Van Der Flier, M.; Van Leeuwen, H.J.; Van Kessel, K.P.; Kimpen, J.L.; Hoepelman, A.I.; Geelen, S.P. Plasma vascular endothelial growth factor in severe sepsis. Shock 2005, 23, 35–38. [Google Scholar] [CrossRef]
- Sato, R.; Nasu, M. A review of sepsis-induced cardiomyopathy. J. Intensiv. Care 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Lowe, D.B.; Storkus, W.J. Chronic inflammation and immunologic-based constraints in malignant disease. Immunotherapy 2011, 3, 1265–1274. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in Sepsis: Potent Immunoregulators and Potential Therapeutic Targets—An Updated View. Mediat. Inflamm. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Senger, D.R.; Van De Water, L.; Brown, L.F.; Nagy, J.A.; Yeo, K.-T.; Yeo, T.-K.; Berse, B.; Jackman, R.W.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993, 12, 303–324. [Google Scholar] [CrossRef]
- Roberts, W.G.; Palade, G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995, 108, 2369–2379. [Google Scholar]
- Ahmad, A.; Rehman, M.U.; Wali, A.F.; El-Serehy, H.A.; Al-Misned, F.A.; Maodaa, S.N.; Aljawdah, H.M.; Mir, T.M.; Ahmad, P. Box–Behnken Response Surface Design of Polysaccharide Extraction from Rhododendron arboreum and the Evaluation of Its Antioxidant Potential. Molecules 2020, 25, 3835. [Google Scholar] [CrossRef]
- Chauhan, N.S. Medicinal & Aromatic Plants of Himachal Pradesh; Indus Publishing Company: New Delhi, India, 1999. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; International Book Publisher: Dehradun, Inida, 2005. [Google Scholar]
- Bhandary, M.R.; Kawabata, J. Antidiabetic activity of Laligurans (Rhododendron arboreum Sm.) flower. J. Food Sci. Tech. Nepal 2008, 4, 61–63. [Google Scholar]
- Verma, N.; Singh, A.P.; Amresh, G.; Sahu, P.K.; Rao, C. Anti-inflammatory and anti-nociceptive activity of Rhododendron arboretum. J. Pharm. Res. 2010, 3, 1376–1380. [Google Scholar]
- Prakash, D.; Upadhyay, G.; Singh, B.N.; Dhakarey, R.; Kumar, S.; Singh, K.K. Free-radical scavenging activities of Himalayan rhododendrons. Curr. Sci. 2007, 92, 526–532. [Google Scholar]
- Nisar, M.; Ali, S.; Qaisar, M. Preliminary Phytochemical Screening of Flowers, Leaves, Bark, Stem and Roots of Rhododendron arboreum. Middle East J. Sci. Res. 2011, 10, 472–476. [Google Scholar]
- Damnjanovic, Z.; Jovanovic, M.; Nagorni, A.; Radojkovic, M.; Sokolović, D.; Djindjic, B.; Smiljkovic, I.; Kamenov, A.; Damnjanovic, I. Correlation of inflammation parameters and biochemical markers of cholestasis with the intensity of lipid peroxidation in patients with choledocholithiasis. Vojn. Pregl. 2013, 70, 170–176. [Google Scholar] [CrossRef]
- Seehofer, D.; Schirmeier, A.; Bengmark, S.; Cho, S.-Y.R.; Koch, M.; Lederer, A.; Rayes, N.; Menger, M.D.; Neuhaus, P.; Nüssler, A.K. Curcumin Attenuates Oxidative Stress and Inflammatory Response in the Early Phase after Partial Hepatectomy with Simultaneous Intraabdominal Infection in Rats. J. Surg. Res. 2010, 159, 497–502. [Google Scholar] [CrossRef]
- Chien, W.-S.; Chen, Y.-H.; Chiang, P.-C.; Hsiao, H.-W.; Chuang, S.-M.; Lue, S.-I.; Hsu, C. Suppression of Autophagy in Rat Liver at Late Stage of Polymicrobial Sepsis. Shock 2011, 35, 506–511. [Google Scholar] [CrossRef]
- Wali, A.F.; Rehman, M.U.; Raish, M.; Kazi, M.; Rao, P.G.M.; AlNemer, O.; Ahmad, P.; Ahmad, A. Zingerone [4-(3-Methoxy-4-hydroxyphenyl)-butan-2] Attenuates Lipopolysaccharide-Induced Inflammation and Protects Rats from Sepsis Associated Multi Organ Damage. Molecules 2020, 25, 5127. [Google Scholar] [CrossRef]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A Bench to Bedside View of Uremic Toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef]
- Radovanovic, T.; Borković-Mitić, S.; Perendija, B.; Despotović, S.G.; Pavlovic, S.Z.; Cakic, P.; Saičić, Z.S. Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river, Serbia. Arch. Biol. Sci. 2010, 62, 97–105. [Google Scholar] [CrossRef]
- Cohen, M.; Lippman, M.; Chabner, B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet 1978, 2, 814–816. [Google Scholar] [CrossRef]
- El-Latif, A.A.E.-A.A.; Sayed, A.A.; Soliman, A.M.; Fahmy, S.R. Exploration of the therapeutic potential effect of Sepia officinalis in animal model of sepsis induced by cecal ligation and puncture. Injury 2016, 47, 2709–2717. [Google Scholar] [CrossRef]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef]
- Cannon, J.G.; Tompkins, R.G.; Gelfand, J.A.; Michie, H.R.; Stanford, G.G.; Van Der Meer, J.W.M.; Endres, S.; Lonnemann, G.; Corsetti, J.; Chernow, B.; et al. Circulating Interleukin-1 and Tumor Necrosis Factor in Septic Shock and Experimental Endotoxin Fever. J. Infect. Dis. 1990, 161, 79–84. [Google Scholar] [CrossRef]
- Michie, H.R.; Manogue, K.R.; Spriggs, D.R.; Revhaug, A.; O’Dwyer, S.; Dinarello, C.A.; Cerami, A.; Wolff, S.M.; Wilmore, D.W. Detection of Circulating Tumor Necrosis Factor after Endotoxin Administration. N. Engl. J. Med. 1988, 318, 1481–1486. [Google Scholar] [CrossRef]
- Chuang, T.-Y.; Cheng, A.-J.; Chen, I.-T.; Lan, T.-Y.; Huang, I.-H.; Shiau, C.-W.; Hsu, C.-L.; Liu, Y.-W.; Chang, Z.-F.; Tseng, P.-H.; et al. Suppression of LPS-induced inflammatory responses by the hydroxyl groups of dexamethasone. Oncotarget 2017, 8, 49735–49748. [Google Scholar] [CrossRef]
- Kern, J.A.; Lamb, R.J.; Reed, J.C.; Daniele, R.P.; Nowell, P.C. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J. Clin. Investig. 1988, 81, 237–244. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H. The Role of Cyclooxygenases in Endotoxin- and Interleukin-1-Induced Hypophagia. Brain Behav. Immun. 2000, 14, 141–152. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. Vivo 2013, 27, 669–684. [Google Scholar]
- Mera, S.; Tatulescu, D.; Cismaru, C.; Bondor, C.; Slavcovici, A.; Zanc, V.; Carstina, D.; Oltean, M. Multiplex cytokine profiling in patients with sepsis. APMIS 2011, 119, 155–163. [Google Scholar] [CrossRef]
- Tang, H.; Bai, Y.; Shen, W.; Wei, Y.; Xu, M.; Zhou, X.; Zhao, J. Clinical significance of combined detection of interleukin-6 and tumour markers in lung cancer. Autoimmunity 2018, 51, 191–198. [Google Scholar] [CrossRef]
- Patterson, C.C.; Smith, A.E.; Yarnell, J.W.G.; Rumley, A.; Ben-Shlomo, Y.; Lowe, G.D.O. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: The Caerphilly Study. Atherosclerosis 2010, 209, 551–557. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Nolan, A.; Weiden, M.D.; Thurston, G.; Gold, J. Vascular Endothelial Growth Factor Blockade Reduces Plasma Cytokines in a Murine Model of Polymicrobial Sepsis. Inflammation 2004, 28, 271–278. [Google Scholar] [CrossRef][Green Version]
- Kim, I.; Moon, S.-O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular Endothelial Growth Factor Expression of Intercellular Adhesion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and E-selectin through Nuclear Factor-κB Activation in Endothelial Cells. J. Biol. Chem. 2001, 276, 7614–7620. [Google Scholar] [CrossRef]
- Reinders, M.E.J.; Sho, M.; Izawa, A.; Wang, P.; Mukhopadhyay, D.; Koss, K.E.; Geehan, C.S.; Luster, A.D.; Sayegh, M.H.; Briscoe, D.M. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Investig. 2003, 112, 1655–1665. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Cool, C.; Taraceviene-Stewart, L.; Geraci, M.W.; Yeager, M.; Bull, T.; Kasper, M.; Tuder, R.M. Janus face of vascular endothelial growth factor: The obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit. Care Med. 2002, 30, S251–S256. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Pickkers, P.; Sprong, T.; Van Eijk, L.; Van Der Hoeven, H.; Smits, P.; Van Deuren, M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 2005, 24, 508–512. [Google Scholar] [CrossRef]
- Thickett, D.R.; Armstrong, L.; Christie, S.J.; Millar, A.B. Vascular Endothelial Growth Factor May Contribute to Increased Vascular Permeability in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 1601–1605. [Google Scholar] [CrossRef]
- Thickett, D.; Armstrong, L.; Millar, A.B. A Role for Vascular Endothelial Growth Factor in Acute and Resolving Lung Injury. Am. J. Respir. Crit. Care Med. 2002, 166, 1332–1337. [Google Scholar] [CrossRef]

| Parameters | Normal Control | LPS 10 mg/kg | RAP 100 mg/kg | RAP200 mg/kg |
|---|---|---|---|---|
| AST (U/L) | 52.19 ± 2.99 | 237.88 ± 9.86 * | 157.66 ± 6.31 *,# | 71.83 ± 4.19 *,# |
| ALT (U/L) | 39.41 ± 2.39 | 215.25 ± 6.91 * | 106.37 ± 4.59 *,# | 72.36 ± 3.69 *,# |
| LDH (U/L) | 305.32 ± 9.56 | 1026.33 ± 31.65 * | 786.25 ± 22.15 *,# | 562.69 ± 19.85 *,# |
| BIL (µmol/L) | 1.55 ± 0.42 | 7.86 ± 1.11 * | 5.44 ± 0.95 *,# | 2.86 ± 0.81 *,# |
| CK (U/L) | 72.88 ± 4.96 | 989.52 ± 20.96 * | 731.43 ± 17.96 *,# | 621.22 ± 21.39 *,# |
| Cr (mg/dL) | 0.37 ± 0.05 | 0.66 ± 0.03 * | 0.50 ± 0.04 *,# | 0.42 ± 0.03 *,# |
| BUN (mg/dL) | 52.23 ± 1.84 | 155.46 ± 6.44 * | 95.43 ± 4.83 *,# | 71.92 ± 3.61 *,# |
| Albumin (g/dL) | 3.92 ± 0.36 | 2.49 ± 0.31 * | 2.86 ± 0.39 *,# | 3.45 ± 0.28 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Wali, A.F.; Rehman, M.U.; Khan, A.; Raish, M.; Kazi, M.; Alnemer, O.; G. M. Rao, P. Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation. Molecules 2020, 25, 6045. https://doi.org/10.3390/molecules25246045
Ahmad A, Wali AF, Rehman MU, Khan A, Raish M, Kazi M, Alnemer O, G. M. Rao P. Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation. Molecules. 2020; 25(24):6045. https://doi.org/10.3390/molecules25246045
Chicago/Turabian StyleAhmad, Ajaz, Adil Farooq Wali, Muneeb U. Rehman, Andleeb Khan, Mohammad Raish, Mohsin Kazi, Osamah Alnemer, and Padma G. M. Rao. 2020. "Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation" Molecules 25, no. 24: 6045. https://doi.org/10.3390/molecules25246045
APA StyleAhmad, A., Wali, A. F., Rehman, M. U., Khan, A., Raish, M., Kazi, M., Alnemer, O., & G. M. Rao, P. (2020). Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation. Molecules, 25(24), 6045. https://doi.org/10.3390/molecules25246045








