Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol
Abstract
1. Introduction
2. Results
2.1. Synthesis of Florfenicol-COS Conjugates (F-COS)
2.2. Characterization of Florfenicol-COS Conjugates (F-COS)
2.2.1. Flo-COOH
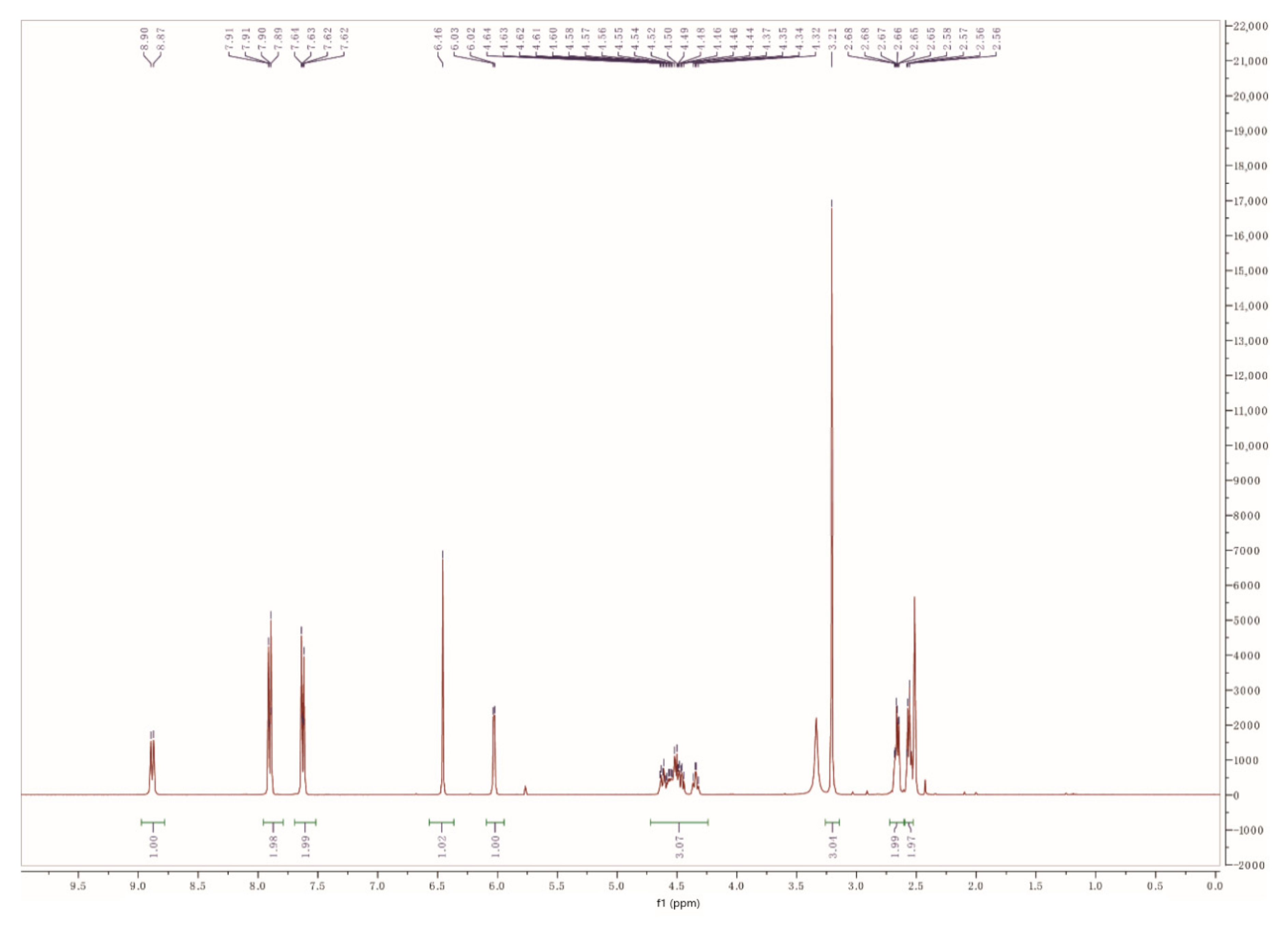
2.2.2. F-COS
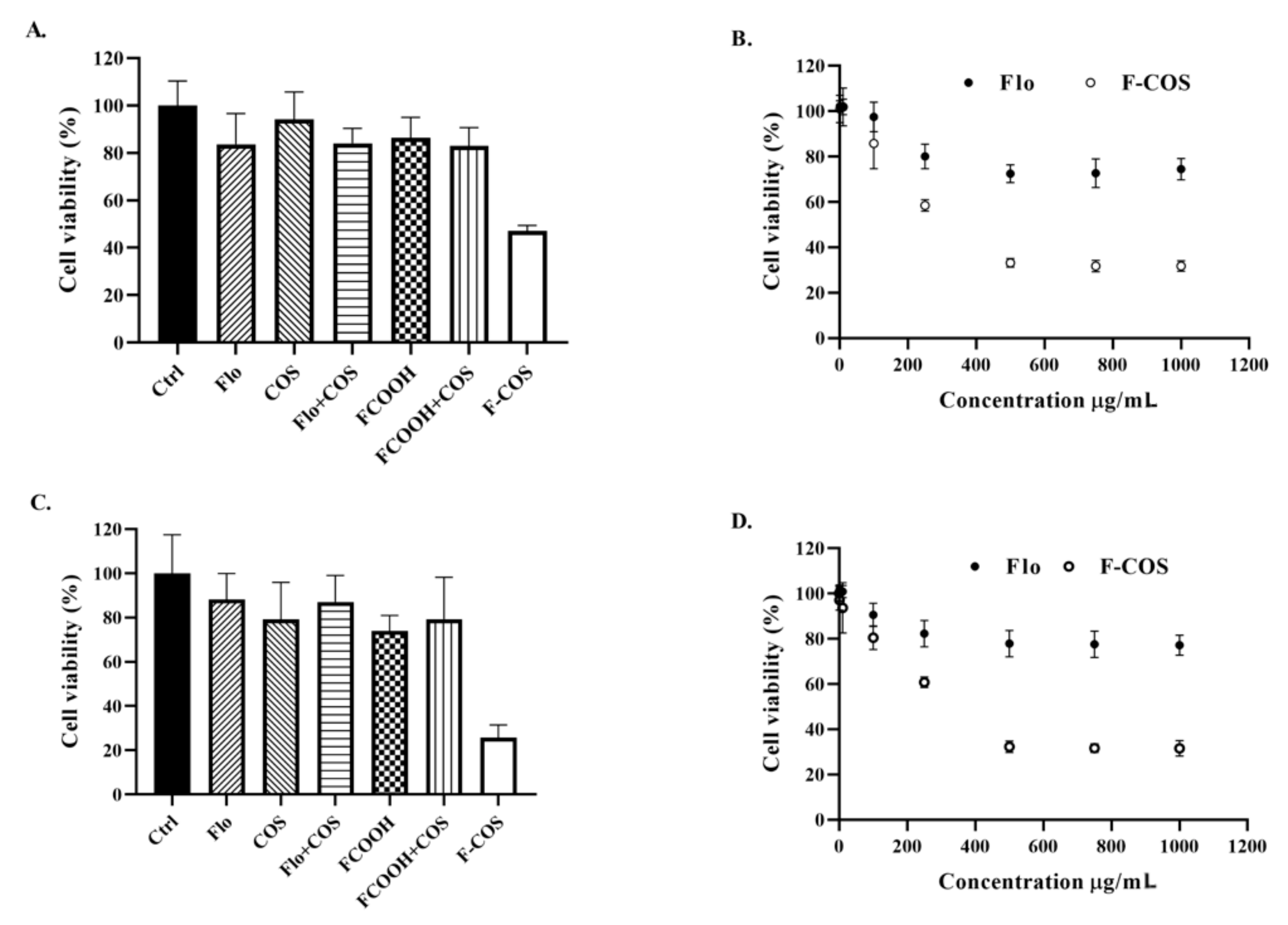
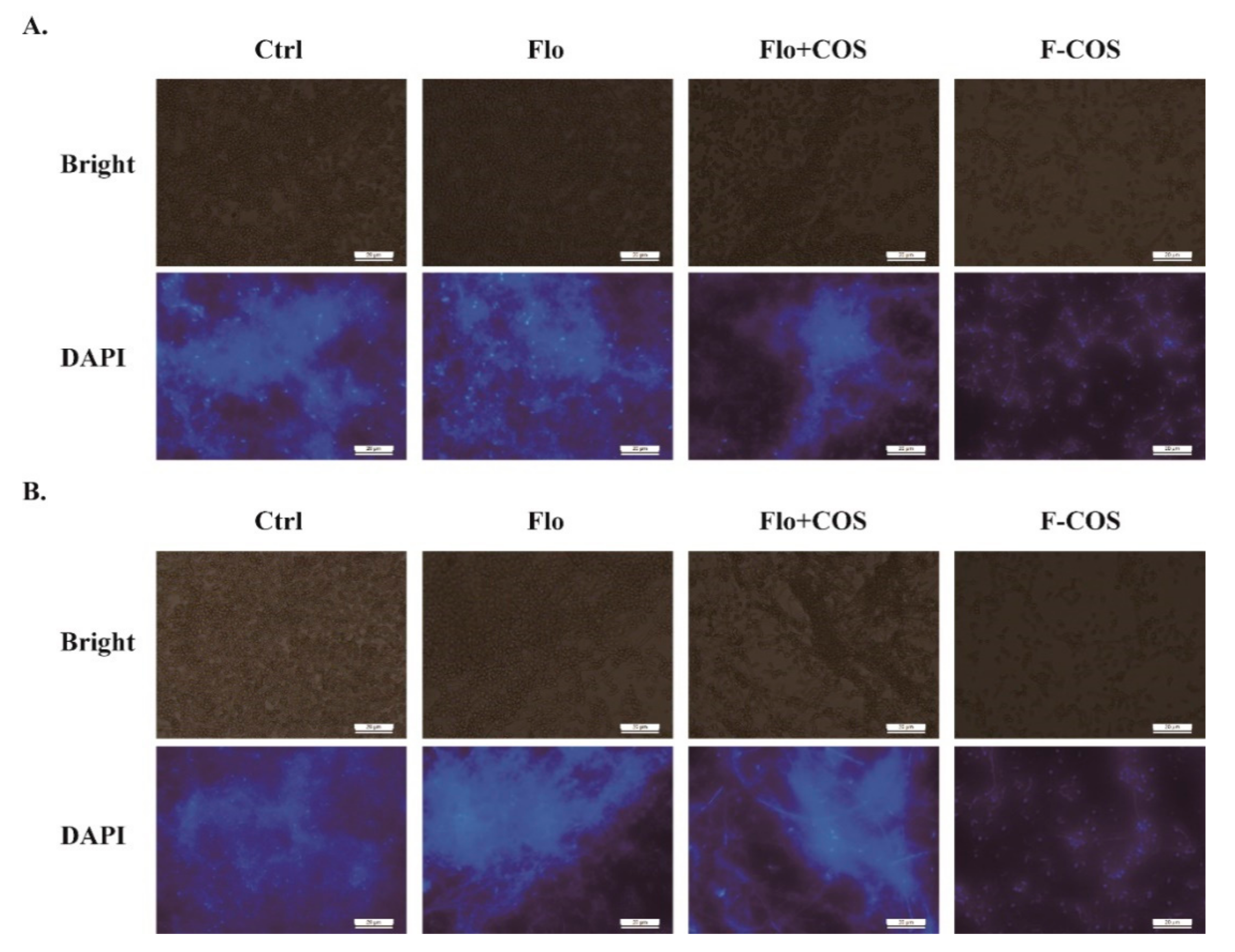
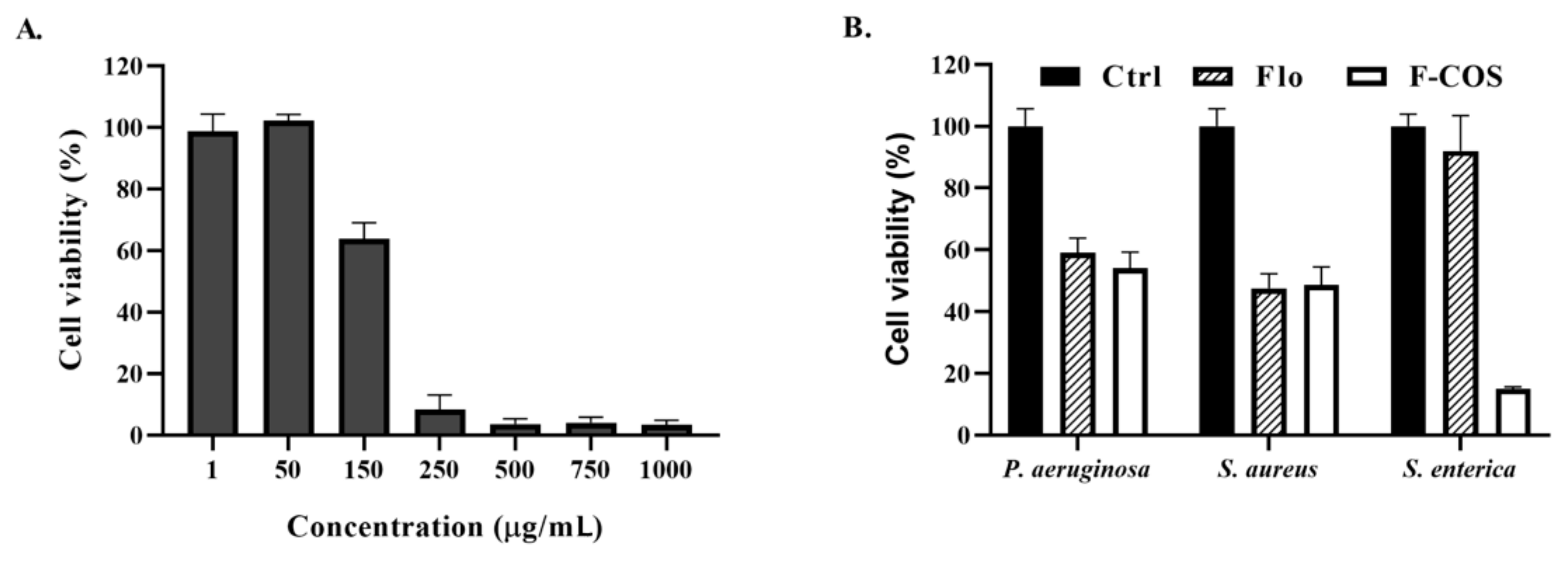
2.3. Antibacterial Activity of F-COS Conjugates against S. hyovaginalis Biofilm and Planktonic Cells
2.4. Broad-Spectrum Anti-Biofilm Activity of F-COS Conjugates against Different Bacteria
3. Discussion
4. Materials and Methods
4.1. Reagents and Material
4.2. Synthesis and Purification of Florfenicol-COS Conjugates (F-COS)
4.2.1. Synthesis of Florfenicol Amber Acid Ester (F-COOH)
4.2.2. Synthesis of Florfenicol-COS Conjugates (F-COS)
4.3. Characterization of Florfenicol-COS Conjugates (F-COS)
4.3.1. Characterization of F-COOH
4.3.2. Characterization of F-COS
4.4. Biofilm Inhibition and Eradication Assays
4.5. Planktonic Bacteria Inhibition Assay
4.6. Fluorescence Microscopy
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brockmeier, S.L.; Loving, C.L.; Nicholson, T.L.; Wang, J.; Peters, S.E.; Weinert, L.; Chaudhuri, R.; Seilly, D.J.; Langford, P.; Rycroft, A.; et al. Use of Proteins Identified through a Functional Genomic Screen To Develop a Protein Subunit Vaccine That Provides Significant Protection against Virulent Streptococcus suis in Pigs. Infect. Immun. 2017, 86, e00559-17. [Google Scholar] [CrossRef]
- Yanase, T.; Morii, D.; Kamio, S.; Nishimura, A.; Fukao, E.; Inose, Y.; Honma, Y.; Kitahara, N.; Yokozawa, T.; Chang, B.; et al. The first report of human meningitis and pyogenic ventriculitis caused by Streptococcus suis: A case report. J. Infect. Chemother. 2018, 24, 669–673. [Google Scholar] [CrossRef]
- Qin, G.C.; Chen, L.X.; Yang, P.; Zuom, H. Synthesis of Water-Soluble Prodrug of Florfenicol. Asian J. Chem. 2011, 23, 5157–5158. [Google Scholar]
- Black, L.A.; Higgins, D.P.; Govendir, M. In vitro activity of chloramphenicol, florfenicol and enrofloxacin against Chlamydia pecorum isolated from koalas (Phascolarctos cinereus). Aust. Vet. J. 2015, 93, 420. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519. [Google Scholar] [CrossRef] [PubMed]
- Van De Riet, J.M.; A Potter, R.; Christie-Fougere, M.; Burns, B.G. Simultaneous Determination of Residues of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Farmed Aquatic Species by Liquid Chromatography/Mass Spectrometry. J. AOAC Int. 2003, 86, 510. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Lim, J.-H.; Kim, M.-S.; Hwang, Y.-H.; Yun, H. Pharmacokinetics of florfenicol and its metabolite, florfenicol amine, in dogs. Res. Vet. Sci. 2008, 84, 85–89. [Google Scholar] [CrossRef]
- Birdane, Y.O.; Birdane, F.M. Pharmacokinetics of florfenicol following intravenous and intramuscular administration in dogs. Veterinární Med. 2016, 60, 323–329. [Google Scholar] [CrossRef]
- Holden, M.T.; Hauser, H.; Sanders, M.; Ngo, T.H.; Cherevach, I.; Cronin, A.; Goodhead, I.; Mungall, K.; Quail, M.A.; Price, C.; et al. Rapid Evolution of Virulence and Drug Resistance in the Emerging Zoonotic Pathogen Streptococcus suis. PLoS ONE 2009, 4, e6072. [Google Scholar] [CrossRef]
- Gottschalk, M.; Segura, M. The pathogenesis of the meningitis caused by Streptococcus suis: The unresolved questions. Veter. Microbiol. 2000, 76, 259–272. [Google Scholar] [CrossRef]
- A Fux, C.; Costerton, J.; Stewart, P.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, H.-C.F.J.; Szewzyk, U.; Steinberg, P.; A Rice, S.; Kjelleberg, S.A.R.S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sengol, J.S.; Balasubramanian, V.; Rajaram, R. Chemical characterization and bioactivity evaluation of bacteriocin from marine biofilm-forming bacteria. Afr. J. Microbiol. Res. 2014, 8, 3617–3624. [Google Scholar] [CrossRef][Green Version]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187. [Google Scholar] [CrossRef]
- Trappetti, C.; Gualdi, L.; Di Meola, L.; Jain, P.K.; Korir, C.C.; Edmonds, P.; Iannelli, F.; Ricci, S.; Pozzi, G.; Oggioni, M.R. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Gao, L.; Cai, H.; Zhou, Y.; Yang, Y.; Xu, C.; Ding, W.; Chen, J.; Muhammad, I.; et al. Rutin Inhibits Streptococcus suis Biofilm Formation by Affecting CPS Biosynthesis. Front. Pharmacol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Yuan, X.; Wei, J.; Zhang, X.; Cheng, G.; Wang, Z.A.; Du, Y.-G. Synthesis and Evaluation of a Chitosan Oligosaccharide-Streptomycin Conjugate against Pseudomonas aeruginosa Biofilms. Mar. Drugs 2019, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Scott, H.; Pansare, S.V.; Glinka, T.W. Florfenicol Prodrug Having Improved Water Solubility. U.S. Patent 7,153,842, 26 December 2006. [Google Scholar]
- Wei, H.T.; Liu, F.; Zhong, X.-T.; Lou, B.; Huang, X.-H. The Preparation of Water-soluble Florfenicol. Chin. J. Vet. Drug 2009, 43, 12–15. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhu, H.; Wang, Y.; Li, P.Y.; Li, C.Q. Preparation of florfenicol soluble powder and determination of its dissolution. Chin. J. Vet. Drug 2004, 3, 223–234. [Google Scholar] [CrossRef]
- Gu, S.X.; Du, J.-W.; Ju, X.-L.; Chen, Q.-P. A Scalable One-Pot Process for the Synthesis of Florfenicol Phosphodiester. Org. Process. Res. Dev. 2014, 18, 552–554. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan Coupling Makes Microbial Biofilms Susceptible to Antibiotics. Sci. Rep. 2013, 3, 3364. [Google Scholar] [CrossRef]
- Cheng, C.; Ying, Y.; Zhou, D.; Zhu, L.; Lu, J.; Li, A.; Bao, Q.; Zhu, M. RamA, a transcriptional regulator conferring florfenicol resistance in Leclercia adecarboxylata R25. Folia Microbiol. 2020, 65, 1051–1060. [Google Scholar] [CrossRef]
- Lin, S.B.; Chen, S.H.; Peng, K.C. Preparation of antibacterial chito-oligosaccharide by altering the degree of deacetylation of beta-chitosan in a Trichoderma harzianum chitinase-hydrolysing process. J. Sci. Food Agric. 2009, 89, 238–244. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, P.; Cheng, G.; Jiao, S.; Ren, L.; Ji, S.; Hu, T.; Liu, H.; Du, Y.-G. Enhanced immune response to inactivated porcine circovirus type 2 (PCV2) vaccine by conjugation of chitosan oligosaccharides. Carbohydr. Polym. 2017, 166, 64. [Google Scholar] [CrossRef]
- Pitts, B.; Hamilton, M.A.; Zelver, N.; Stewart, P.S. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 2003, 54, 269–276. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of synthesized F-COS products are available from the authors. |


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Liu, J.; Li, R.; Zhou, J.; Wei, J.; Jiao, S.; Wang, Z.A.; Du, Y. Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol. Molecules 2020, 25, 6043. https://doi.org/10.3390/molecules25246043
Yuan X, Liu J, Li R, Zhou J, Wei J, Jiao S, Wang ZA, Du Y. Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol. Molecules. 2020; 25(24):6043. https://doi.org/10.3390/molecules25246043
Chicago/Turabian StyleYuan, Xianghua, Jing Liu, Ruilian Li, Junlin Zhou, Jinhua Wei, Siming Jiao, Zhuo A. Wang, and Yuguang Du. 2020. "Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol" Molecules 25, no. 24: 6043. https://doi.org/10.3390/molecules25246043
APA StyleYuan, X., Liu, J., Li, R., Zhou, J., Wei, J., Jiao, S., Wang, Z. A., & Du, Y. (2020). Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol. Molecules, 25(24), 6043. https://doi.org/10.3390/molecules25246043





