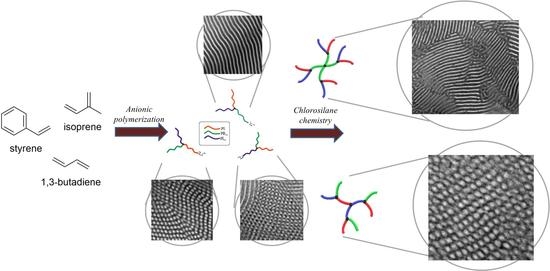
Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison
Abstract
:1. Introduction
2. Results and Discussion
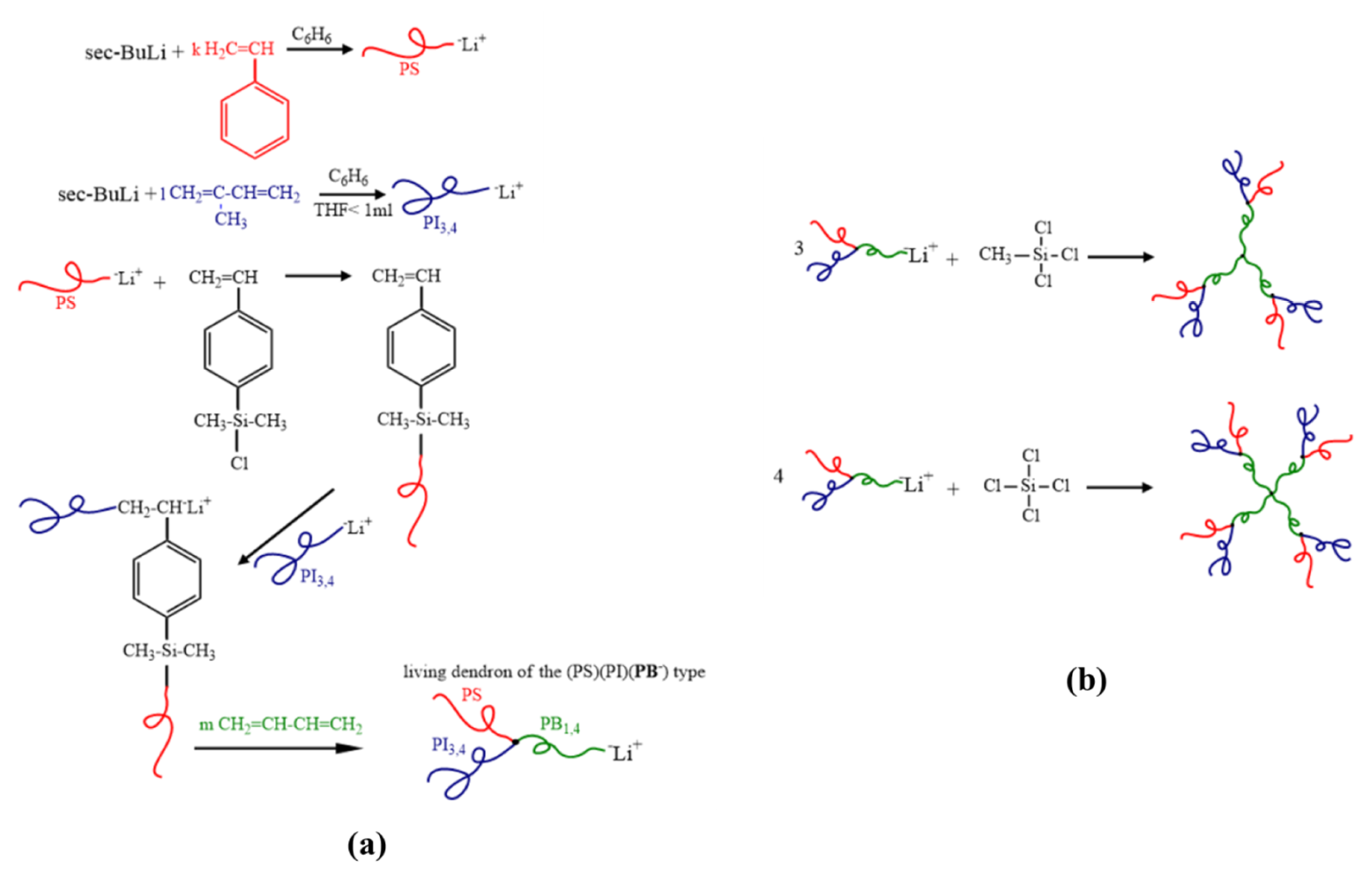
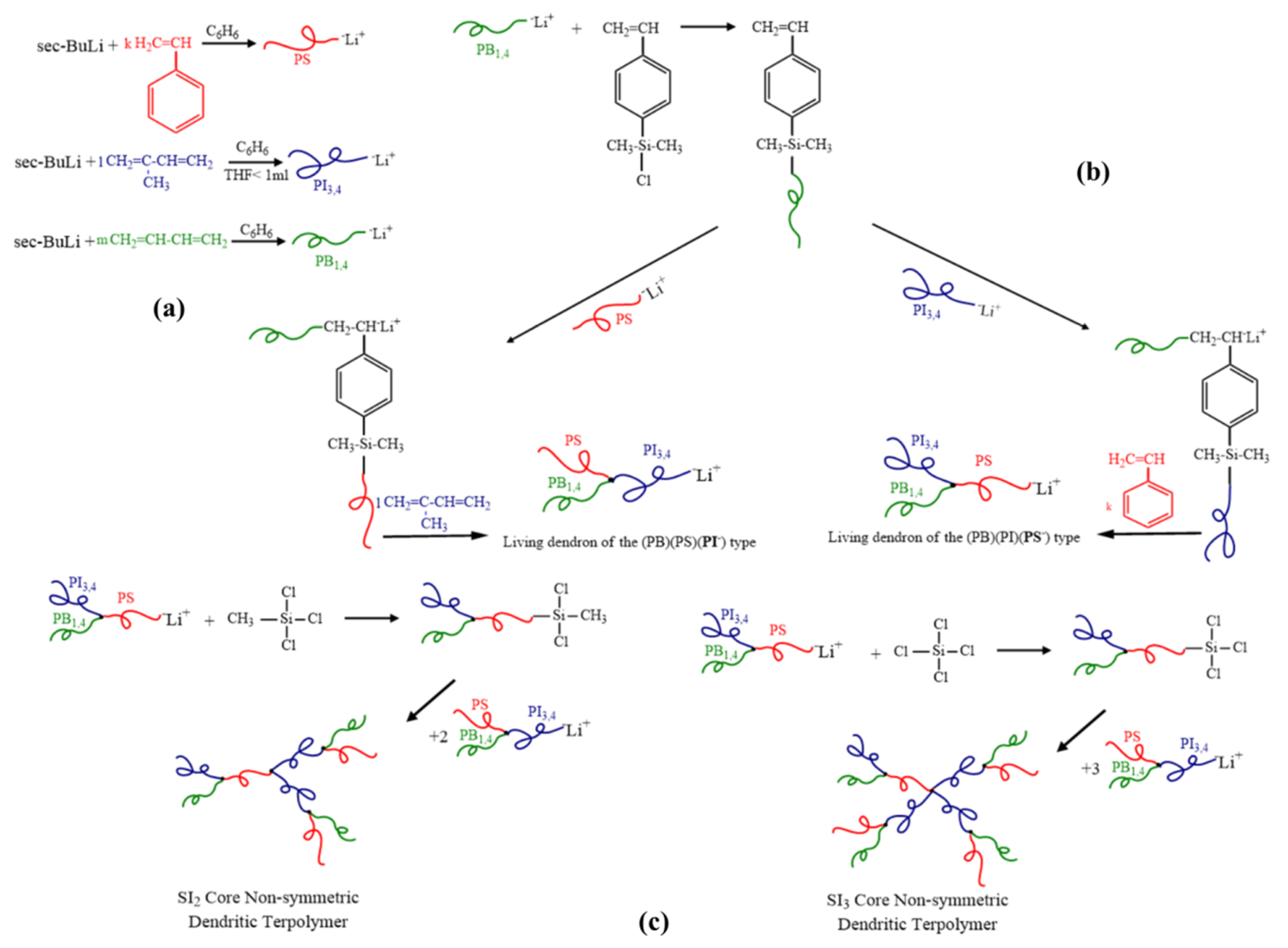
2.1. Synthesis
2.2. Thermal Characterization
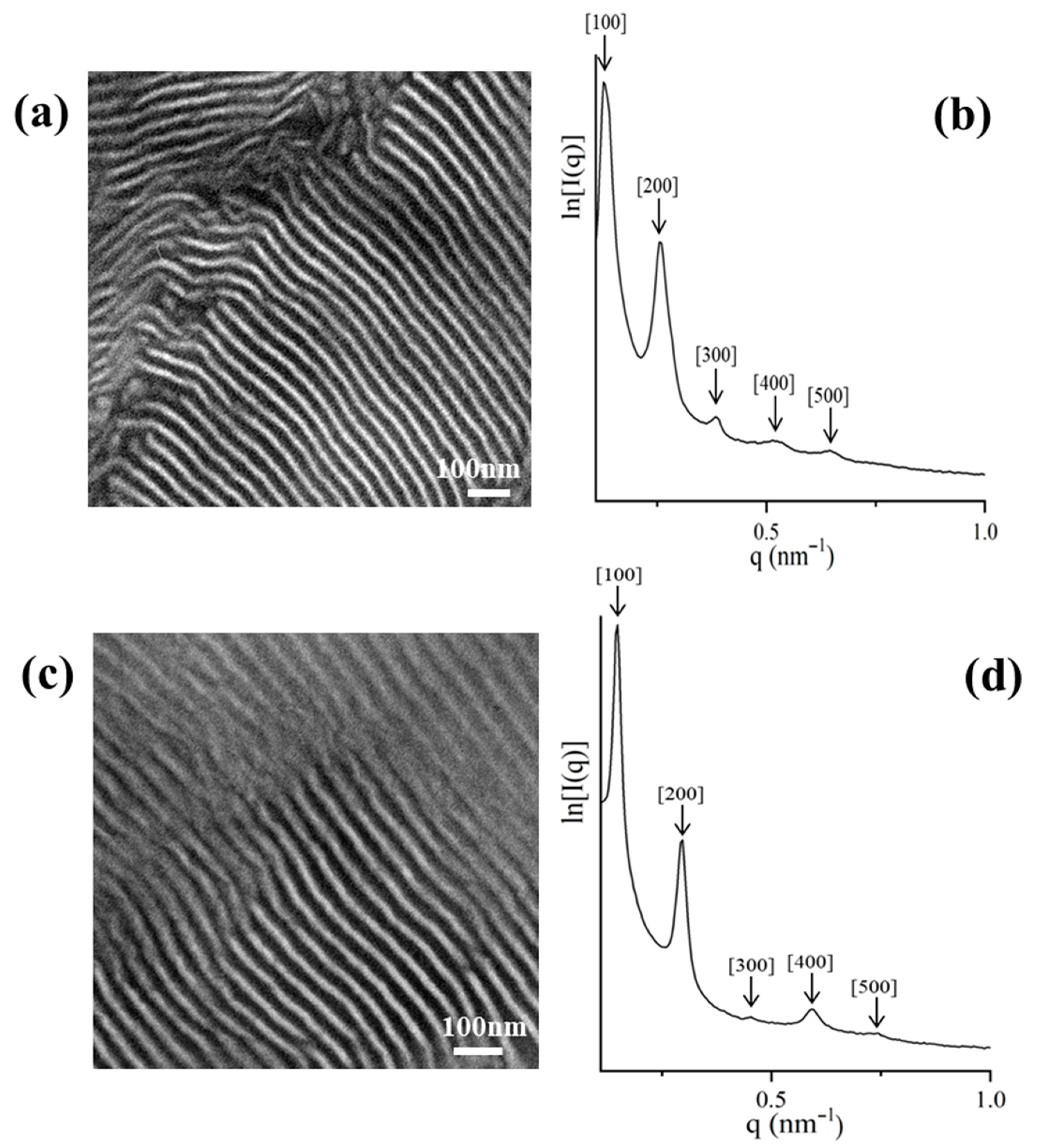
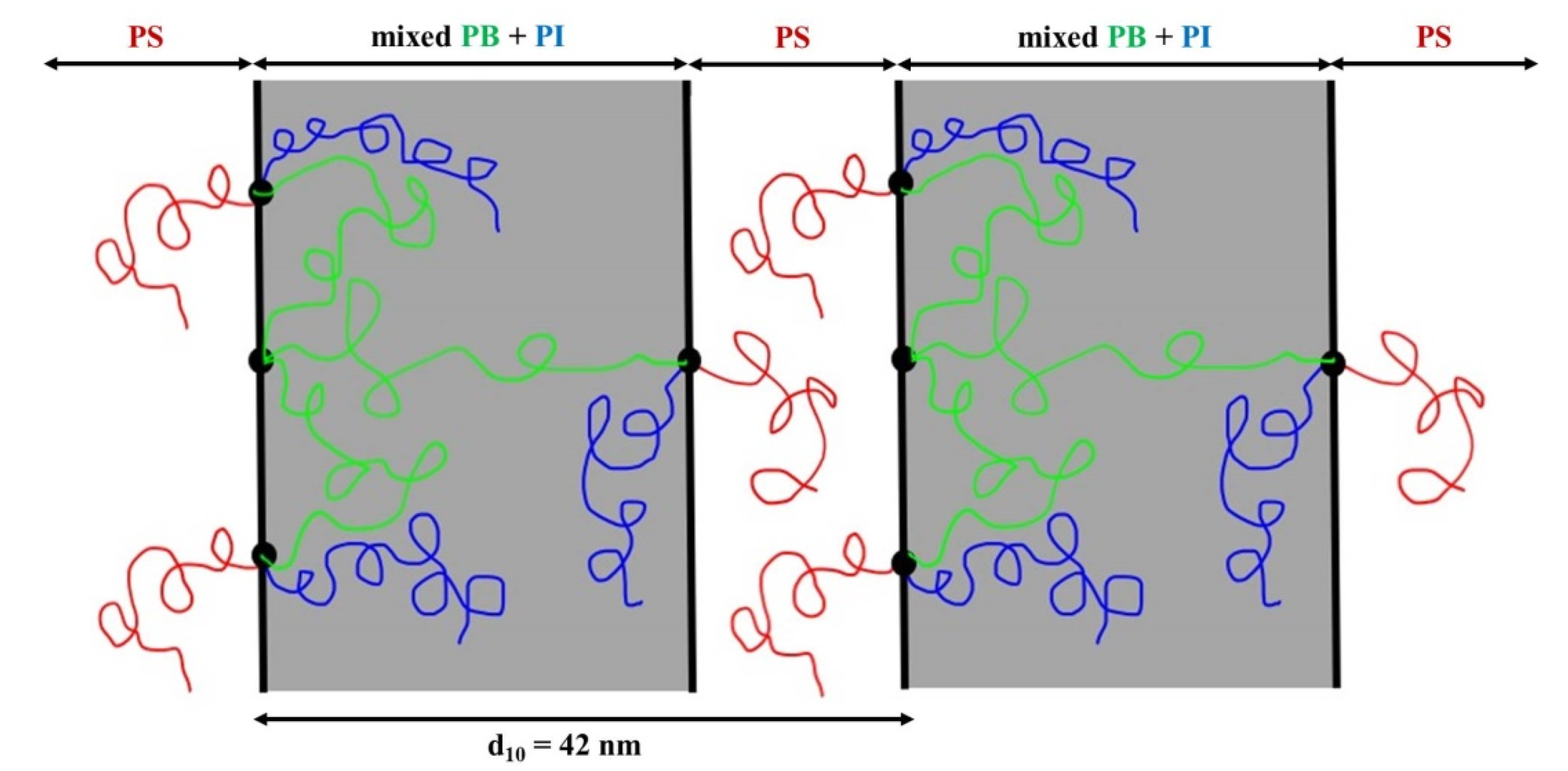
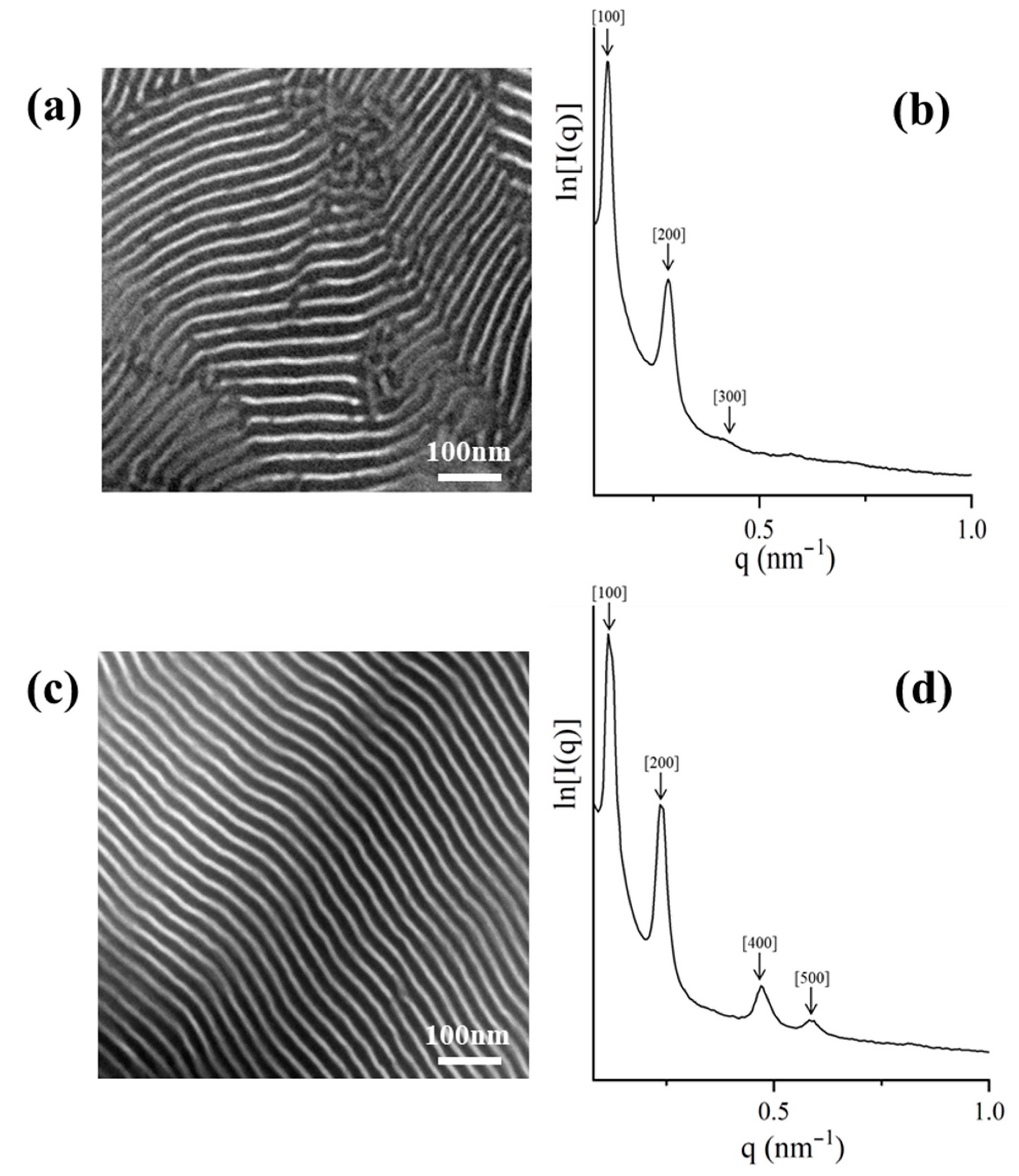
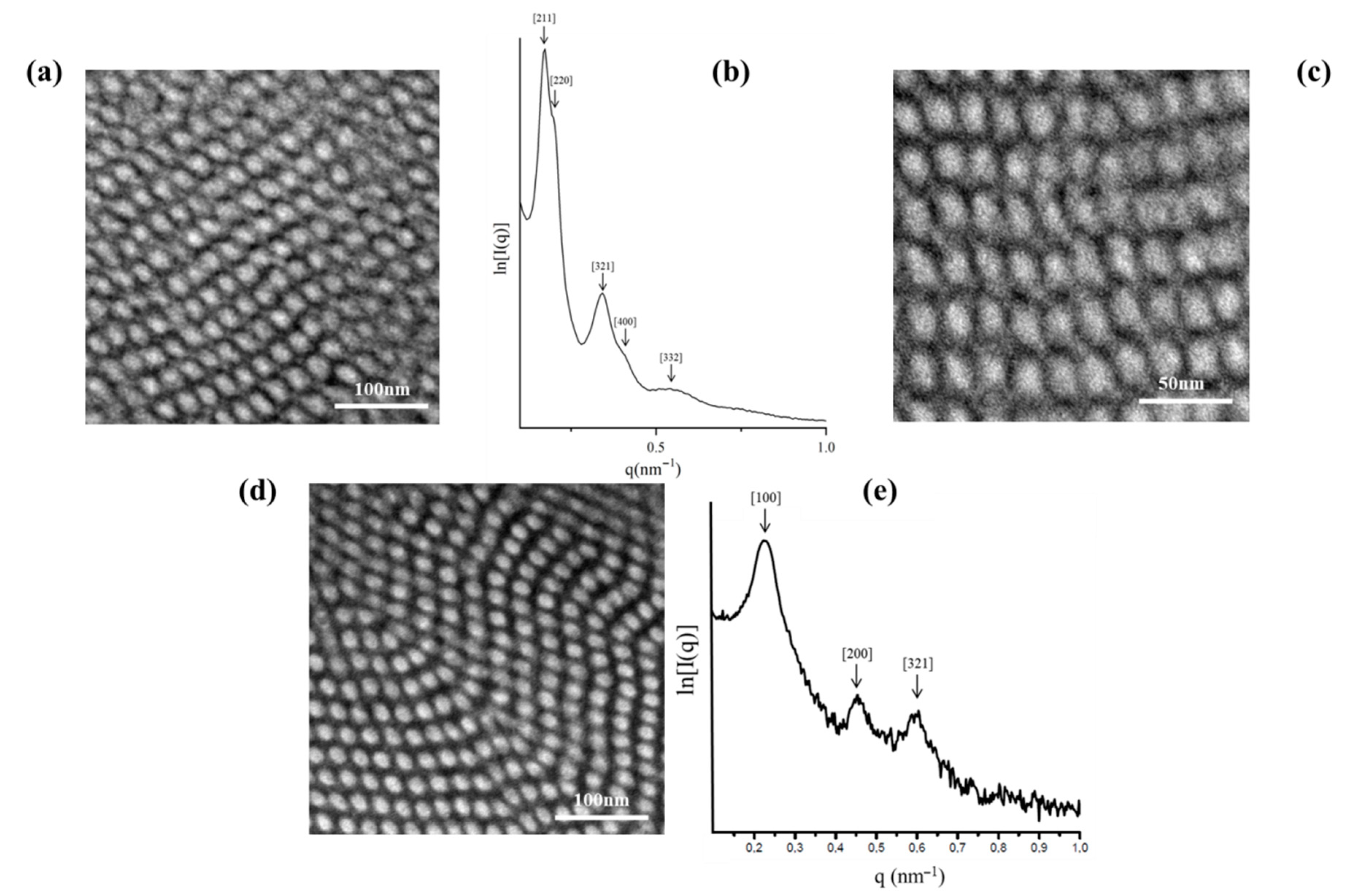
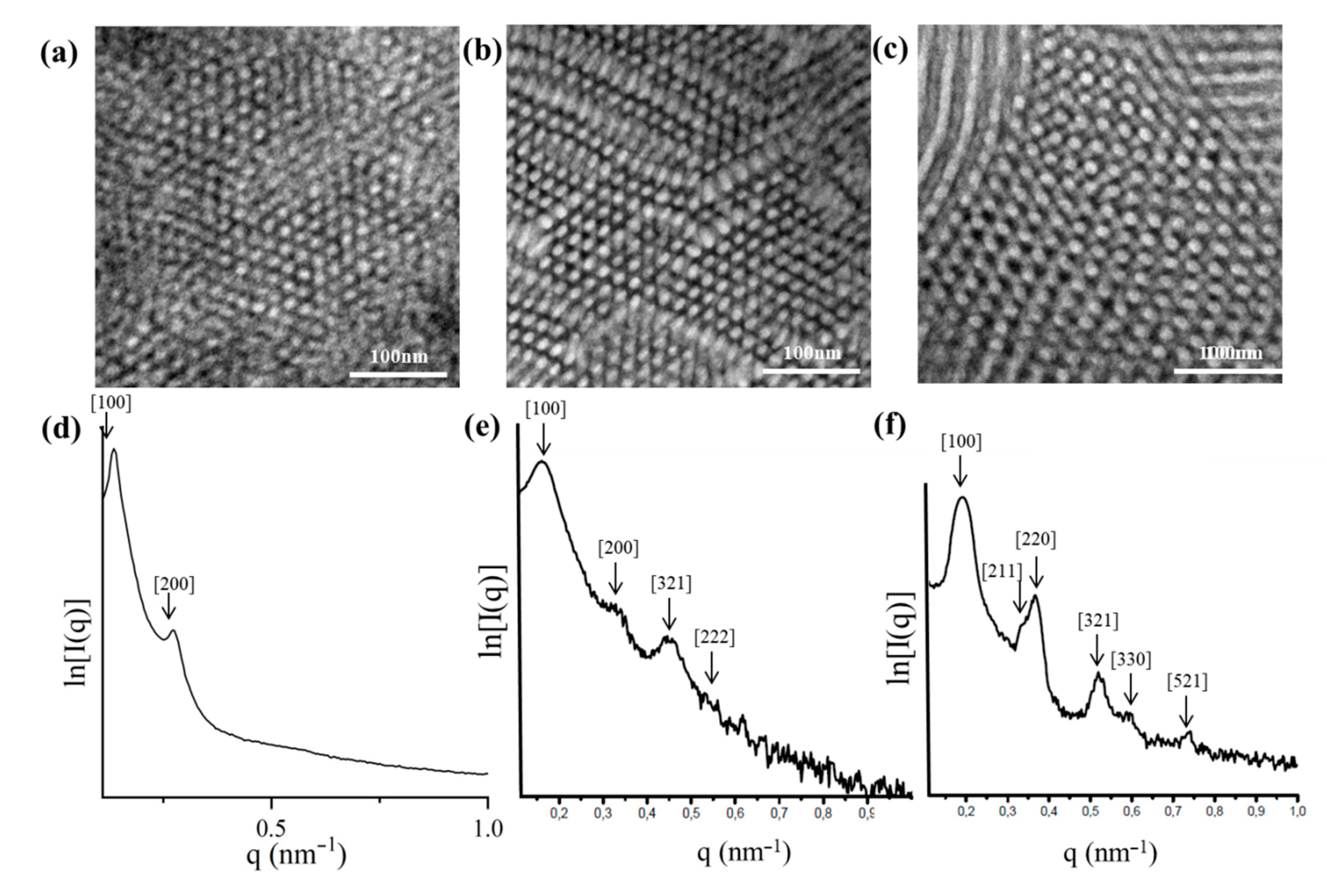
2.3. TEM and SAXS Results
2.3.1. [(PS)(PI)(PB)]3-B3 core
2.3.2. [(PS)(PI)(PB)]4-B4 core
2.3.3. [(PB)(PI)(PSc)][(PS)(PB)(PIc)]2 (SI2 core)
2.3.4. [(PB)(PI)(PSc)][(PS)(PB)(PIc)]3 (SI3 core)
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaefgen, J.R.; Flory, P.J. Synthesis of Multichain Polymers and Investigation of their Viscosities. J. Am. Chem. Soc. 1948, 70, 2709–2718. [Google Scholar] [CrossRef]
- Morton, M.; Helminiak, T.E.; Gadkary, S.D.; Bueche, F. Preparation and Properties of Monodisperse Branched Polystyrene. J. Polym. Sci. 1962, 57, 471–482. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Roovers, J. Synthesis and Solution Properties of Linear, Four-Branched, and Six-Branched Star Polyisoprenes. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 2521–2533. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Guyot, A.; Fetters, L.J. Star-branched Polymers. 1. The Synthesis of Star Polyisoprenes Using Octa-and Dodecachlorosilanes as Linking Agents. Macromolecules 1978, 11, 668–672. [Google Scholar] [CrossRef]
- Bauer, B.J.; Hadjichristidis, N.; Fetters, L.J.; Roovers, J. Starbranched Polymers. 5. The Theta Temperature Depression for 8-and 12-arm Polyisoprenes in Dioxane. J. Am. Chem. Soc. 1980, 102, 2410–2413. [Google Scholar] [CrossRef]
- Roovers, J.; Hadjichristidis, N.; Fetters, L.J. Analysis and Dilute Solution Properties of 12-and 18-arm-star Polystyrenes. Macromolecules 1983, 16, 214–220. [Google Scholar] [CrossRef]
- Roovers, J.; Zhou, L.L.; Toporowski, P.M.; van der Zwan, M.; Iatrou, H.; Hadjichristidis, N. Regular Star Polymers with 64 and 128 Arms. Models for Polymeric Micelles. Macromolecules 1993, 26, 4324–4331. [Google Scholar]
- Liu, N.; Ma, C.-H.; Sun, R.-W.; Huang, J.; Li, C.-L.; Wu, Z.-Q. Facile synthesis of well-defined ABC miktoarm star terpolymers bearing poly(ε-caprolactone), polystyrene and stereoregular helical poly(phenyl isocyanide) blocks. Polym. Chem. 2016, 7, 2447–2451. [Google Scholar] [CrossRef]
- Chernyy, S.; Kirkensgaard, J.J.K.; Mahalik, J.P.; Kim, H.; Arras, M.M.L.; Kumar, R.; Sumpter, B.G.; Smith, G.S.; Mortensen, K.; Russell, T.P.; et al. Bulk and Surface Morphologies of ABC Miktoarm Star Terpolymers Composed of PDMS, PI, and PMMA Arms. Macromolecules 2018, 51, 1041–1051. [Google Scholar] [CrossRef]
- Li, Y.; von der Lühe, M.; Schacher, F.H.; Ling, J. 3-Miktoarm Star Terpolymers via Janus Polymerization: One-Step Synthesis and Self-Assembly. Macromolecules 2018, 51, 4938–4944. [Google Scholar] [CrossRef]
- Kim, H.; Arras, M.M.L.; Mahalik, J.P.; Wang, W.Y.; Yu, D.M.; Chernyy, S.; Goswami, M.; Kumar, R.; Sumpter, B.G.; Hong, K.L.; et al. Studies on the 3-lamellar morphology of miktoarm terpolymers. Macromolecules 2018, 51, 7491–7499. [Google Scholar] [CrossRef]
- Yang, Y.L.; Tsao, H.K.; Sheng, Y.J. Morphology and Wetting Stability of Nanofilms of ABC Miktoarm Star Terpolymers. Macromolecules 2020, 53, 594–601. [Google Scholar] [CrossRef]
- Ntetsikas, K.; Zapsas, G.; Bilalis, P.; Gnanou, Y.; Feng, X.; Edwin, L.; Thomas, E.L.; Hadjichristidis, N. Complex Star Architectures of Well-Defined Polyethylene-Based Co/Terpolymers. Macromolecules 2020, 53, 4355–4365. [Google Scholar] [CrossRef]
- Liu, G.; Sun, H.; Rangou, S.; Ntetsikas, K.; Avgeropoulos, A.; Wang, S.-Q. Studying the Origin of “Strain Hardening”: Basic Difference between Extension and Shear. J. Rheol. 2013, 57, 89–104. [Google Scholar] [CrossRef]
- Sun, H.; Ntetsikas, K.; Avgeropoulos, A.; Wang, S.-Q. Breakdown of Time-Temperature Equivalence in Startup Uniaxial Extension of Entangled Polymer Melts. Macromolecules 2013, 46, 4151–4159. [Google Scholar] [CrossRef]
- Sun, H.; Liu, G.; Ntetsikas, K.; Avgeropoulos, A.; Wang, S.-Q. Rheology of Entangled Polymers Not Far above Glass Transition Temperature: Transient Elasticity and Intersegmental Viscous Stress. Macromolecules 2014, 47, 5839–5850. [Google Scholar] [CrossRef]
- Sun, H.; Lin, P.; Liu, G.; Ntetsikas, K.; Misichronis, K.; Kang, N.; Liu, J.; Avgeropoulos, A.; Mays, J.; Wang, S.-Q. Failure Behavior After Stepwise Uniaxial Extension of Entangled Polymer Melts. J. Rheol. 2015, 59, 751–767. [Google Scholar] [CrossRef]
- Rahman, M.S.; Aggarwal, R.; Larson, R.G.; Dealy, J.M.; Mays, J.W. Synthesis and Dilute Solution Properties of Well-Defined H Shaped Polybutadienes. Macromolecules 2008, 41, 8225–8230. [Google Scholar]
- Chen, X.; Rahman, M.S.; Lee, H.; Mays, J.W.; Chang, T.; Larson, R. Combined Synthesis, TGIC Characterization, and Rheological Measurement and Prediction of Symmetric H Polybutadienes and Their Blends with Linear and Star-Shaped Polybutadienes. Macromolecules 2011, 44, 7799–7809. [Google Scholar] [CrossRef]
- Rahman, M.S.; Lee, H.; Chen, X.; Chang, T.; Larson, R.; Mays, J.W. Model Branched Polymers: Synthesis and Characterization of Asymmetric H-Shaped Polybutadienes. ACS Macro Lett. 2012, 1, 537–540. [Google Scholar] [CrossRef]
- Chen, X.; Lee, H.; Rahman, M.S.; Chang, T.; Mays, J.W.; Larson, R. Analytical Rheology of Asymmetric H-Shaped Model Polybutadiene Melts. Macromolecules 2012, 45, 5744–5756. [Google Scholar] [CrossRef]
- Roovers, J.; Toporowski, P.M. Preparation and Characterization of H-Shaped Polystyrene. Macromolecules 1981, 14, 1174–1178. [Google Scholar] [CrossRef]
- Iatrou, H.; Hadjichristidis, N. Synthesis of a Model S-Miktoarm Star Terpolymer. Macromolecules 1992, 25, 4649–4651. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Dewald, J.R.; Hall, M.R.; Martin, S.J.; Smith, P.B. Reprints of the 1st SPSJ International Polymer Conference, Kyoto, Japan. Soc. Polym. Sci. 1984, 65. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic. Macromol. Polym. J. 1985, 17, 117–132. [Google Scholar]
- Hawker, C.; Fréchet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef]
- Six, J.L.; Gnanou, Y. From Star-Shaped to Dendritic Poly(ethylene oxide)s: Toward Increasingly Branched Architectures by Anionic Polymerization. Macromol. Symp. 1995, 95, 137–150. [Google Scholar] [CrossRef]
- Feng, X.; Taton, D.; Chaikof, E.L.; Gnanou, Y. Toward an Easy Access to Dendrimer-Like Poly(ethylene oxide)s. J. Am. Chem. Soc. 2005, 127, 10956–10966. [Google Scholar] [CrossRef]
- Trollsås, M.; Hedrick, J.L. Dendrimer-Like Star Polymers. J. Am. Chem. Soc. 1998, 120, 4644–4651. [Google Scholar] [CrossRef]
- Chalari, I.; Hadjichristidis, N. Synthesis of well-defined second-generation dendritic polymers of isoprene (I) and styrene (S): (S2I)3, (SI′I)3, (I″I′I)3, and (I′2I)4. J. Polym. Sci. A Polym. Chem. 2002, 40, 1519–1526. [Google Scholar] [CrossRef]
- Rangou, S.; Theodorakis, P.E.; Gergidis, L.N.; Avgeropoulos, A.; Efthymiopoulos, P.; Smyrnaios, D.; Kosmas, M.; Vlahos, C. Synthesis, molecular characterization and theoretical study of first generation dendritic homopolymers of butadiene and isoprene with different microstructures. Polymer 2007, 48, 652–663. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Rangou, S.; Krikorian, V.; Thomas, E.L. Synthesis and Self-Assembly of 2nd Generation Dendritic Homopolymers and Copolymers of Polydienes with Different Isomeric Microstructures. Macromol. Symp. 2008, 267, 16–20. [Google Scholar] [CrossRef]
- Rangou, S.; Avgeropoulos, A. Synthesis of Dendritic Terpolymers Consisting of Polystyrene, Polybutadiene, and Polyisoprene with Different Isomerisms. J. Polym. Sci. A Polym. Chem. 2009, 47, 1567–1574. [Google Scholar] [CrossRef]
- Urbani, C.N.; Bell, C.A.; Lonsdale, D.; Whittaker, M.R.; Monteiro, M.J. Self-Assembly of Amphiphilic Polymeric Dendrimers Synthesized with Selective Degradable Linkages. Macromolecules 2008, 41, 76–86. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Matmour, R.; Francis, R.; Taton, D.; Gnanou, Y. Synthesis of Dendrimer-Like Polystyrene by Atom Transfer Radical Polymerization and Investigation of their Viscosity Behavior. Macromolecules 2005, 38, 3120–3128. [Google Scholar] [CrossRef]
- Frechet, J.M.J.; Tomalia, D.A. (Eds.) Dendrimers and Other Dendritic Polymers; Wiley Series in Polymer Science, Wiley: West Sussex, UK, 2002; pp. 361–382. [Google Scholar]
- Roovers, J.; Comanita, B. Dendrimers and Dendrimer-Polymer Hybrids. In Advances in Polymer Science; Springer: Verlag Berlin-Heidelberg, Germany, 1999; Volume 142, pp. 179–228. [Google Scholar]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar]
- Jang, W.-D.; Selim, K.M.K.; Lee, C.-H.; Kang, I.-K. Bioinsired application of dendrimers: From bio-minicry to biomedical applications. Prog. Pol. Sci. 2009, 34, 1–23. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, A.D. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Lotocki, V.; Kakkar, A. Miktoarm Star Polymers: Branched Architectures in Drug Delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef]
- Astruc, D.; Charda, F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3023. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef] [PubMed]
- Kovvali, A.S.; Chen, H.; Sirkar, K.K. Dendrimer Membranes: A CO2-Selective Molecular Gate. J. Am. Chem. Soc. 2000, 122, 7594–7595. [Google Scholar] [CrossRef]
- Parrott, M.C.; Benhabbour, S.R.; Saab, C.; Lemon, J.A.; Parker, S.; Valliant, J.F.; Adronov, A. Synthesis, Radiolabeling, and Bio-imaging of High Generation Polyester Dendrimers. J. Am. Chem. Soc. 2009, 131, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. molecules 2020, 25, 3333. [Google Scholar] [CrossRef] [PubMed]
- Iatrou, H.; Avgeropoulos, A.; Hadjichristidis, N. Synthesis of Model Super H-Shaped Block Copolymers. Macromolecules 1994, 27, 6232–6233. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Poulos, Y.; Hadjichristidis, N.; Roovers, J. Synthesis of Model 16-Miktoarm (Vergina) Star Copolymers of the A8B8 Type. Macromolecules 1996, 29, 6076–6078. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Dair, B.J.; Hadjichristidis, N.; Thomas, E.L. Tricontinuous Double Gyroid Cubic Phase in Triblock Copolymers of the ABA Type. Macromolecules 1997, 30, 5634–5642. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Paraskeva, S.; Hadjichristidis, N.; Thomas, E.L. Synthesis and Microphase Separation of Linear Triblock Terpolymers of Polystyrene, High 1,4-Polybutadiene, and High 3,4-Polyisoprene. Macromolecules 2002, 35, 4030–4035. [Google Scholar] [CrossRef]
- Altintas, O.; Demirel, A.L.; Hizal, G.; Tunka, U. Dendrimer-like miktoarm star terpolymers: A3-(B-C)3 via click reaction strategy. J. Polym. Sci. A Polym. Chem. 2008, 46, 5916–5928. [Google Scholar] [CrossRef]
- Matsuo, A.; Watanabe, T.; Hirao, A. Synthesis of Well-Defined Dendrimer-like Branched Polymers and Block Copolymer by the Iterative Approach Involving Coupling Reaction of Living Anionic Polymer and Functionalization. Macromolecules 2004, 37, 6283–6290. [Google Scholar] [CrossRef]
- Hirao, A.; Matsuo, A.; Watanabe, T. Precise Synthesis of Dendrimer-like Star-Branched Poly(methyl methacrylate)s up to Seventh Generation by an Iterative Divergent Approach Involving Coupling and Transformation Reactions. Macromolecules 2005, 38, 8701–8711. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, H.; Tang, P.; Qiu, F.; Yanga, Y. Self-assembly of ABC dendrimer by real-space self-consistent mean field theory in a two-dimensional space. Soft Matter 2011, 7, 10076–10084. [Google Scholar] [CrossRef]
- van Hest, J.C.M.; Baars, M.W.P.L.; Elissen-Román, C.; van Genderen, M.H.P.; Meijer, E.W. Acid-Functionalized Amphiphiles Derived from Polystyrene-Poly(propylene imine) Dendrimers, with a pH-Dependent Aggregation. Macromolecules 1995, 28, 6689–6691. [Google Scholar] [CrossRef] [Green Version]
- van Hest, J.C.M.; Delnoye, D.A.P.; Baars, M.W.P.L.; Elissen-Román, C.; van Genderen, M.H.P.; Meijer, E.W. Polystyrene-PoIy(propylene imine) Dendrimers: Synthesis, Characterization, and Association Behavior of a New Class of Amphiphiles. Chem. Eur. J. 1996, 2, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Román, C.; Fischer, H.R.; Meijer, E.W. Microphase Separation of Diblock Copolymers Consisting of Polystyrene and Acid-Functionalized Poly(propylene imine) Dendrimers. Macromolecules 1999, 32, 5525–5531. [Google Scholar] [CrossRef]
- Mackay, M.E.; Hong, Y.; Jeong, M.; Tande, B.M.; Wagner, N.J.; Hong, S.; Gido, S.P.; Vestberg, R.; Hawker, C.J. Microphase Separation of Hybrid Dendron-Linear Diblock Copolymers into Ordered Structures. Macromolecules 2002, 35, 8391–8399. [Google Scholar] [CrossRef]
- Pochan, D.J.; Pakstis, L.; Huang, E.; Hawker, C.; Vestberg, R.; Pople, J. Architectural Disparity Effects in the Morphology of Dendrimer-Linear Coil Diblock Copolymers. Macromolecules 2002, 35, 9239–9242. [Google Scholar] [CrossRef]
- Johnson, M.A.; Iyer, J.; Hammond, P.T. Microphase Segregation of PEO-PAMAM Linear-Dendritic Diblock Copolymers. Macromolecules 2004, 37, 2490–2501. [Google Scholar] [CrossRef]
- Cho, B.-K.; Jain, A.; Nieberle, J.; Mahajan, S.; Wiesner, U.; Gruner, S.M.; Türk, S.; Räder, H.J. Synthesis and Self-Assembly of Amphiphilic Dendrimers Based Aliphatic Polyether-Type Dendritic Cores. Macromolecules 2004, 37, 4227–4234. [Google Scholar] [CrossRef]
- Chung, Y.-W.; Lee, B.-I.; Kim, H.-Y.; Wiesner, U.; Cho, B.-K. Influence of Crystalline Peripheral Chain Length on the Solid-State Assemblies of Amphiphilic Dendrons. J. Polym. Sci. A Polym. Chem. 2007, 45, 4988–4994. [Google Scholar] [CrossRef]
- Cho, B.-K.; Kim, S.-H.; Lee, E. Salt-induced microphase separation of amorphous dendritic poly(ethylene oxide)-block-linear polystyrene copolymers. J. Polym. Sci. A Polym. Chem. 2010, 48, 2372–2376. [Google Scholar] [CrossRef]
- Moschovas, D.; Manesi, G.-M.; Karydis-Messinis, A.; Zapsas, G.; Ntetsikas, K.; Zafeiropoulos, N.E.; Piryazev, A.; Thomas, E.L.; Hadjichristidis, N.; Ivanov, D.A.; et al. Alternating Gyroid Network Structure in an ABC Miktoarm Terpolymer Comprised of Polystyrene and Two Polydienes. Nanomaterials 2020, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Physical Properties of Polymers Handbook, Part III: Thermodynamic Properties, 2nd ed.; Springer: New York, NY, USA, 2007; pp. 289–304. [Google Scholar]
- Zapsas, G.; Moschovas, D.; Ntetsikas, K.; Rangou, S.; Lee, J.-H.; Thomas, E.L.; Zafeiropoulos, N.E.; Avgeropoulos, A. Immiscible Polydiene Blocks in Linear Copolymer and Terpolymer Sequences. J. Polym. Sci. B Polym. Phys. 2015, 53, 1238–1246. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.Q.; Burgaz, E.; Gido, S.P.; Staudinger, U.; Weidisch, R.; Uhrig, D.; Mays, J.W. Morphology and Tensile Properties of Multigraft Copolymers with Regularly Spaced Tri-, Tetra-, and Hexafunctional Junction Points. Macromolecules 2006, 39, 4428–4436. [Google Scholar] [CrossRef] [Green Version]
- Iatrou, H.; Hadjichristidis, N. Morphology and Miscibility of Miktoarm Styrene-Diene Copolymers and Terpolymers. Macromolecules 1993, 26, 5812–5815. [Google Scholar]
- Neumann, C.; Abetz, V.; Stadler, R. Phase behavior of ABC-triblock copolymers with two inherently miscible blocks. Colloid Polym. Sci. 1998, 276, 19–27. [Google Scholar] [CrossRef]
- Cohen, R.E.; Wilfong, D.E. Properties of Block Copolymers and Homopolymer Blends Comprised of 1,2-Polybutadiene and 1,4-Polybutadiene. Macromolecules 1982, 15, 370–375. [Google Scholar] [CrossRef]
- Dair, J.D.; Avgeropoulos, A.; Hadjichristidis, N.; Thomas, E.L. Mechanical properties of the double gyroid phase in oriented thermoplastic elastomers. J. Mater Sci. 2000, 35, 5207–5213. [Google Scholar] [CrossRef]
- Mogi, Y.; Nomura, M.; Kotsuji, H.; Ohnishi, K.; Matsushita, Y.; Noda, I. Superlattice Structures in Morphologies of the ABC Triblock Copolymers. Macromolecules 1994, 27, 6755–6760. [Google Scholar] [CrossRef]
- Brinkmann, S.; Stadler, R.; Thomas, E.L. New Structural Motif in Hexagonally Ordered Cylindrical Ternary (ABC) Block Copolymer Microdomains. Macromolecules 1998, 31, 6566–6572. [Google Scholar] [CrossRef]
- Matsen, M.W.; Gardiner, J.M. Anomalous domain spacing difference between AB diblock and homologous A2B2 starblock copolymers. J. Chem. Phys. 2000, 113, 1673–1676. [Google Scholar] [CrossRef]
- Hayashida, K.; Dotera, T.; Takano, A.; Matsushita, Y. Polymeric Quasicrystal: Mesoscopic Quasicrystalline Tiling in ABC Star Polymers. Phys. Rev. Lett. 2007, 98, 195502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Symmetric Terpolymers | ||||||
|---|---|---|---|---|---|---|
| Dendritic Sample | kg/mol a | kg/mol a | kg/mol a,b | kg/mol b | kg/mol b | Đ c |
| B3-core | 13.0 | 13.4 | 11.3 | 37.7 | 113.1 | 1.05 |
| B4-core | 14.4 | 18.0 | 15.7 | 48.1 | 192.4 | 1.08 |
| Asymmetric Terpolymers | |||||||
|---|---|---|---|---|---|---|---|
| Dendritic Sample | “Living Dendron” | kg/mol a | kg/mol a | kg/mol a | kg/mol a | kg/mol b | Đ c |
| (SI2)-core | 1 × (PB)(PI)(PS−) | 50.0 | 11.6 | 15.6 | 77.2 | 141.2 | 1.07 |
| 2 × (PS)(PB)(PI−) | 12.0 | 10.0 | 10.0 | 32.0 | |||
| (SI3)-core | 1 × (PB)(PI)(PS−) | 40.0 | 13.3 | 14.5 | 67.8 | 214.0 | 1.06 |
| 3 × (PS)(PB)(PI−) | 12.5 | 12.2 | 25.0 | 48.7 | |||
| χ | PS | 1,4-PB | 1,4-PI | 3,4-PI |
|---|---|---|---|---|
| 1,4-PB | 0.0483 a | 0 | ||
| 1,4-PI | 0.0706 a | 0.0012 b | 0 | |
| 3,4-PI | 0.1109 a | 0.0105 b | 0.0052 b | 0 |
Sample Availability: Samples of the dendrons and the final dendritic terpolymers are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangou, S.; Moschovas, D.; Moutsios, I.; Manesi, G.-M.; Tsitoni, K.; Bovsunovskaya, P.V.; Ivanov, D.A.; Thomas, E.L.; Avgeropoulos, A. Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison. Molecules 2020, 25, 6030. https://doi.org/10.3390/molecules25246030
Rangou S, Moschovas D, Moutsios I, Manesi G-M, Tsitoni K, Bovsunovskaya PV, Ivanov DA, Thomas EL, Avgeropoulos A. Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison. Molecules. 2020; 25(24):6030. https://doi.org/10.3390/molecules25246030
Chicago/Turabian StyleRangou, Sofia, Dimitrios Moschovas, Ioannis Moutsios, Gkreti-Maria Manesi, Konstantina Tsitoni, Polina V. Bovsunovskaya, Dimitri A. Ivanov, Edwin L. Thomas, and Apostolos Avgeropoulos. 2020. "Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison" Molecules 25, no. 24: 6030. https://doi.org/10.3390/molecules25246030
APA StyleRangou, S., Moschovas, D., Moutsios, I., Manesi, G.-M., Tsitoni, K., Bovsunovskaya, P. V., Ivanov, D. A., Thomas, E. L., & Avgeropoulos, A. (2020). Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison. Molecules, 25(24), 6030. https://doi.org/10.3390/molecules25246030