The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts
Abstract
1. Introduction
2. Results and Discussion
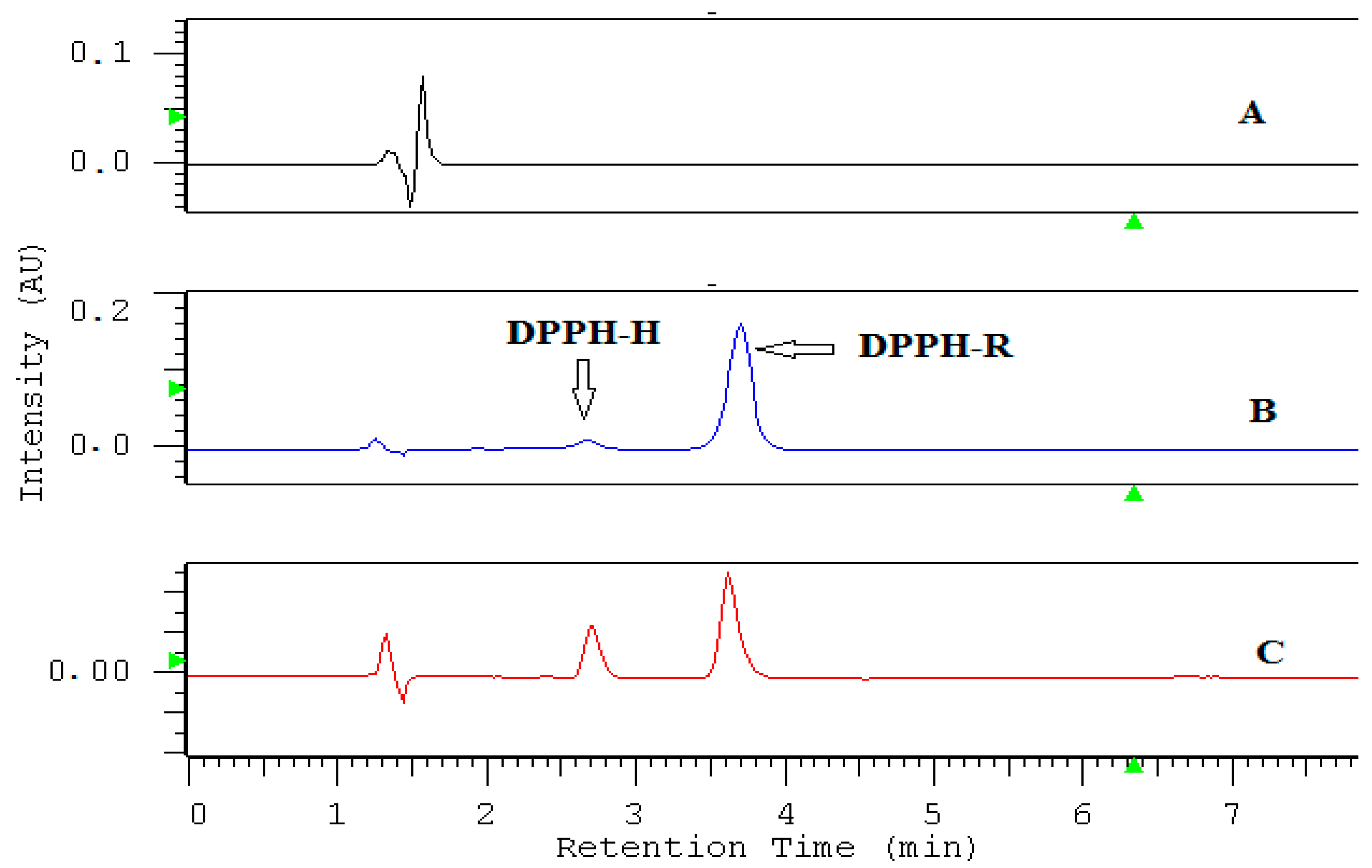
2.1. HPLC-DAD Detection Conditions
2.2. Antioxidant Properties of Standard AOs Measured by the DPPH-HPLC-DAD
2.2.1. Optimization of the Incubation Time
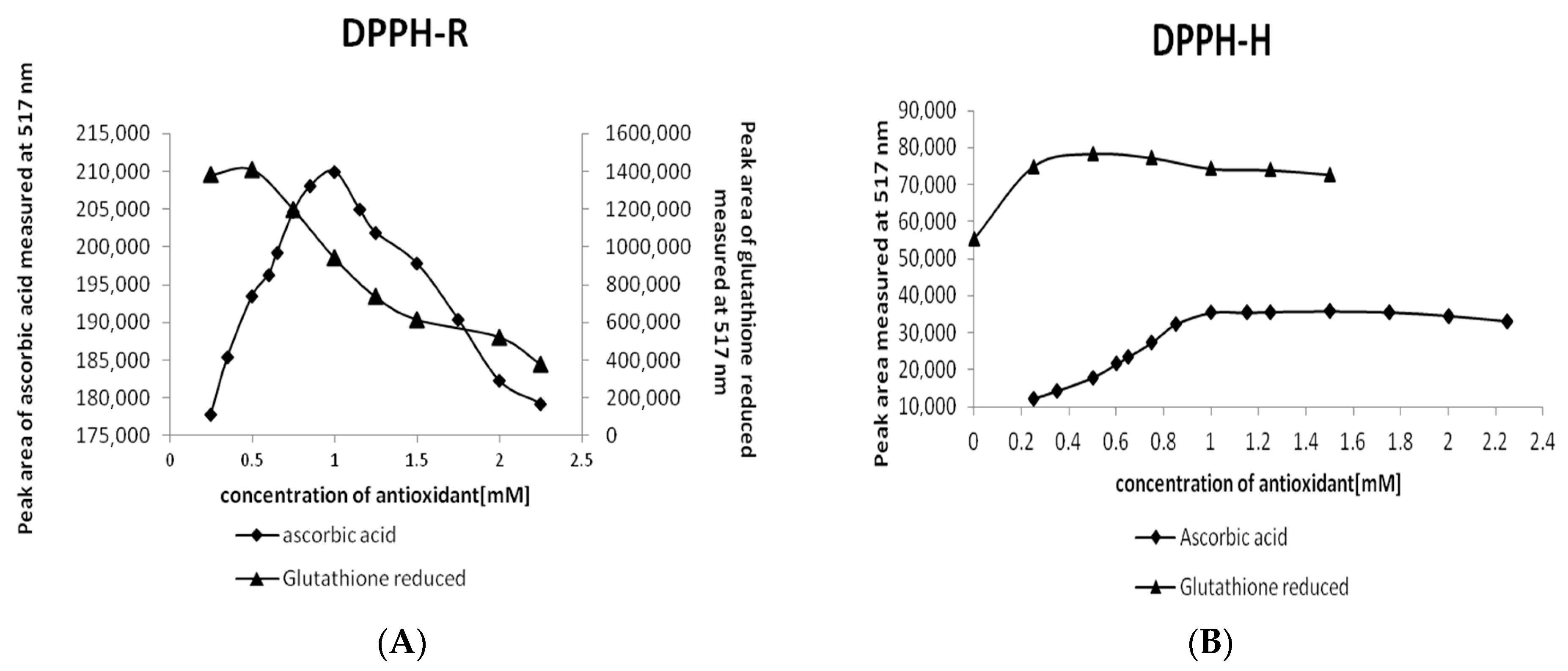
2.2.2. Optimization of the AO Concentration
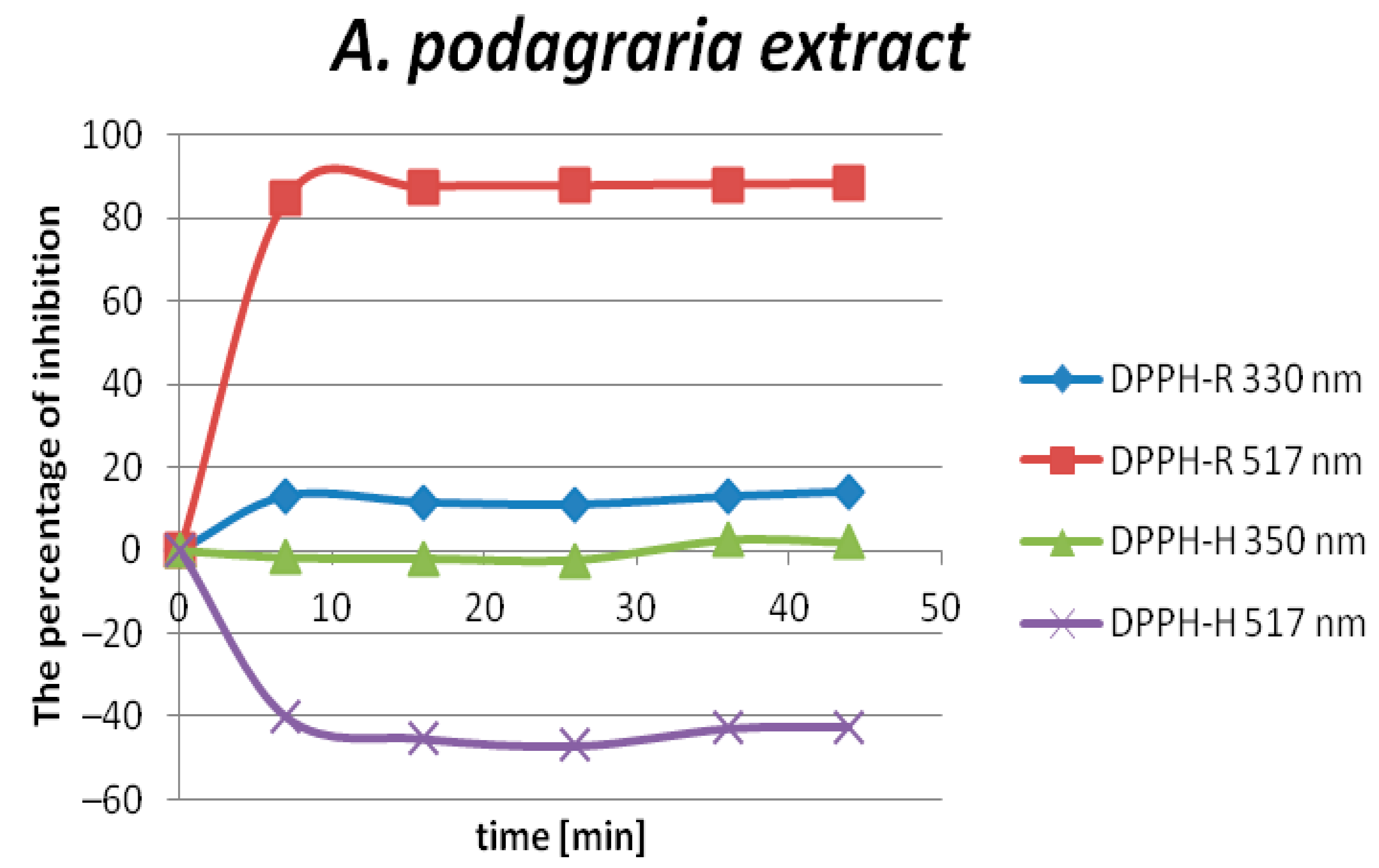
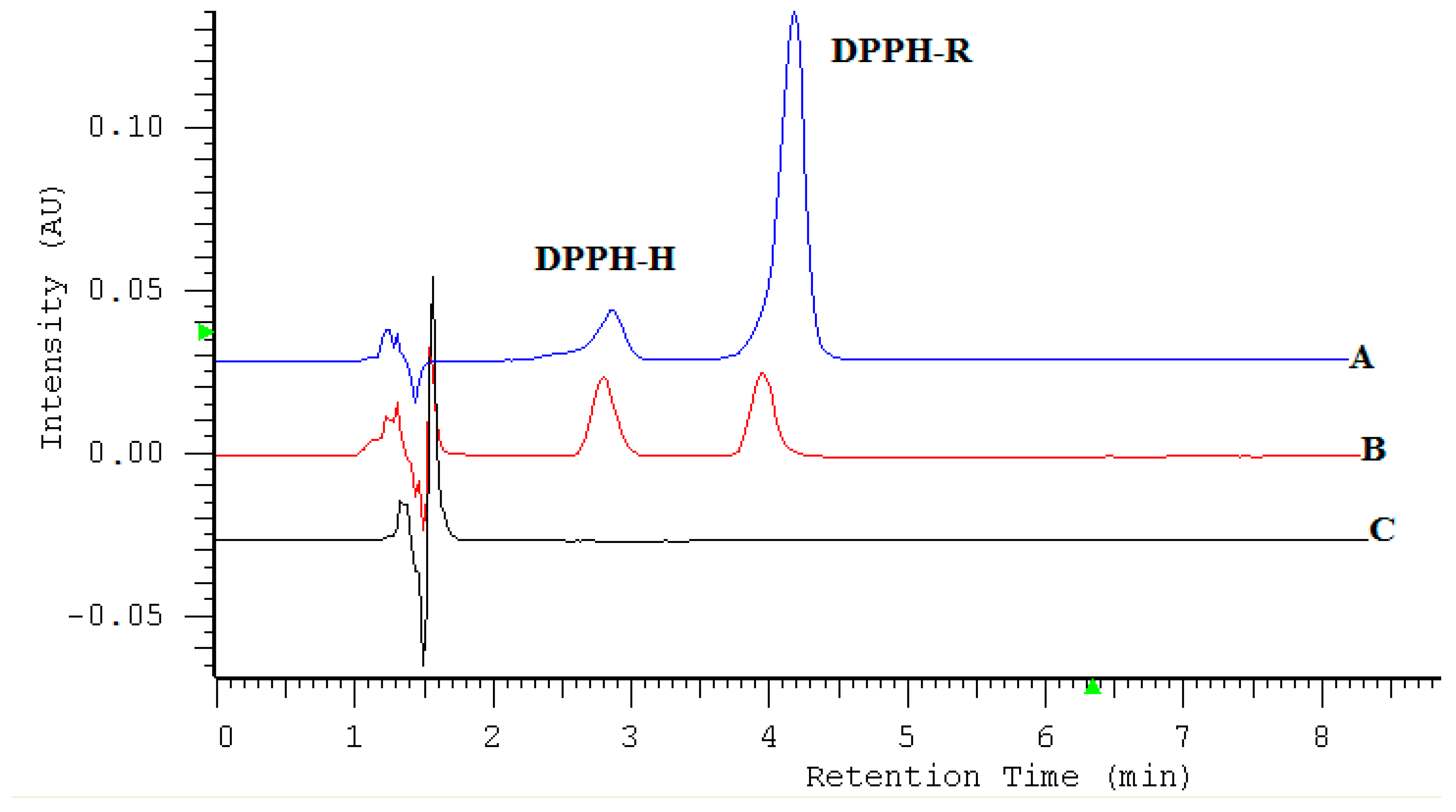
2.3. The Determination of the Antioxidant Capacity of Aegopodium podagraria L. Extracts by DPPH-HPLC-DAD
3. Materials and Methods
3.1. Chemicals
3.2. Collection of the Plant Material and Sample Preparation
3.3. Determination of DPPH Radical Scavenging Activity
3.4. HPLC Measurement
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dham, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease. Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Squiban, A.B. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Cao, X.; Liu, Y. Screen for Potential Candidate Alternatives of Sargentodoxa cuneata from Its Six Adulterants Based on Their Phenolic Compositions and Antioxidant Activities. Int. J. Mol. Sci. 2019, 20, 5427. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisnero-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Goldschmidt, S.; Renn, K. Zweiwertiger Stickstoff: Über das α, α-Diphenyl-β-trinitrophenyl-hydrazyl. (IV. Mitteilung über Amin-Oxydation). Chem. Ber. 1922, 55, 628–643. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH• assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Osman, A.M. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) with (+)-catechin: Evidence for the formation of a covalent adduct between (DPPH•) and the oxidized form of the polyphenol. Biochem. Biophys. Res. Commun. 2011, 412, 473–478. [Google Scholar] [CrossRef]
- Passari, A.K.; Leo, V.V.; Singh, G.; Samanta, L.; Ram, H.; Siddaiah, C.N.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; Fathi Abd_Allah, E.; et al. In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties. Int. J. Mol. Sci. 2020, 21, 7364. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Gois Ruivo da Silva, M.; Skrt, M.; Komes, D.; Poklar Ulrih, N.; Pogačnik, L. Enhanced Yield of Bioactivities from Onion (Allium cepa L.) Skin and Their Antioxidant and Anti-α-Amylase Activities. Int. J. Mol. Sci. 2020, 21, 2909. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. Scavenging of dpph• Radicals by Vitamin E Is Accelerated by Its Partial Ionization: the Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2005, 7, 4951–4954. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Mackie, I.D.; DiLabio, G.A.; Ingold, K.U. Reaction of Phenols with the 2,2-Diphenyl-1-picrylhydrazyl Radical. Kinetics and DFT Calculations Applied to Determine ArO-H Bond Dissociation Enthalpies and Reaction Mechanism. J. Org. Chem. 2008, 73, 9270–9282. [Google Scholar] [CrossRef] [PubMed]
- Liwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstractions. 1. The Reactions of Phenols with 2,2-Diphenyl-1-picrylhydrazyl (dpph•) in Alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH.Free Radical Method. Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated beta-cyclodextrin as the solubility enhancer. J Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M. Developing a rational basis for selection of antioxidant screening and testing methods. Acta Hortic. 2006, 709, 79–94. [Google Scholar] [CrossRef]
- Viirlaid, S.; Mahlapuu, R.; Kilk, K.; Kuznetsov, A.; Soomets, U.; Jarv, J. Mechanism and stoichiometry of 2,2-diphenyl-1-picrylhydrazyl radical scavenging by glutathione and its novel α-glutamyl derivative. Bioorg. Chem. 2009, 37, 126–132. [Google Scholar] [CrossRef]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC Method for Evaluation of the Free Radical-scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Boudier, A.; Tournebize, J.; Bartosz, G.; El Hani, S.; Bengueddour, R.; Sapin-Minet, A.; Leroy, P. High performance liquid chromatographic method to evaluate the hydrogen atom transfer during reaction between 1,1-diphenyl-2-picryl-hydrazyl radical and antioxidants. Anal. Chim. Acta 2012, 711, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, X.; Netrusov, A.I.; Zhou, Q.; Guo, D.; Liu, X.; He, H.; Xin, X.; Wang, Y.; Chen, L. Screening and Identifying Antioxidative Components in Ginkgo biloba Pollen by DPPH-HPLC-PAD Coupled with HPLC-ESI-MS2. PloS ONE 2017, 17, e0170141. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Janda, K.; Styburski, D.; Łukomska, A. Goutweed (Aegopodium podagraria L.)-botanical characteristics and prohealthy properties. Postepy Hig. Med. Dosw. 2020, 74, 28–35. [Google Scholar] [CrossRef]
- Priori, N.M.; Lundgaard, N.H.; Light, M.E.; Stafford, G.I.; van Staden, J.; Jäger, A.K. The polyacetylene falcarindiol with COX-1 activity isolated from Aegopodium podagraria L. J. Ethnopharmacol. 2007, 113, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Orav, A.; Viitak, A.; Vaher, M. Identification of bioactive compounds in the leaves and stems of Aegopodium podagraria by various analytical techniques. Proc. Chem. 2010, 2, 152–160. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Paramonov, E.A.; Khalilova, A.Z.; Odinokov, V.N.; Khalilov, L.M. Identification and biological activity of volatile organic compounds isolated from plants and insects. III. Chromatography-mass spectrometry of volatile compounds of Aegopodium podagraria. Chem. Nat. Comp. 2000, 36, 584–586. [Google Scholar] [CrossRef]
- Kapetanos, C.; Karioti, A.; Bojović, S.; Marin, P.; Veljić, M.; Skaltsa, H. Chemical and principal-component analyses of the essential oils of Apioideae Taxa (Apiaceae) from Central Balkan. Chem. Biodivers. 2008, 5, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kwiatkowski, P.; Sienkiewicz, M.; Janda, K. The content of polyphenols in extract from goutweed (Aegopodium podagraria L.) and their antistaphylococcal activity. Post. Fitoter 2018, 1, 3–9. [Google Scholar] [CrossRef]
- Augspole, I.; Duma, M.; Ozola, B.; Cinkmanis, I. Phenolic profile of fresh and frozen nettle, goutweed, dandelion and chickweed leaves. In Proceedings of the Food Science and Technology in a Changing World, 11th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 27–28 April 2017. [Google Scholar]
- Pogozhikh, N.I.; Tovchiga, O.V.; Evlash, V.V.; Stepanova, S.I.; Koyro, O.O. Substantiation of the Rational Drying Conditions for the Herbal Raw Material of Goutweed (Aegopodium podagraria L.) Aerial Part. South Asian Res. J. Nat. Prod. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Corp, N.; Pendry, B. The role of Western herbal medicine in the treatment of gout. J. Herb. Med. 2013, 3, 157–170. [Google Scholar] [CrossRef]
- Tovchiga, O.V. Interaction of Aegopodium podagraria L. (goutweed) preparations with central nervous system depressants. Ukr. Biopharmac. J. 2016, 1, 31–36. [Google Scholar] [CrossRef]
- Valyova, M.; Tashev, A.; Stoyanov, S.; Yordanova, S.; Ganeva, Y. In vitro free-radical scavenging activity of Aegopodium podagraria L. and Orlaya grandifl ora (L.) Hoffm. (Apiaceae). J. Chem. Technol. Metall. 2016, 51, 271–274. [Google Scholar]
- Wróblewska, A.; Janda, K.; Makuch, E.; Walasek, M.; Miądlicki, P.; Jakubczyk, K. Effect of extraction method on the antioxidative activity of ground elder (Aegopodium podagraria L.). Pol. J. Chem. Technol. 2019, 21, 13–18. [Google Scholar] [CrossRef]
- Dębia, K.; Janda, K.; Siwiec, E.; Wolska, J.; Baranowska-Bosiacka, I.; Jakubczyk, K.; Chlubek, D.; Gutowska, I. Do brewing temperature and the morphological part of the ground elder plant have an influence on the fluoride content of ground elder infusions? Fluoride 2018, 51, 153–163. [Google Scholar]
- Stefanovic, O.; Comic, L.; Stanojevic, D.; Čomić, L. Antibacterial activity of Aegopodium podagraria L. extracts and interaction between extracts and antibiotics. Turk. J. Biol. 2009, 33, 145–150. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Tovchiga, O.V. Metabolic Effects of Goutweed (Aegopodium podagraria L.) Tincture and Metformin in Dexamethasone-Treated Rats. J. Dis. Med. Plants 2016, 2, 117–126. [Google Scholar] [CrossRef]
- Tovchiga, O.V. The influence of goutweed (Aegopodium podagraria L.) preparations on the metabolic processes in alloxan-induced diabetic mice. Pharmacol. Med. Toxicol. 2012, 5, 73–78. [Google Scholar]
- Tovchiga, O.; Shtrygol, S. The influence of Aegopodium podagraria L. extract and tincture, on behavioural reactions of random-bred mice. J. Chem. Pharm. Res. 2015, 7, 370–384. [Google Scholar]
- Tovchiga, O.V. The influence of goutweed (Aegopodium podagraria L.) tincture and metformin on the carbohydrate and lipid metabolism in dexamethasone-treated rats. BMC Complement. Altern. Med. 2016, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Tovchiga, O.V.; Shtrygol, S.Y.; Taran, A.V.; Yudkevich, T.K. The renal effects of the goutweed (Aegopodium podagraria L.) tincture and metformin in dexamethasone-treated rats. Clin. Pharm. 2016, 20, 39–45. [Google Scholar] [CrossRef]
- Tovchiga, O.V. The effects of goutweed (Aegopodium podagraria L.) preparations on glycemia in intact rats and against the background of metformin. News Pharm. 2017, 2, 54–62. [Google Scholar] [CrossRef]
- Tovchiga, O.V.; Shtrygol, S.Y. The effect of medicines with goutweed (Aegopodium podagraria L.) on the physical endurance, cognitive functions and the level of depression in animals. News Pharm. 2016, 1, 71–76. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Šircelj, H.; Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Slatnar, A. Lipophilic antioxidants in edible weeds from agricultural areas. Turk. J. Agric. For. 2018, 42, 1–10. [Google Scholar] [CrossRef]
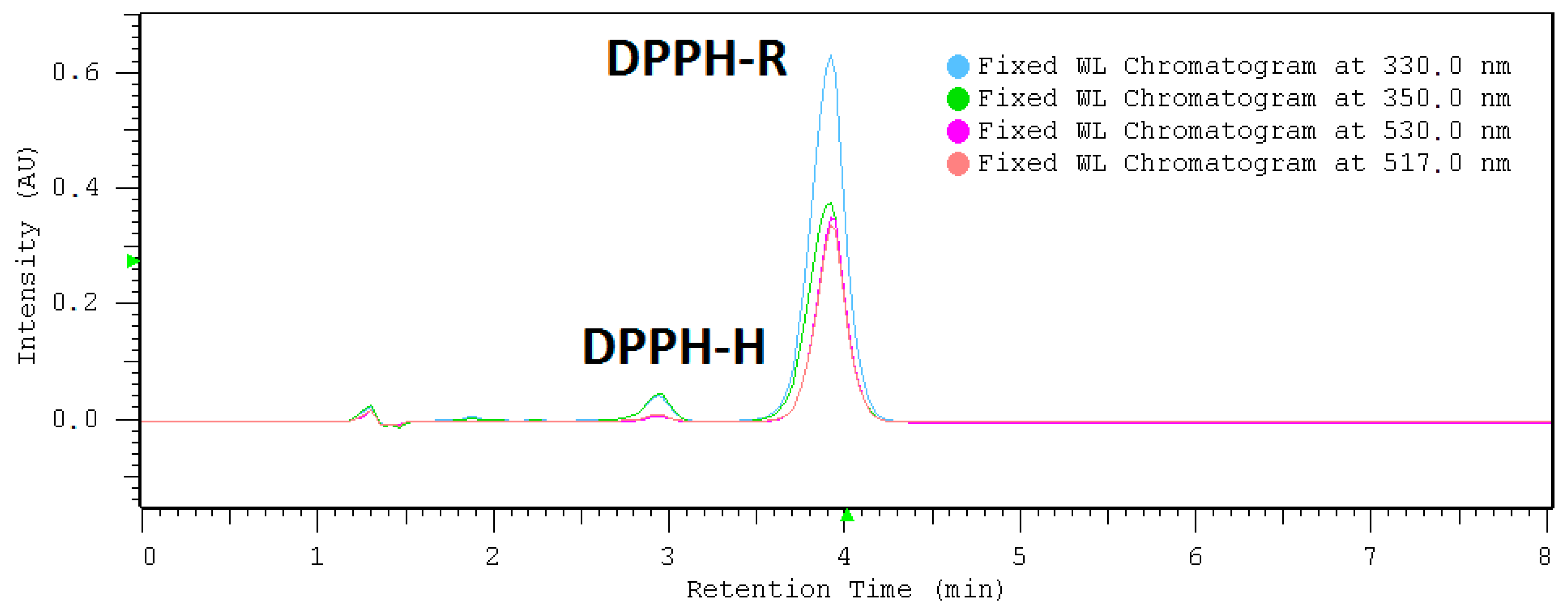
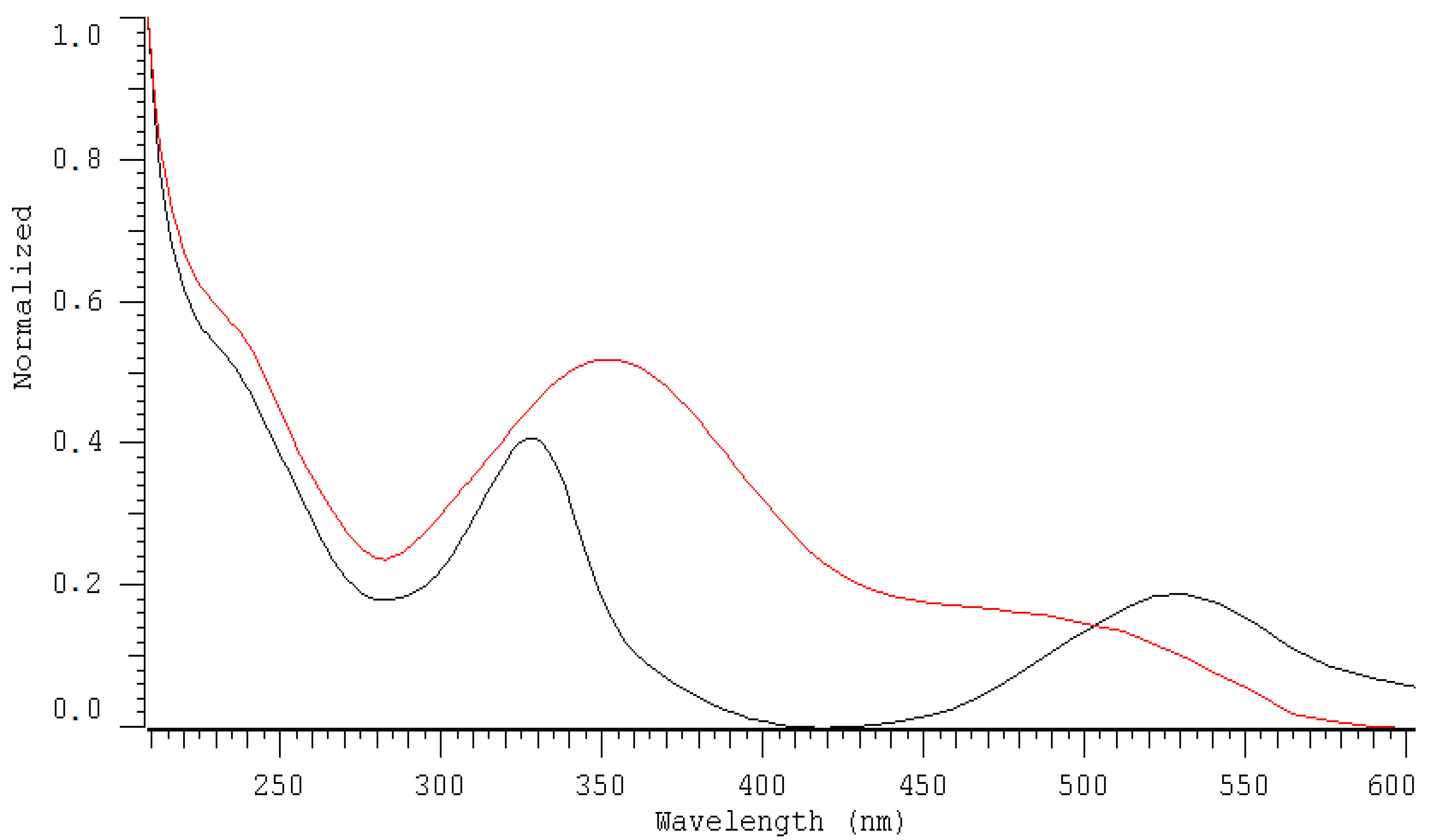
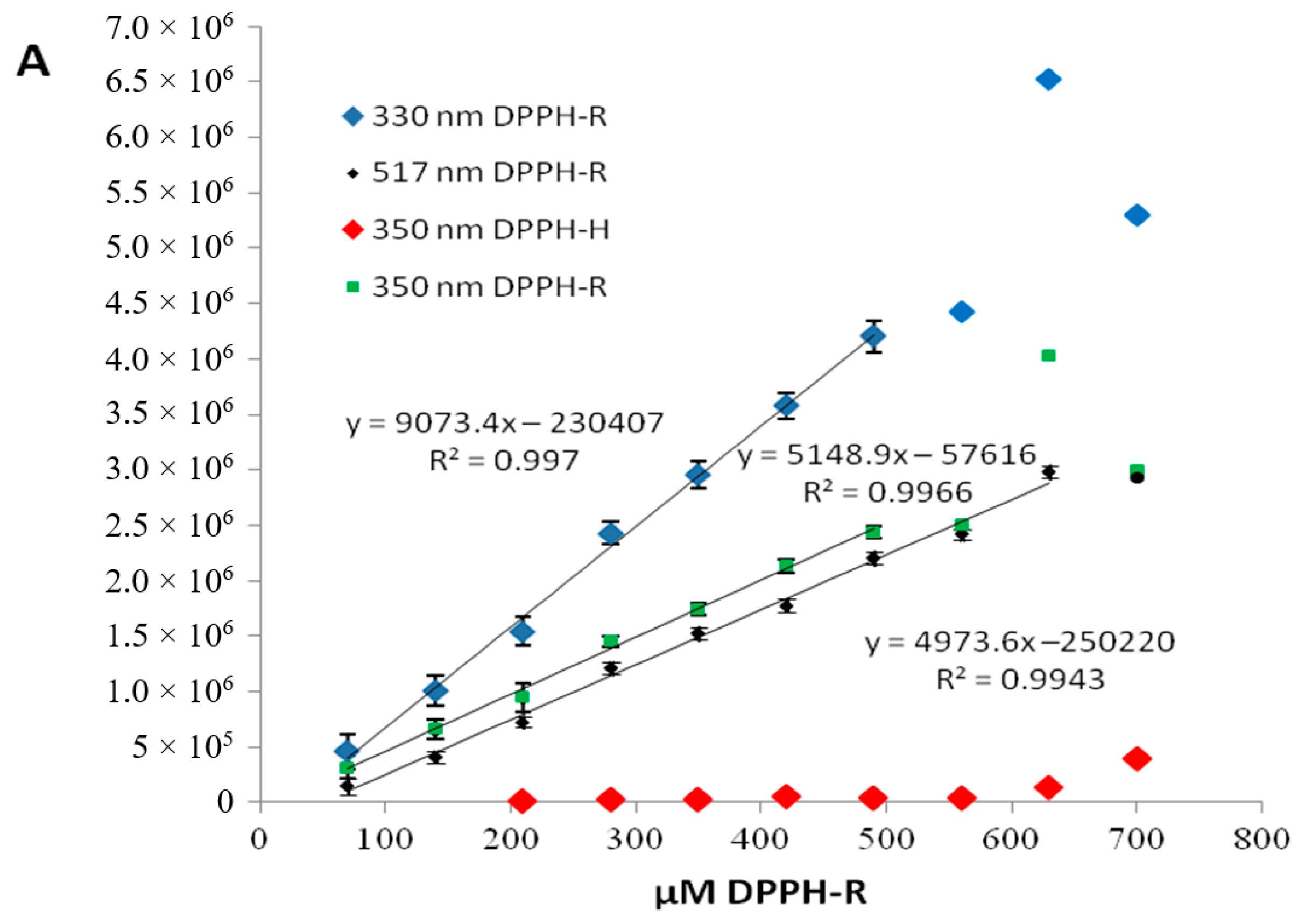
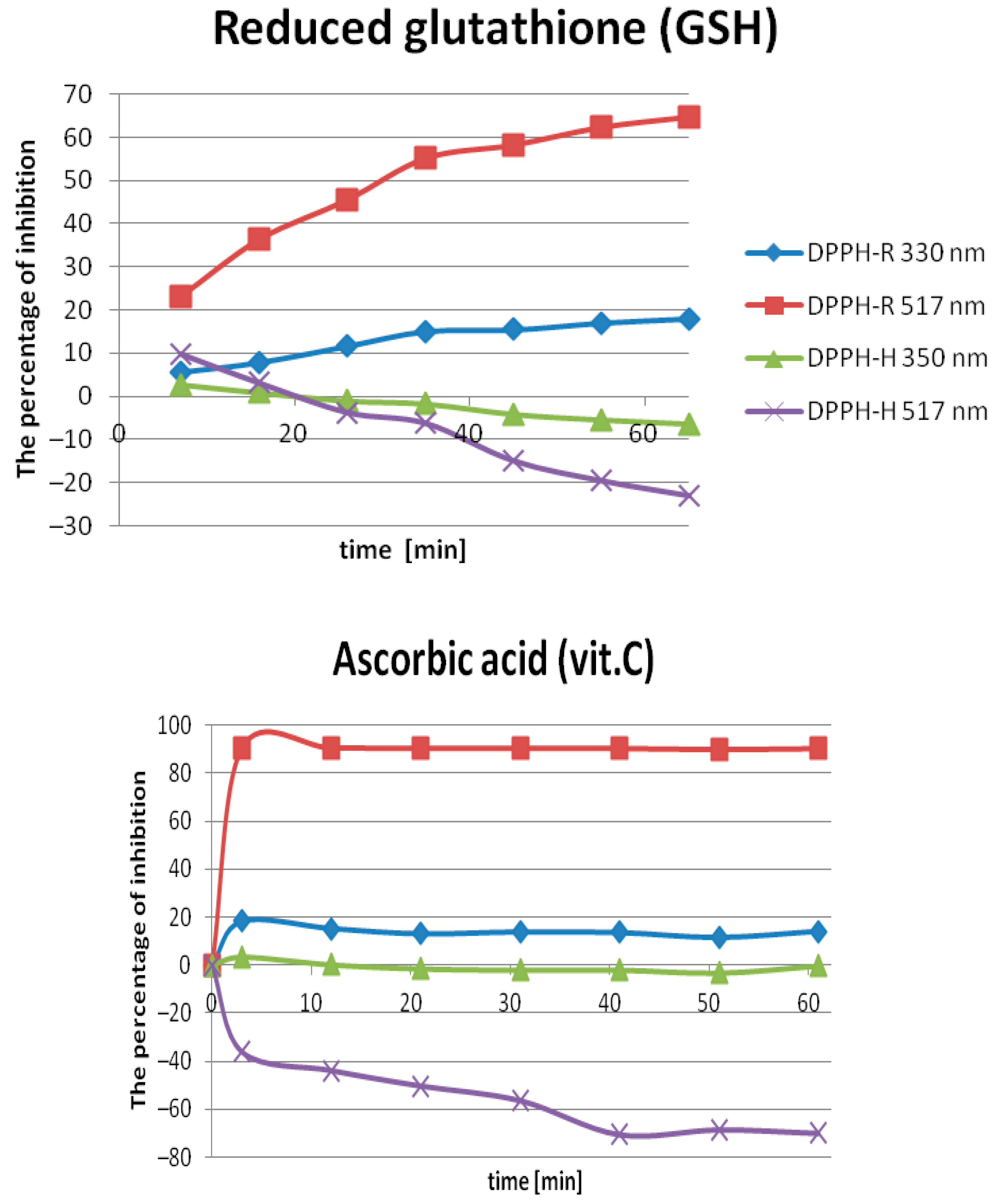
| DAD nm | LOD µmol L−1 | LOQ µmol L−1 | Linearity Range: µmol L−1 | Linearity Equation: (y = ax + b) * | ||||
|---|---|---|---|---|---|---|---|---|
| a ± SD | b ± SD | R2 | F | se | ||||
| The detection conditions of DPPH-R in MeOH | ||||||||
| 330 350 517 | 25.6 26.9 37.2 | 77.4 81.5 112.7 | 70–490 70–490 70–630 | 9073.44 ± 224.42 5148.86 ± 134.11 4973.57 ± 142.24 | −230,407.43 ± 70,255.84 −57,615.57 ± 41,983.95 −250,219.75 ± 56,030.77 | 0.997 0.997 0.994 | 1634.58 1473.95 1222.59 | 83,127.83 49,676.07 77,126.04 |
| The detection conditions of DPPH-R in presence of AO ** | ||||||||
| 330 517 | 6.4 9.5 | 19.3 28.9 | 3.5–315 17.5–315 | 5,700,012.9 ± 69,506.8 301,066.5 ± 4882.4 | −4386.17 ± 10,996.12 −953.57 ± 871.17 | 0.992 0.998 | 6725.07 3802.36 | 27,621.50 1630.22 |
| The detection conditions of DPPH-H in presence of AO ** | ||||||||
| 350 517 | 6.4 19.2 | 19.5 58.1 | 7–315 28–315 | 501,769.22 ± 5957.58 122,833.91 ± 3820.14 | 88.77 ± 978.05 −926.77 ± 714.59 | 0.999 0.992 | 2260.64 1033.89 | 7093.61 1164.66 |
| Detection Wavelength | 330 nm | 350 nm | 517 nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH Conc. | k | As | N(EUP) | k | As | N(EUP) | k | As | N(EUP) |
| 70 µM | 3.31 | 0.76 | 1411 | 3.29 | 0.8 | 1174 | 3.31 | 0.79 | 3590 |
| 140 µM | 2.49 | 0.81 | 1278 | 2.49 | 0.8 | 1010 | 2.49 | 0.88 | 2014 |
| 210 µM | 2.05 | 0.88 | 2429 | 2.05 | 0.86 | 2043 | 2.05 | 0.95 | 3188 |
| 280 µM | 2.05 | 0.89 | 2435 | 2.05 | 0.87 | 2080 | 2.07 | 0.87 | 3062 |
| 350 µM | 2.05 | 0.85 | 2493 | 2.05 | 0.84 | 2118 | 2.05 | 0.91 | 3051 |
| 420 µM | 2.05 | 0.85 | 2409 | 2.05 | 0.84 | 1927 | 2.05 | 0.94 | 3220 |
| 490 µM | 2.05 | 0.86 | 2450 | 2.05 | 0,85 | 2039 | 2.05 | 0.94 | 3044 |
| 560 µM | 2.05 | 0.91 | 2671 | 2.05 | 0.89 | 2348 | 2.05 | 1.00 | 3111 |
| 630 µM | 2.03 | 0.87 | 1710 | 2.03 | 0.85 | 1350 | 2.05 | 0.90 | 3000 |
| 700 µM | 2.03 | 0.91 | 2532 | 2.02 | 0.89 | 2217 | 2.05 | 0.88 | 3051 |
| AOs | Linearity Range mmol L−1 | Linearity Equation: (y = ax + b) | ||||
|---|---|---|---|---|---|---|
| a ± SD | b ± SD | R2 | F | se | ||
| Ascorbic acid Reduced glutathione | 1.0–2.25 0.25–1.5 | −25,006.8 ± 1153.1 −731,164.8 ± 63277.0 | 234,157.8 ± 1862.3 1,544,272.5 ± 61,607.2 | 0.9895 0.9709 | 470.30 133.52 | 1308.17 66,176.70 |
| The Antioxidant Capacity | Extract I | Extract II | Extract III |
|---|---|---|---|
| Sample conc. | 100 µL/mL | 100 µL/mL | 100 µL/mL |
| DPPH-R conc. | 350 µM | 350 µM | 350 µM |
| The peak inhibition * | 29.69% | 33.38% | 77.24% |
| IC50 ** ± RSD % | 336.81 ± 0.61 µL/mL | 149,79 ± 0.31 µL/mL | 64.74 ± 0.22 µL/mL |
| Equivalent conc. of ascorbic acid | 0.49 mM (86.30 µg/mL) | 0.55 mM (96.87 µg/mL) | 1.27 mM (223.68 µg/mL) |
Sample Availability: Samples of the A.podagraria L. extracts are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. https://doi.org/10.3390/molecules25246005
Flieger J, Flieger M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules. 2020; 25(24):6005. https://doi.org/10.3390/molecules25246005
Chicago/Turabian StyleFlieger, Jolanta, and Michał Flieger. 2020. "The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts" Molecules 25, no. 24: 6005. https://doi.org/10.3390/molecules25246005
APA StyleFlieger, J., & Flieger, M. (2020). The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules, 25(24), 6005. https://doi.org/10.3390/molecules25246005







