Bio-Catalysis in Multicomponent Reactions
Abstract
1. Introduction
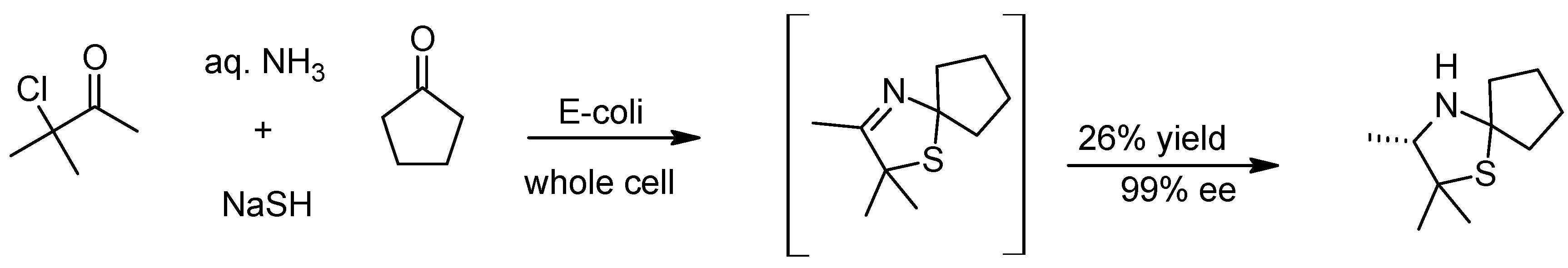
2. The Asinger Reaction
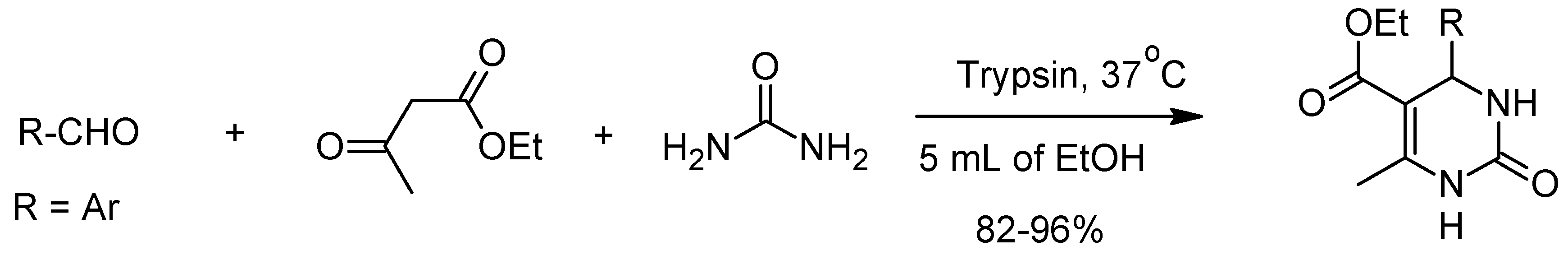
3. The Biginelli Reaction
4. The Hantzsch Reaction
5. The Strecker Reaction
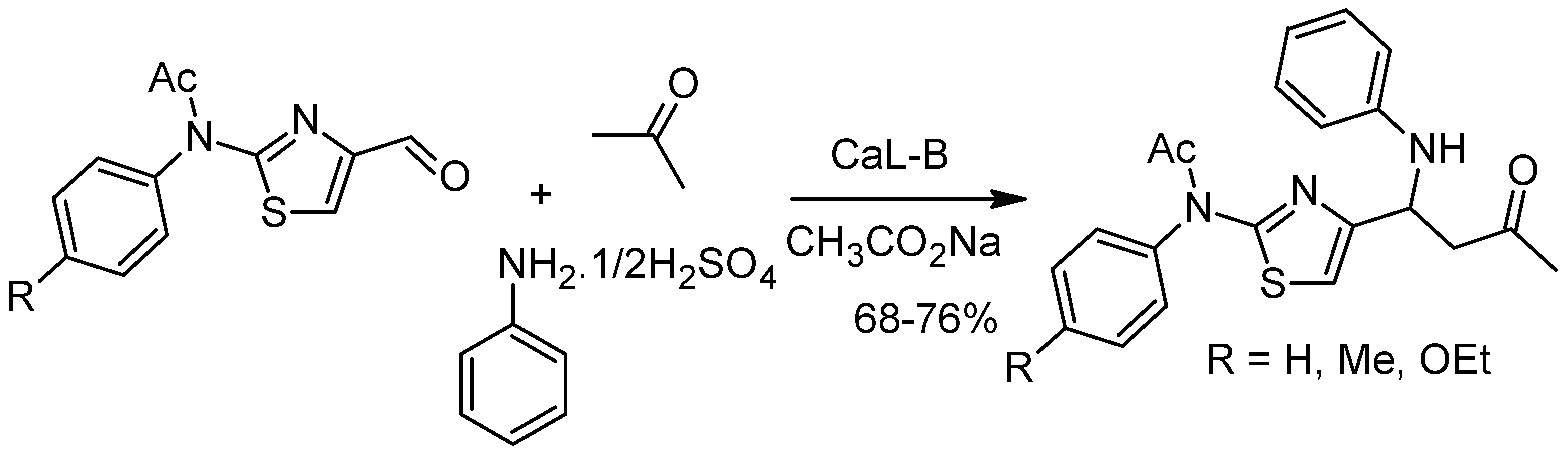
6. The Mannich Reaction
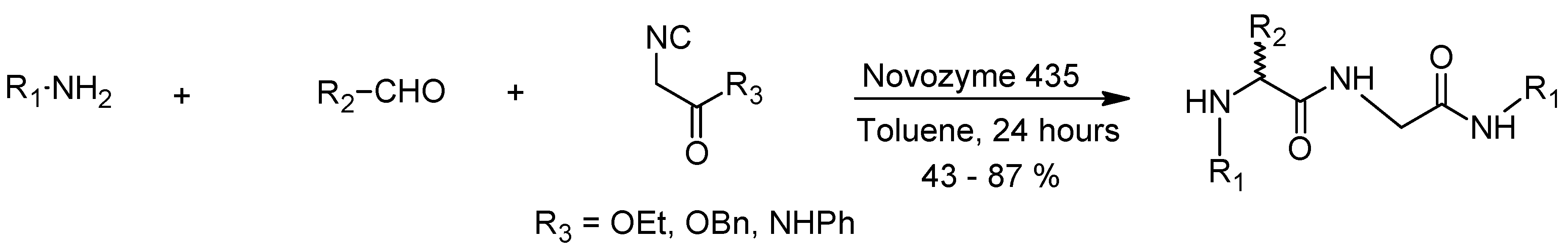
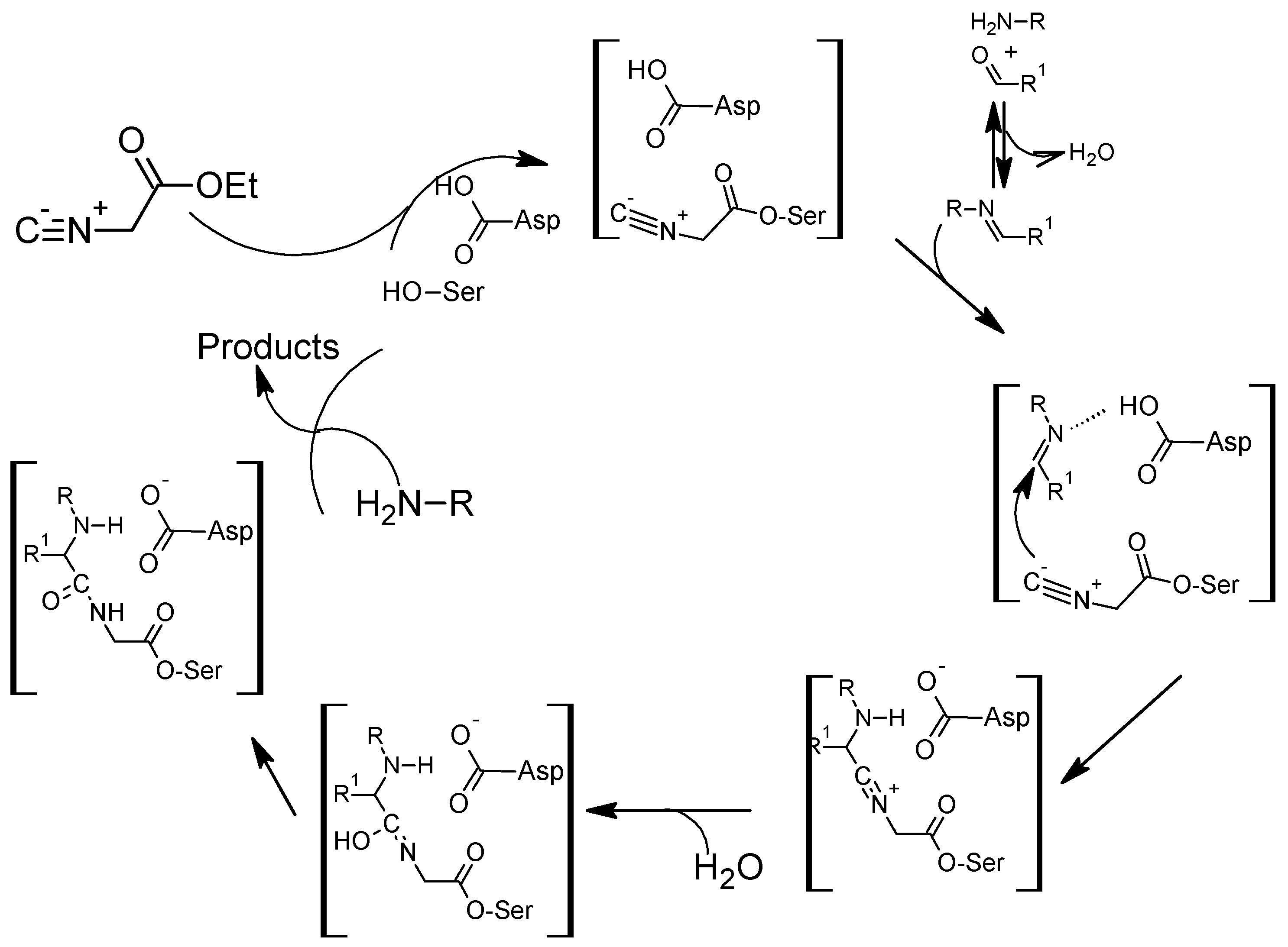
7. The Ugi Reaction
8. Multicomponent No Named Reactions
8.1. Three-Component Reactions
8.2. Four-Component Reactions
8.3. Five-Component Reactions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, W.; Verrier, C.; Wang, L.; Ahmar, M.; Tan, J.-N.; Popowycz, F.; Queneau, Y. 5-(Hydroxymethyl) furfural and 5-(glucosyloxymethyl) furfural in multicomponent reactions. In Recent Trends in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–100. [Google Scholar] [CrossRef]
- Nouali, F.; Kibou, Z.; Boukoussa, B.; Choukchou-Braham, N.; Bengueddach, A.; Villemin, D.; Hamacha, R. Efficient multicomponent synthesis of 2-aminopyridines catalysed by basic mesoporous materials. Res. Chem. Intermed. 2020, 46, 3179–3191. [Google Scholar] [CrossRef]
- Wu, P.; Givskov, M.; Nielsen, T.E. Reactivity and synthetic applications of multicomponent petasis reactions. Chem. Rev. 2019, 119, 11245–11290. [Google Scholar] [CrossRef]
- Santra, S. Baker’s yeast catalyzed multicomponent reactions: A new hope? ChemistrySelect 2019, 4, 12630–12637. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, X.; Zhang, Z.; Song, P.; Li, Y. Trypsin-catalyzed multicomponent reaction: A novel and efficient one-pot synthesis of thiazole-2-imine derivatives. J. Biotechnol. 2017, 241, 14–21. [Google Scholar] [CrossRef]
- Lamberth, C. Multicomponent reactions in crop protection chemistry. Biorg. Med. Chem. 2020, 115471. [Google Scholar] [CrossRef]
- Strecker, A. Ueber einen neuen aus Aldehyd-Ammoniak und Blausäure entstehenden Körper. Justus Liebigs Ann. Der Chem. 1854, 91, 349–351. (In German) [Google Scholar] [CrossRef]
- Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Liebigs Ann. Der Chem. 1850, 75, 27–45. (In German) [Google Scholar] [CrossRef]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef]
- Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly efficient synthesis of substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) catalyzed by Hf(OTf)4: Mechanistic insights into reaction pathways under metal lewis acid catalysis and solvent-free conditions. Molecules 2019, 24, 364. [Google Scholar] [CrossRef]
- Dige, N.C.; Mahajan, P.G.; Raza, H.; Hassan, M.; Vanjare, B.D.; Hong, H.; Lee, K.H.; latip, J.; Seo, S.-Y. Synthesis and characterization of new 4H-chromene-3-carboxylates ensuring potent elastase inhibition activity along with their molecular docking and chemoinformatics properties. Bioorg. Chem. 2020, 100, 103906. [Google Scholar] [CrossRef]
- Ali, M.; Khan, K.M.; Mahdavi, M.; Jabbar, A.; Shamim, S.; Salar, U.; Taha, M.; Perveen, S.; Larijani, B.; Faramarzi, M.A. Synthesis, in vitro and in silico screening of 2-amino-4-aryl-6-(phenylthio) pyridine-3,5-dicarbonitriles as novel α-glucosidase inhibitors. Bioorg. Chem. 2020, 100, 103879. [Google Scholar] [CrossRef]
- Sellitepe, H.E.; Doğan, İ.S.; Eroğlu, G.; Barut, B.; Özel, A. Synthesis, characterization and investigation of cholinesterase enzyme inhibition and antioxidant activities of some 4-aryl-1,4-dihydropyridine derivatives. Marmara Pharm. J. 2019, 23. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Biocatalysis—Key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef]
- López-Iglesias, M.; Gotor-Fernández, V. Recent advances in biocatalytic promiscuity: Hydrolase-catalyzed reactions for nonconventional transformations. Chem. Rec. 2015, 15, 743–759. [Google Scholar] [CrossRef]
- Forti, L.; Cramarossa, M.R.; Filippucci, S.; Tasselli, G.; Turchetti, B.; Buzzini, P. Chapter 6-Nonconventional yeast-promoted biotransformation for the production of flavor compounds. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 165–187. [Google Scholar] [CrossRef]
- Sulman, E.M.; Matveeva, V.G.; Bronstein, L.M. Design of biocatalysts for efficient catalytic processes. Curr. Opin. Chem. Eng. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Waldvogel, S.R. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zarganes-Tzitzikas, T.; Chandgude, A.L.; Dömling, A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 2015, 15, 981–996. [Google Scholar] [CrossRef]
- Asinger, F. Über eine einfache Synthese von Thiazolinen (3,4), Vortrag GDCH–Jahrestagung. Angew. Chem. 1956, 68, 377. (In German) [Google Scholar]
- Asinger, F.; Thiel, M. Einfache synthesen und chemisches verhalten neuer heterocyclischer ringsysteme. Angew. Chem. 1958, 70, 667–683. [Google Scholar] [CrossRef]
- Schlemminger, I.; Janknecht, H.-H.; Maison, W.; Saak, W.; Martens, J. Synthesis of the first enantiomerically pure 3-thiazolines via Asinger reaction. Tetrahedron Lett. 2000, 41, 7289–7292. [Google Scholar] [CrossRef]
- Elander, R. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Liu, Z.-Q. Two Neglected multicomponent reactions: Asinger and Groebke reaction for constructing thiazolines and imidazolines. Curr. Org. Synth. 2015, 12, 20–60. [Google Scholar] [CrossRef]
- Weigert, W.M.; Offermanns, H.; Degussa, P.S. d-Penicillamine—Production and properties. Angew. Chem. Int. Ed. Engl. 1975, 14, 330–336. [Google Scholar] [CrossRef]
- Nakatani, S.; Hidaka, K.; Ami, E.I.; Nakahara, K.; Sato, A.; Nguyen, J.-T.; Hamada, Y.; Hori, Y.; Ohnishi, N.; Nagai, A. Combination of non-natural d-amino acid derivatives and allophenylnorstatine-dimethylthioproline scaffold in HIV protease inhibitors have high efficacy in mutant HIV. J. Med. Chem. 2008, 51, 2992–3004. [Google Scholar] [CrossRef]
- Zumbrägel, N.; Gröger, H. One-pot synthesis of a 3-thiazolidine through combination of an Asinger-type multi-component-condensation reaction with an enzymatic imine reduction. J. Biotechnol. 2019, 291, 35–40. [Google Scholar] [CrossRef]
- Biginelli, C. Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital 1893, 23, 360–413. [Google Scholar]
- Ould M’hamed, M.; Alshammari, A.G.; Lemine, O. Green high-yielding one-pot approach to Biginelli reaction under catalyst-free and solvent-free ball milling conditions. Appl. Sci. 2016, 6, 431. [Google Scholar] [CrossRef]
- Felluga, F.; Benedetti, F.; Berti, F.; Drioli, S.; Regini, G. Efficient Biginelli synthesis of 2-aminodihydropyrimidines under microwave irradiation. Synlett 2018, 29, 1047–1054. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A. Bakers’ yeast catalyzed synthesis of polyhydroquinoline derivatives via an unsymmetrical Hantzsch reaction. Tetrahedron Lett. 2007, 48, 3887–3890. [Google Scholar] [CrossRef]
- Li, W.; Zhou, G.; Zhang, P.; Lai, Y.; Xu, S. One-pot synthesis of dihydropyrimidiones via environmentally friendly enzyme-catalyzed bignelli reaction. Catal. Catal. React. 2011, 83, 2067–2077. [Google Scholar] [CrossRef]
- Wang, L.-M.; Sheng, J.; Zhang, L.; Han, J.-W.; Fan, Z.-Y.; Tian, H.; Qian, C.-T. Facile Yb (OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 2005, 61, 1539–1543. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.-F. Ceric ammonium nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron 2006, 62, 7293–7299. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Damu, G.L.V.; Rao, C.V. An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions. Arkivoc 2006, 2, 201–206. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, N.; Kumar, R.; Sinha, A.K. Biocatalysts for multicomponent Biginelli reaction: Bovine serum albumin triggered waste-free synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Amino Acids 2013, 44, 1031–1037. [Google Scholar] [CrossRef]
- Xie, Z.-B.; Wang, N.; Wu, W.-X.; Le, Z.-G.; Yu, X.-Q. Trypsin-catalyzed tandem reaction: One-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones by in situ formed acetaldehyde. J. Biotechnol. 2014, 170, 1–5. [Google Scholar] [CrossRef]
- Borse, B.; Borude, V.; Shukla, S. Synthesis of novel dihydropyrimidin-2 (1H)-ones derivatives using lipase and their antimicrobial activity. Curr. Chem. Lett. 2012, 1, 59–68. [Google Scholar] [CrossRef]
- Mostafa, A.A.-F.; SathishKumar, C.; Al-Askar, A.A.; Sayed, S.R.; SurendraKumar, R.; Idhayadhulla, A. Synthesis of novel benzopyran-connected pyrimidine and pyrazole derivatives via a green method using Cu (ii)-tyrosinase enzyme catalyst as potential larvicidal, antifeedant activities. RSC Adv. 2019, 9, 25533–25543. [Google Scholar] [CrossRef]
- SathishKumar, C.; Keerthana, S.; Ahamed, A.; Arif, I.A.; SurendraKumar, R.; Idhayadhulla, A. CuII-tyrosinase enzyme catalyst-mediated synthesis of 2-thioxopyrimidine derivatives with potential mosquito larvicidal activity: Spectroscopic and computational investigation as well as molecular docking interaction with OBPs of culex quinquefasciatus. ChemistrySelect 2020, 5, 4567–4574. [Google Scholar] [CrossRef]
- Ruijter, E.; Orru, R.V. Discovery of MCRs. Multicomponent React. Org. Synth. 2015, 13–38. [Google Scholar] [CrossRef]
- Tamaddon, F.; Arab, D.; Ahmadi-AhmadAbadi, E. Urease immobilization on magnetic micro/nano-cellulose dialdehydes: Urease inhibitory of Biginelli product in Hantzsch reaction by urea. Carbohydr. Polym. 2020, 229, 115471. [Google Scholar] [CrossRef]
- Vargas, A.Y.; Rojas, H.A.; Romanelli, G.P.; Martínez, J.J. Synthesis of 1,4-dihydropyrimidines with immobilized urease: Effect of method immobilization on magnetic supports. Green Process. Synth. 2017, 6, 377–384. [Google Scholar] [CrossRef]
- Xie, Z.-B.; Fu, L.-H.; Meng, J.; Lan, J.; Hu, Z.-Y.; Le, Z.-G. Efficient biocatalytic strategy for one-pot Biginelli reaction via enhanced specific effects of microwave in a circulating reactor. Bioorg. Chem. 2020, 101, 103949. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, T.F.; Sidhom, P.A.; Esmat, E.A.; El-Mahdy, N.A. Synthesis, docking simulation, biological evaluations and 3D-QSAR study of 1, 4-dihydropyridines as calcium channel blockers. Chem. Pharm. Bull. 2017, 65, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Allais, C.; Liéby-Muller, F.; Constantieux, T.; Rodriguez, J. Dual heterogeneous catalysis for a regioselective three-component synthesis of Bi- and Tri(hetero) arylpyridines. Adv. Synth. Catal. 2012, 354, 2537–2544. [Google Scholar] [CrossRef]
- Shen, L.; Cao, S.; Wu, J.; Zhang, J.; Li, H.; Liu, N.; Qian, X. A revisit to the Hantzsch reaction: Unexpected products beyond 1,4-dihydropyridines. Green Chem. 2009, 11, 1414–1420. [Google Scholar] [CrossRef]
- Lee, J.H. Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers’ yeast. Tetrahedron Lett. 2005, 46, 7329–7330. [Google Scholar] [CrossRef]
- Bridgwood, K.L.; Veitch, G.E.; Ley, S.V. Magnesium nitride as a convenient source of ammonia: Preparation of dihydropyridines. Org. Lett. 2008, 10, 3627–3629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Liu, B.-K.; Yin, C.; Wu, Q.; Lin, X.-F. Candida antarctica lipase B-catalyzed the unprecedented three-component Hantzsch-type reaction of aldehyde with acetamide and 1,3-dicarbonyl compounds in non-aqueous solvent. Tetrahedron 2011, 67, 2689–2692. [Google Scholar] [CrossRef]
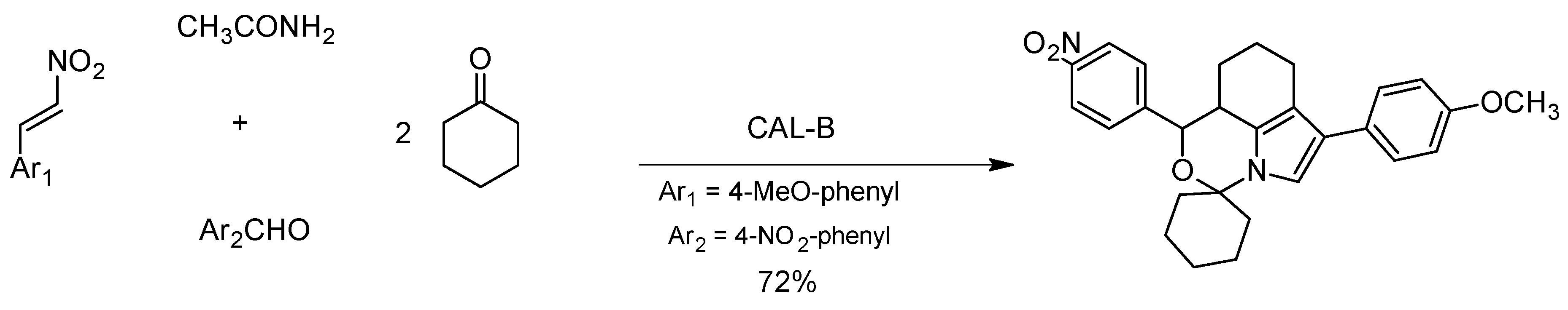
- Liang, Y.-R.; Hu, Y.-J.; Zhou, X.-H.; Wu, Q.; Lin, X.-F. One-pot construction of spirooxindole backbone via biocatalytic domino reaction. Tetrahedron Lett. 2017, 58, 2923–2926. [Google Scholar] [CrossRef]
- Javanshir, S.; Saghiran Pourshiri, N.; Dolatkhah, Z.; Farhadnia, M. Caspian isinglass, a versatile and sustainable biocatalyst for domino synthesis of spirooxindoles and spiroacenaphthylenes in water. Mon. Für Chem. Chem. Mon. 2017, 148, 703–710. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Yang, F.; Guo, C.; Zhao, Z.; Ji, D.; Zhou, F.; Wang, Z.; Zhao, R.; Wang, L. Synthesis of dihydropyrano [4,3-b] pyranes via a multi-component reaction catalyzed by lipase. Green Chem. Lett. Rev. 2017, 10, 54–58. [Google Scholar] [CrossRef]
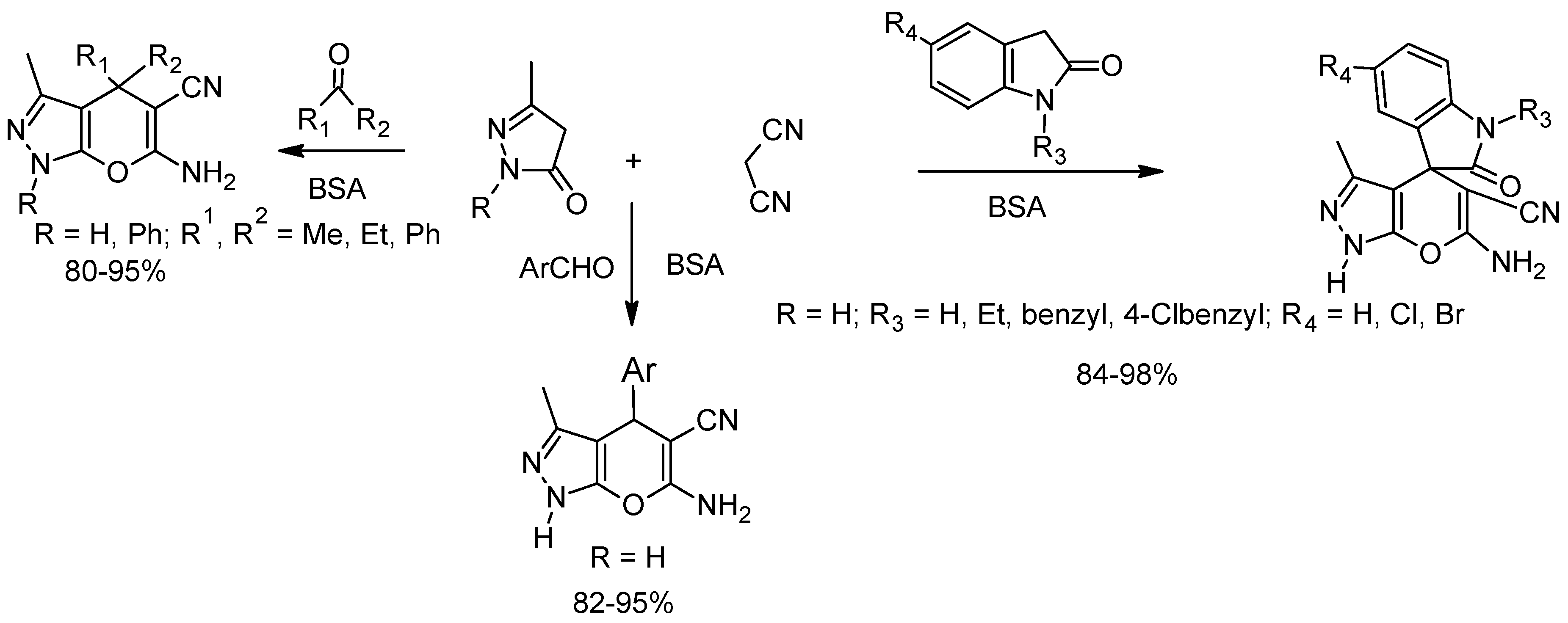
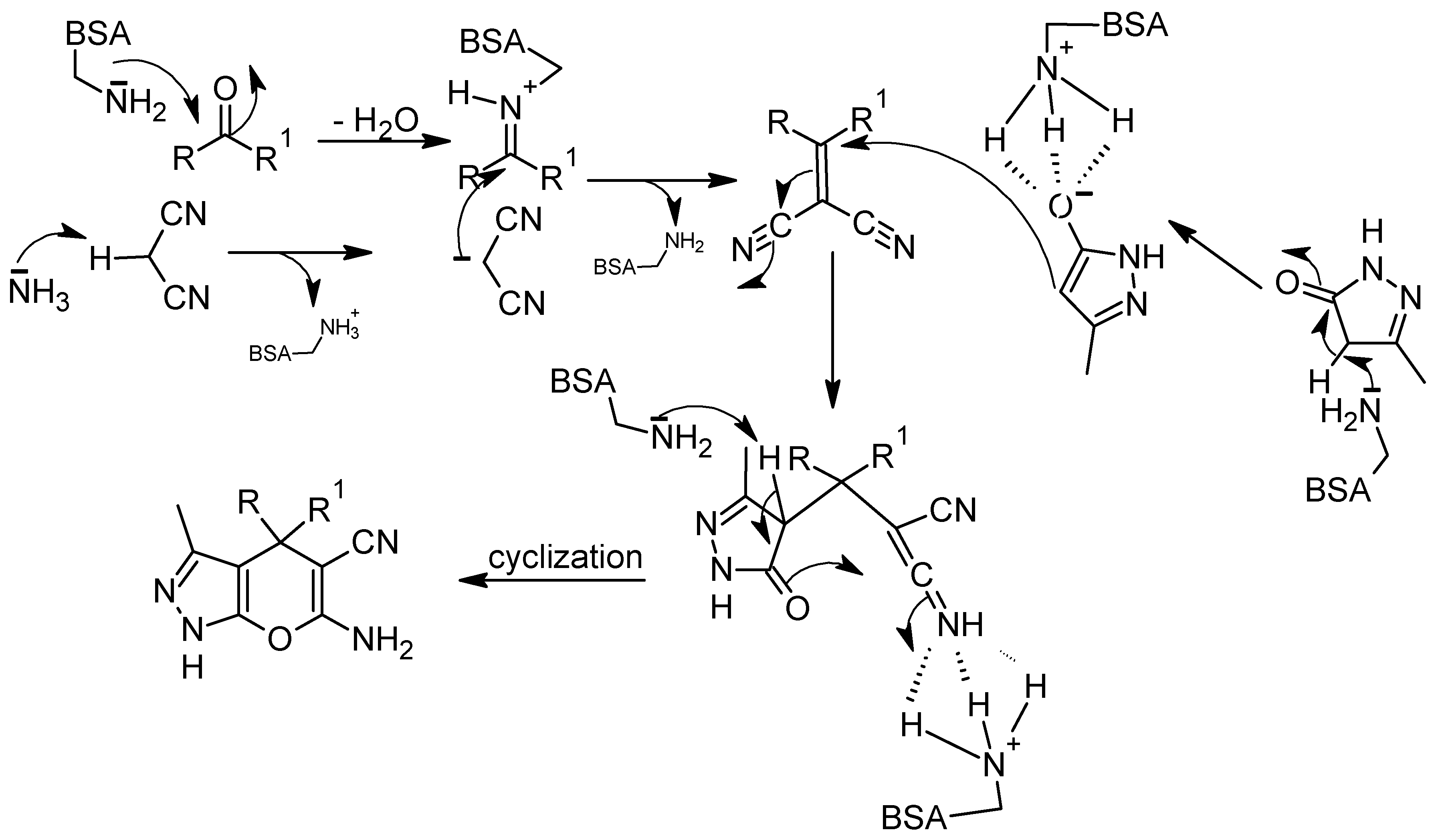
- Yang, Z.-J.; Gong, Q.-T.; Wang, Y.; Yu, Y.; Liu, Y.-H.; Wang, N.; Yu, X.-Q. Biocatalytic tandem multicomponent reactions for one-pot synthesis of 2-Amino-4H-Pyran library and in vitro biological evaluation. Mol. Catal. 2020, 491, 110983. [Google Scholar] [CrossRef]
- Mogharabi-Manzari, M.; Ghahremani, M.H.; Sedaghat, T.; Shayan, F.; Faramarzi, M.A. A Laccase heterogeneous magnetic fibrous silica-based biocatalyst for green and one-pot cascade synthesis of chromene derivatives. Eur. J. Org. Chem. 2019, 2019, 1741–1747. [Google Scholar] [CrossRef]
- Salehi, N.; Mirjalili, B.B.F. Nano-ovalbumin: A green biocatalyst for biomimetic synthesis of tetrahydrodipyrazolo pyridines in water. Res. Chem. Intermed. 2018, 44, 7065–7077. [Google Scholar] [CrossRef]
- Zetzsche, L.; Yazarians, J.; Hinze, M.; Narayan, A. Engineering biocatalysts for selective CC bond formation. In Abstracts of Papers of the American Chemical Society; ACS: Washington, DC, USA, 2019. [Google Scholar]
- Soons, P.A.; Mulders, T.M.T.; Uchida, E.; Schoemaker, H.C.; Cohen, A.F.; Breimer, D.D. Stereoselective pharmacokinetics of oral felodipine and nitrendipine in healthy subjects: Correlation with nifedipine pharmacokinetics. Eur. J. Clin. Pharmacol. 1993, 44, 163–169. [Google Scholar] [CrossRef]
- Valdivia, H.H.; Coronado, R. Internal and external effects of dihydropyridines in the calcium channel of skeletal muscle. J. Gen. Physiol. 1990, 95, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Stoltefuss, J.; Goldmann, S.; Triggle, D.J. Pharmacologic and radioligand binding studies of 1,4-dihydropyridines in rat cardiac and vascular preparations: Stereoselectivity and voltage dependence of antagonist and activator interactions. Mol. Pharmacol. 1992, 41, 535–541. [Google Scholar]
- Vongvilai, P.; Ramström, O. Dynamic asymmetric multicomponent resolution: Lipase-mediated amidation of a double dynamic covalent system. J. Am. Chem. Soc. 2009, 131, 14419–14425. [Google Scholar] [CrossRef]
- Kawahara, N.; Asano, Y. Chemoenzymatic method for enantioselective synthesis of (R)-2-phenylglycine and (R)-2-phenylglycine amide from benzaldehyde and KCN using difference of enzyme affinity to the enantiomers. ChemCatChem 2018, 10, 5014–5020. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Gao, X.; Zeng, S.; Zhang, H.; Liu, J.; Jiao, Q. Dynamic kinetic resolution for asymmetric synthesis of l-Noncanonical amino acids from d-Ser using tryptophan synthase and alanine racemase. Eur. J. Org. Chem. 2019, 2019, 6618–6625. [Google Scholar] [CrossRef]
- Shu, S.; Zhao, L.; Zhou, S.; Wu, C.; Liu, H.; Wang, J. Recyclable and stable α-methylproline-derived chiral ligands for the chemical dynamic kinetic resolution of free C, N-unprotected α-amino acids. Molecules 2019, 24, 2218. [Google Scholar] [CrossRef] [PubMed]
- Mutalikdesai, A.; Nassir, M.; Saady, A.; Hassner, A.; Gedanken, A. Sonochemically modified ovalbumin enhances enantioenrichment of some amino acids. Ultrason. Sonochem. 2019, 58, 104603. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.; Zadsirjan, V. (Eds.) 5-Asymmetric multicomponent reactions. In Recent Advances in Applications of Name Reactions in Multicomponent Reactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–422. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; He, T.; Li, C.; Feng, X.-W.; Wang, N.; Yu, X.-Q. Lipase-catalysed direct Mannich reaction in water: Utilization of biocatalytic promiscuity for C-C bond formation in a “one-pot” synthesis. Green Chem. 2009, 11, 777–779. [Google Scholar] [CrossRef]
- He, T.; Li, K.; Wu, M.-Y.; Feng, X.-W.; Wang, N.; Wang, H.-Y.; Li, C.; Yu, X.-Q. Utilization of biocatalytic promiscuity for direct Mannich reaction. J. Mol. Catal. B Enzym. 2010, 67, 189–194. [Google Scholar] [CrossRef]
- Chai, S.-J.; Lai, Y.-F.; Zheng, H.; Zhang, P.-F. A Novel trypsin-catalyzed three-component mannich reaction. Helv. Chim. Acta 2010, 93, 2231–2236. [Google Scholar] [CrossRef]
- López-Iglesias, M.; Busto, E.; Gotor, V.; Gotor-Fernández, V. Use of protease from bacillus licheniformis as promiscuous catalyst for organic synthesis: Applications in C-C and C-N bond formation reactions. Adv. Synth. Catal. 2011, 353, 2345–2353. [Google Scholar] [CrossRef]
- Xue, Y.; Li, L.-P.; He, Y.-H.; Guan, Z. Protease-catalysed direct asymmetric mannich reaction in organic solvent. Sci. Rep. 2012, 2, 761. [Google Scholar] [CrossRef]
- Guan, Z.; Song, J.; Xue, Y.; Yang, D.-C.; He, Y.-H. Enzyme-catalyzed asymmetric Mannich reaction using acylase from Aspergillus melleus. J. Mol. Catal. B Enzym. 2015, 111, 16–20. [Google Scholar] [CrossRef]
- Denisa, L.; Laszlo Csaba, B.; Paizs, C.; Irimie, F.D.; Zaharia, V. Heterocycles 38. Biocatalytic synthesis of new heterocyclic mannich bases and derivatives. Molecules 2015, 20, 12300–12313. [Google Scholar] [CrossRef]
- Leonte, D.; TŐTŐS, R.; Zaharia, V. Heterocycles 50. Synthesis and characterization of new 2-phenylaminothiazole derived mannich bases by biocatalytic multicomponent reactions. Studia Univ. Babes-Bolyai Chem. 2019, 64. [Google Scholar] [CrossRef]
- Wu, L.-L.; Xiang, Y.; Yang, D.-C.; Guan, Z.; He, Y.-H. Biocatalytic asymmetric Mannich reaction of ketimines using wheat germ lipase. Catal. Sci. Technol. 2016, 6, 3963–3970. [Google Scholar] [CrossRef]
- Ugi, I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Kłossowski, S.; Wiraszka, B.; Berłożecki, S.; Ostaszewski, R. Model studies on the first enzyme-catalyzed Ugi reaction. Org. Lett. 2013, 15, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, Y.; Xu, J.; Tang, D.-Y.; Shao, J.-W.; Li, H.-Y.; Chen, Z.-Z.; Xu, Z.-G. An acid-catalyzed 1,4-addition isocyanide-based multicomponent reaction in neat water. Green Chem. 2020, 22, 3716–3720. [Google Scholar] [CrossRef]
- Żądło-Dobrowolska, A.; Kłossowski, S.; Koszelewski, D.; Paprocki, D.; Ostaszewski, R. Enzymatic Ugi reaction with amines and cyclic imines. Chem. A Eur. J. 2016, 22, 16684–16689. [Google Scholar] [CrossRef]
- Wilk, M.; Brodzka, A.; Koszelewski, D.; Madej, A.; Paprocki, D.; Żądło-Dobrowolska, A.; Ostaszewski, R. The influence of the isocyanoesters structure on the course of enzymatic Ugi reactions. Bioorg. Chem. 2019, 93, 102817. [Google Scholar] [CrossRef]
- Pratap, U.R.; Jawale, D.V.; Netankar, P.D.; Mane, R.A. Baker’s yeast catalyzed one-pot three-component synthesis of polyfunctionalized 4H-pyrans. Tetrahedron Lett. 2011, 52, 5817–5819. [Google Scholar] [CrossRef]
- Yang, F.; Wang, H.; Jiang, L.; Yue, H.; Zhang, H.; Wang, Z.; Wang, L. A green and one-pot synthesis of benzo [g] chromene derivatives through a multi-component reaction catalyzed by lipase. RSC Adv. 2015, 5, 5213–5216. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Z.; Wang, Z.; Guo, C.; Wang, C.; Zhao, R.; Wang, L. Lipase-catalyzed synthesis of indolyl 4H-chromenes via a multicomponent reaction in ionic liquid. Catalysts 2017, 7, 185. [Google Scholar] [CrossRef]
- Chavan, A.S.; Kharat, A.S.; Bhosle, M.R.; Mane, R.A. Baker’s yeast catalyzed one-pot synthesis of bioactive 2-[benzylidene(or pyrazol-4-ylmethylene)hydrazono]-1,3-thiazolidin-4-one-5-yl-acetic acids. Heterocycl. Commun. 2018, 24, 103. [Google Scholar] [CrossRef]
- Dalal, K.S.; Tayade, Y.A.; Wagh, Y.B.; Trivedi, D.R.; Dalal, D.S.; Chaudhari, B.L. Bovine serum albumin catalyzed one-pot, three-component synthesis of dihydropyrano [2-c] pyrazole derivatives in aqueous ethanol. RSC Adv. 2016, 6, 14868–14879. [Google Scholar] [CrossRef]
- Dalal, K.S.; Padvi, S.A.; Wagh, Y.B.; Dalal, D.S.; Chaudhari, B.L. Lipase from porcine pancreas: An efficient biocatalyst for the synthesis of ortho-aminocarbonitriles. ChemistrySelect 2018, 3, 10378–10382. [Google Scholar] [CrossRef]
- Ani, D.; Noble, V.T.; Vidya, S. Green protocols for the synthesis of 3,3′-spirooxindoles-2016-mid 2019. Curr. Green Chem. 2019, 6, 210–225. [Google Scholar] [CrossRef]
- Bora, P.P.; Bihani, M.; Bez, G. Multicomponent synthesis of dihydropyrano[2,3-c]pyrazoles catalyzed by lipase from Aspergillus niger. J. Mol. Catal. B Enzym. 2013, 92, 24–33. [Google Scholar] [CrossRef]
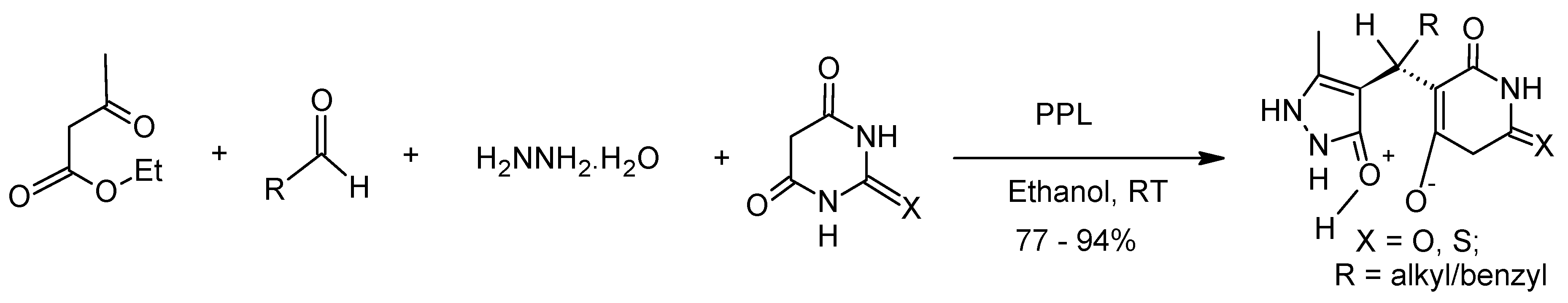
- Bihani, M.; Bora, P.P.; Verma, A.K.; Baruah, R.; Boruah, H.P.D.; Bez, G. PPL catalyzed four-component PASE synthesis of 5-monosubstituted barbiturates: Structure and pharmacological properties. Bioorganic Med. Chem. Lett. 2015, 25, 5732–5736. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Wang, D.; Li, Y. Bovine serum albumin: An efficient and green biocatalyst for the one-pot four-component synthesis of pyrano [2-c] pyrazoles. Chin. J. Catal. 2016, 37, 1461–1467. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, X.Y.; Wu, Q.; Lin, X.F. One-pot synthesis of spirooxazino derivatives via enzyme-initiated multicomponent reactions. Adv. Synth. Catal. 2014, 356, 999–1005. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, G.J.; Wang, J.L.; Wu, Q.; Lin, X.F. Lipase/acetamide-catalyzed carbon-carbon bond formations: A mechanistic view. Adv. Synth. Catal. 2013, 355, 864–868. [Google Scholar] [CrossRef]
- Li, C.; Feng, X.-W.; Wang, N.; Zhou, Y.-J.; Yu, X.-Q. Biocatalytic promiscuity: The first lipase-catalysed asymmetric aldol reaction. Green Chem. 2008, 10, 616–618. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Wang, J.-L.; Lin, X.-F.; Wu, Q. Lipase-initiated one-pot synthesis of spirooxazino derivatives: Redesign of multicomponent reactions to expand substrates scope and application potential. Tetrahedron 2016, 72, 3318–3323. [Google Scholar] [CrossRef]










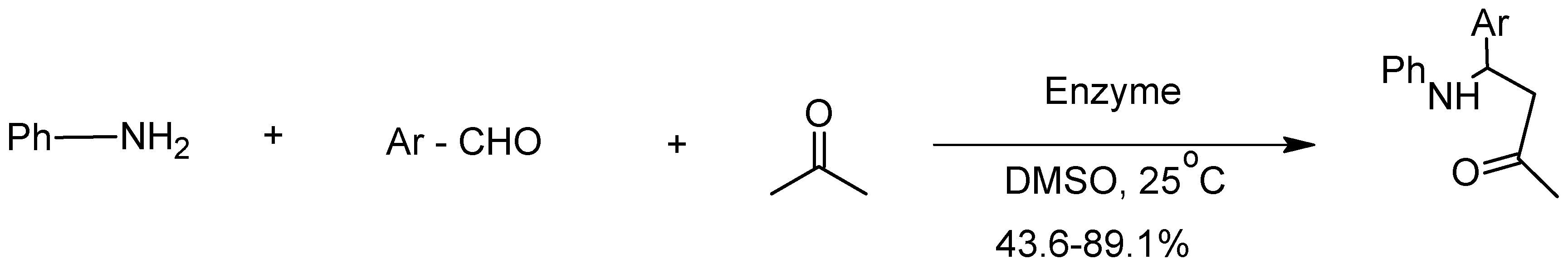

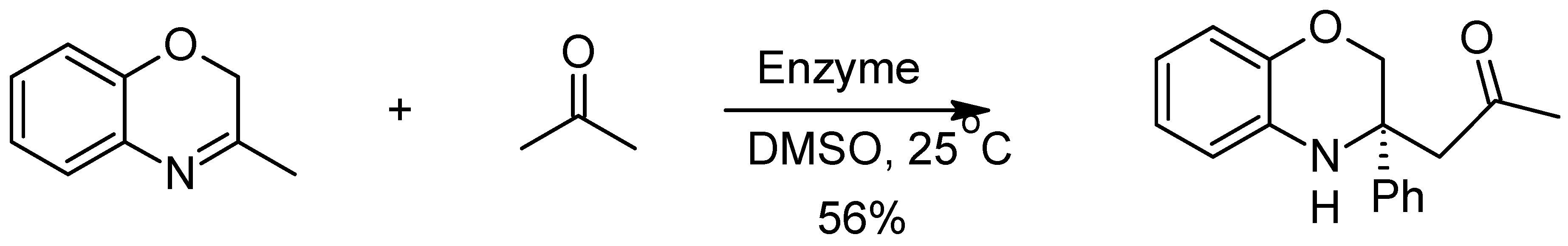

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumbam, N.D.; Masamba, W. Bio-Catalysis in Multicomponent Reactions. Molecules 2020, 25, 5935. https://doi.org/10.3390/molecules25245935
Jumbam ND, Masamba W. Bio-Catalysis in Multicomponent Reactions. Molecules. 2020; 25(24):5935. https://doi.org/10.3390/molecules25245935
Chicago/Turabian StyleJumbam, Ndze Denis, and Wayiza Masamba. 2020. "Bio-Catalysis in Multicomponent Reactions" Molecules 25, no. 24: 5935. https://doi.org/10.3390/molecules25245935
APA StyleJumbam, N. D., & Masamba, W. (2020). Bio-Catalysis in Multicomponent Reactions. Molecules, 25(24), 5935. https://doi.org/10.3390/molecules25245935