The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix
Abstract
1. Introduction
2. Results
2.1. Determination of Alkaloids in STR Extracts
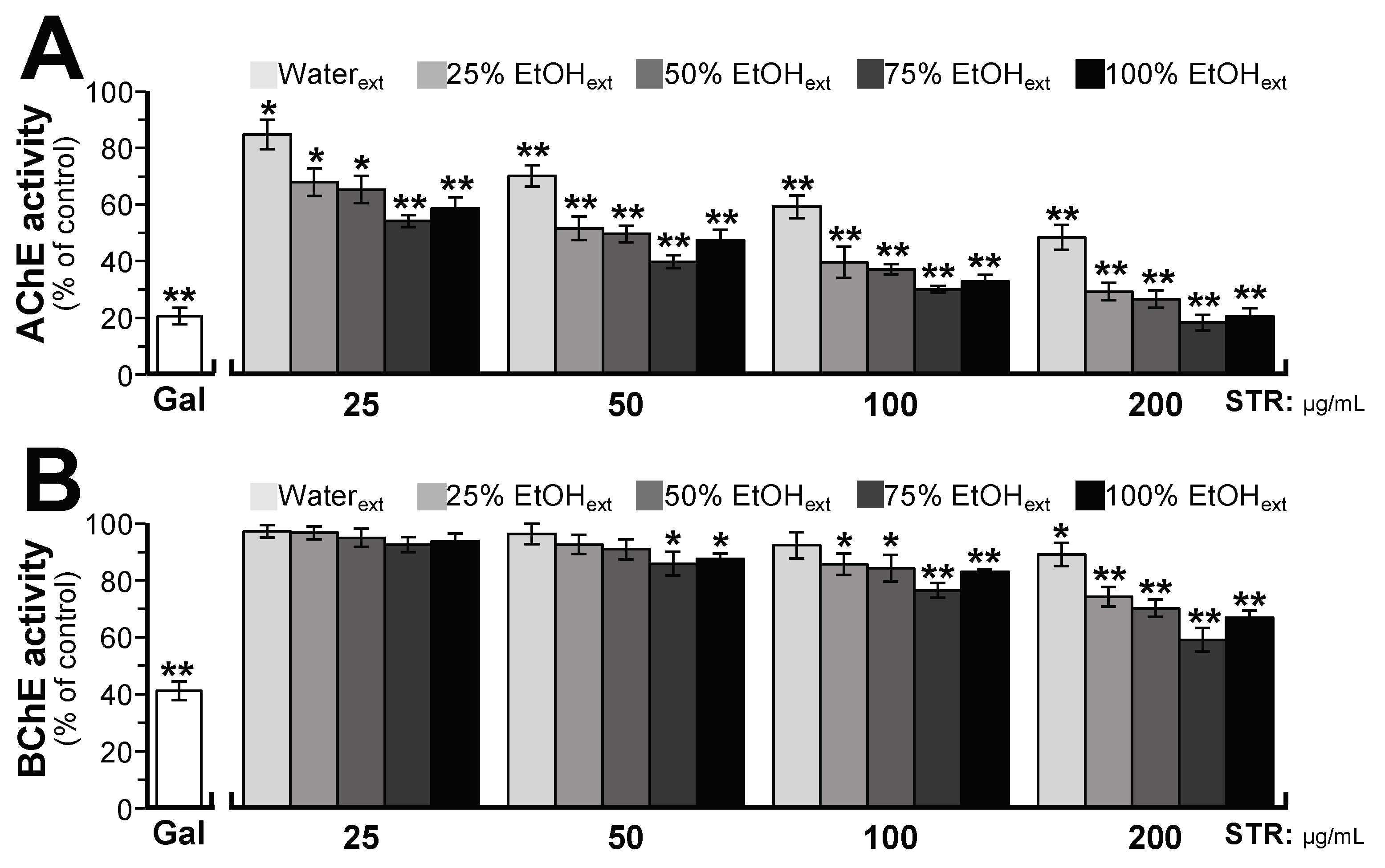
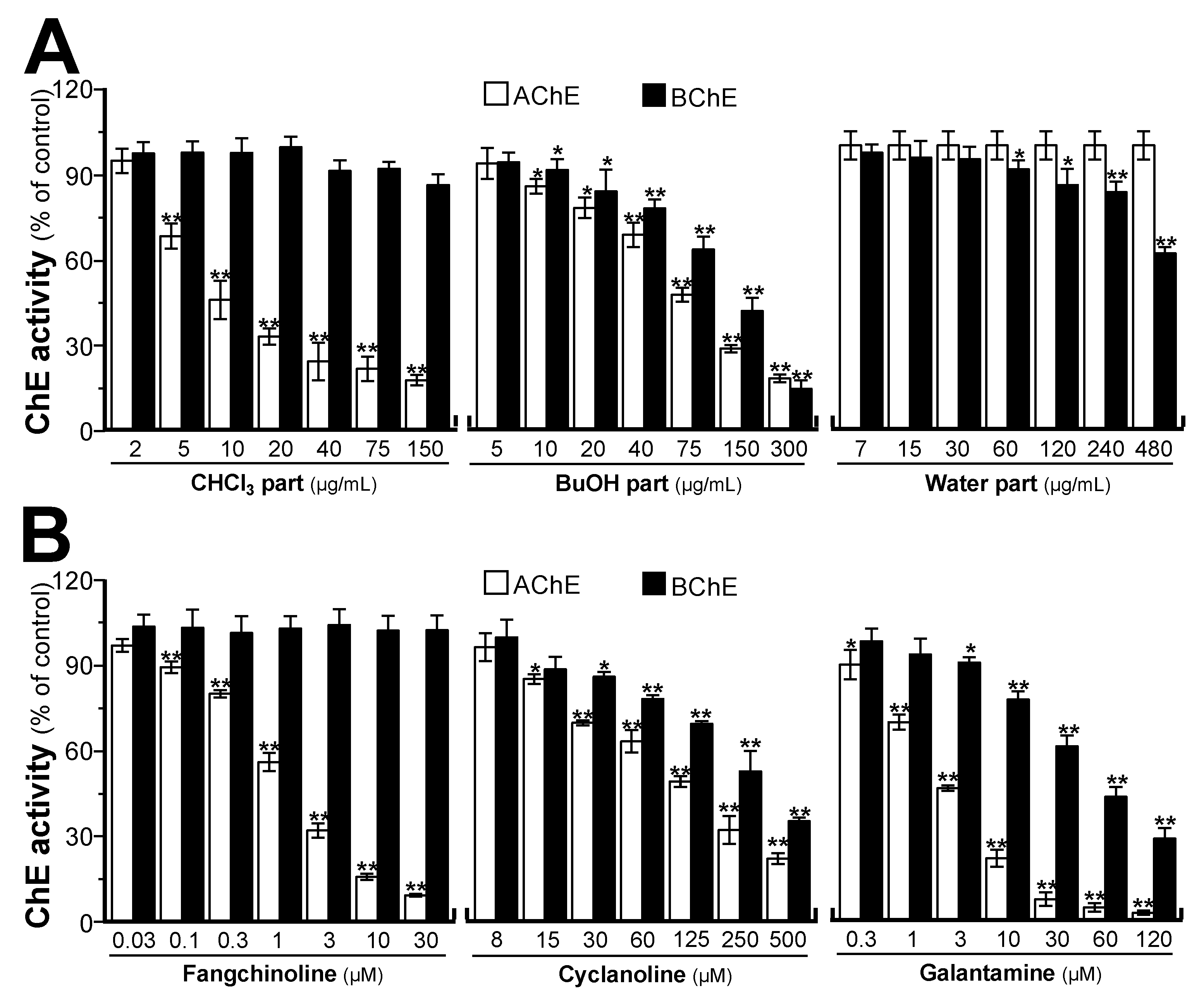
2.2. STR Extracts Inhibit Cholinesterase Activity
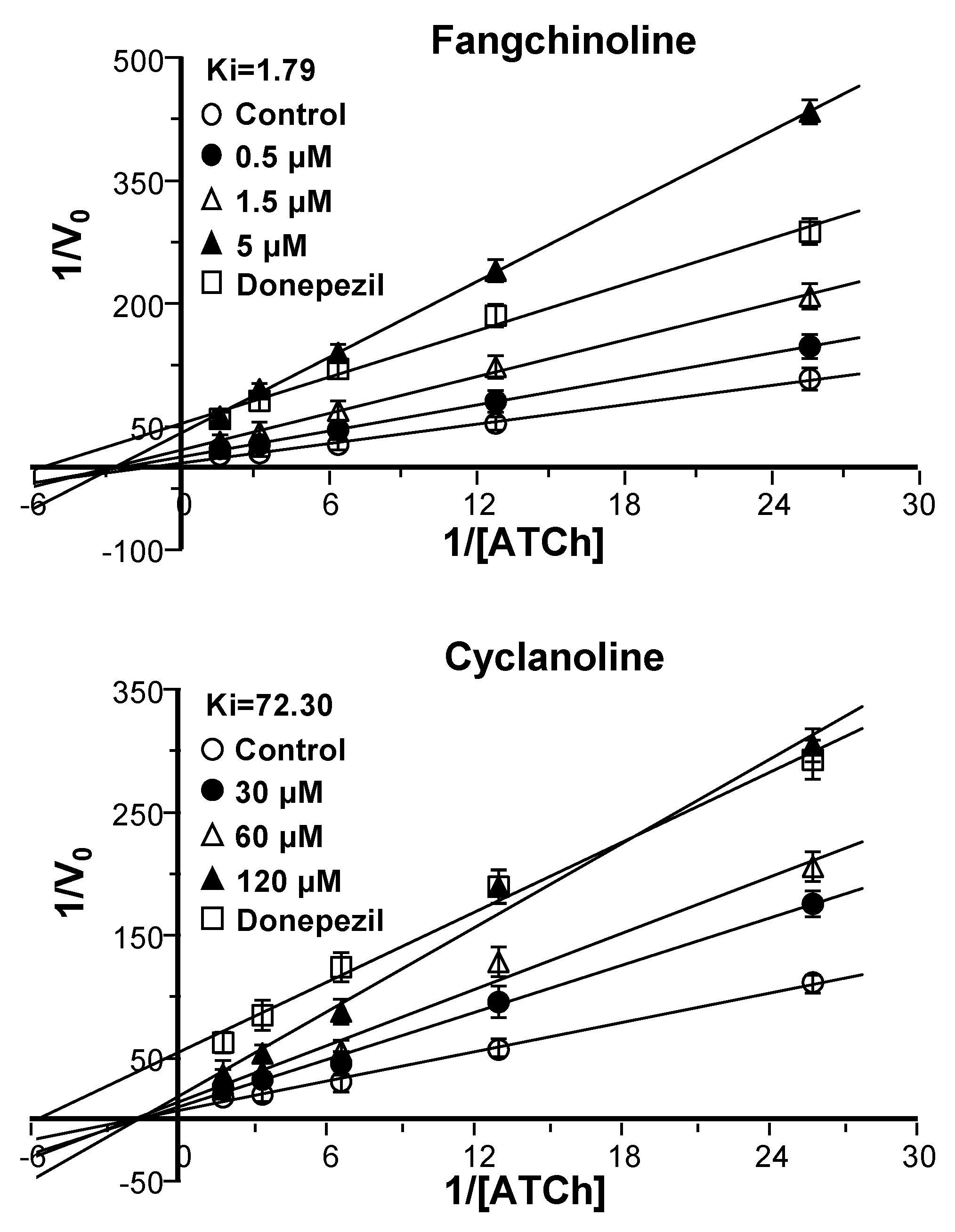
2.3. Synergy of Fangchinoline and Donepezil or Huperzine A
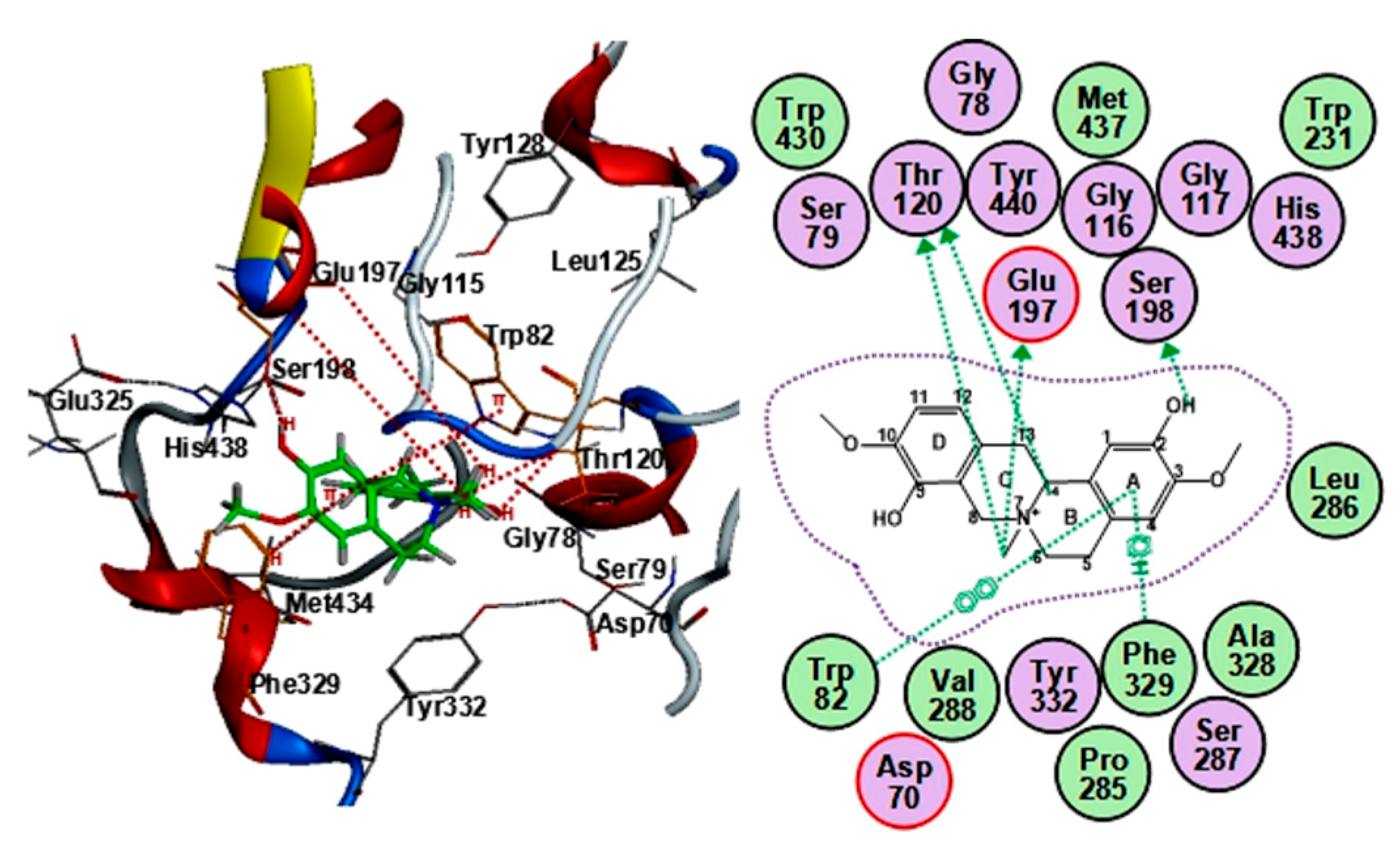
2.4. Cholinesterase Binding Site Analysis of STR Alkaloids
3. Discussion
4. Materials and Methods
4.1. Chemicals and STR Extract Preparation
4.2. Determination of Alkaloids in Different STR Extracts
4.3. Preparation of Different STR Extracts and Alkaloids for Cholinesterase Activity Assay
4.4. Cholinesterase Activity Assay
4.5. Synergistic Effect Analysis by Median-Effect Principle
4.6. Molecular Docking
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.P.; Ding, H.L. Alzheimer’s disease: Epidemiology, genetics, and beyond. Neurosci. Bull. 2008, 24, 105–109. [Google Scholar] [CrossRef]
- Giannakopoulos, P. Best predictors of cognitive status in AD: Amyloid, neurofibrillary tangles or neuronal loss. Int. Psychogeriatr. 2003, 15, 192. [Google Scholar]
- Reale, M.; Brenner, T.; Greig, N.H.; Inestrosa, N.; Paleacu, D. Neuroinflammation, AD, and dementia. Int. J. Alzheimers Dis. 2010, 2010, 1340–1347. [Google Scholar] [CrossRef]
- Checler, F.; Turner, A.J. Journal of Neurochemistry special issue on Alzheimer’s disease: ‘Amyloid cascade hypothesis-20 years on’. J. Neurochem. 2012, 120 (Suppl. S1), iii–iv. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm. (Vienna) 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Levy, M.L.; Cummings, J.L.; Kahn-Rose, R. Neuropsychiatric symptoms and cholinergic therapy for Alzheimer’s Disease. Gerontology 1999, 45, 15–22. [Google Scholar] [CrossRef]
- Gauthier, S.; Juby, A.; Morelli, L.; Rehel, B.; Schecter, R.; ExtendInvestigators. A large, naturalistic, community-based study of rivastigmine in mild-to-moderate AD: The extendstudy. Curr. Med. Res. Opin. 2006, 22, 2251–2265. [Google Scholar] [CrossRef]
- Lu, P.H.; Edland, S.D.; Teng, E.; Tingus, K.; Petersen, R.C.; Cummings, J.L.; Alzheimer’s Disease Cooperative Study Group. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009, 72, 2115–2121. [Google Scholar] [CrossRef]
- Digiacomo, M.; Chen, Z.; Wang, S.; Lapucci, A.; Macchia, M.; Yang, X.; Chu, J.; Han, Y.; Pi, R.; Rapposelli, S. Synthesis and pharmacological evaluation of multifunctional tacrine derivatives against several disease pathways of AD. Bioorg. Med. Chem. Lett. 2015, 25, 807–810. [Google Scholar] [CrossRef]
- Shaddock, J.G.; Feuers, R.J.; Chou, M.W.; Swenson, D.H.; Casciano, D.A. Genotoxicity of tacrine in primary hepatocytes isolated from B6C3F1 mice and aged ad libitum and calorie restricted Fischer 344 rats. Mutat. Res. 1995, 344, 79–88. [Google Scholar] [CrossRef]
- Dierckx, R.I.R.; Vandewoude, M.F.J. Donepezil-related toxic hepatitis. Acta Clin. Belg. 2008, 63, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.M.; Tooze, J.A.; Flowers, L.; Hill, D.F.; Mcmullen, K.P.; Shaw, E.G.; Parsons, S.K. Toxicity and efficacy of the acetylcholinesterase (AChE) inhibitor donepezil in childhood brain tumor survivors: A pilot study. Pediatr. Blood Cancer 2012, 59, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Brinton, R.D. Inflammation: Bridging age, menopause and apoeε4 genotype to Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.L.; Walker, K.A.; Moghekar, A.R.; Pettigrew, C.; Soldan, A.; Albert, M.S.; Walston, J.D. Plasma markers of inflammation linked to clinical progression and decline during preclinical AD. Front. Aging Neurosci. 2019, 11, 229. [Google Scholar] [CrossRef]
- Chou, R.C.; Kane, M.; Ghimire, S.; Gautam, S.; Gui, J. Treatment for rheumatoid arthritis and risk of Alzheimer’s disease: A nested case-control analysis. CNS Drugs 2016, 30, 1111–1120. [Google Scholar] [CrossRef]
- Huang, L.-C.; Chang, Y.-H.; Yang, Y.-H. Can disease-modifying anti-rheumatic drugs reduce the risk of developing dementia in patients with rheumatoid arthritis. Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 703–709. [Google Scholar] [CrossRef]
- Cuya, T.; Baptista, L.; Celmar Costa França, T. A molecular dynamics study of components of the ginger (Zingiber officinale) extract inside human acetylcholinesterase: Implications for Alzheimer disease. J. Biomol. Struct. Dyn. 2018, 36, 3843–3855. [Google Scholar] [CrossRef]
- Tian, F.; Xu, H. Effect of Huanglian Jiedu Decoction combined with leflunomide on the levels of IL-1β and TNF-α in serum of patients with rheumatoid arthritis. Shaanxi J. Tradit. Chin. Med. 2017, 038, 1229–1231. [Google Scholar]
- Rungsaeng, P.; Sangvanich, P.; Karnchanatat, A. Zingipain, a ginger protease with acetylcholinesterase inhibitory activity. Appl. Biochem. Biotechnol. 2013, 170, 934–950. [Google Scholar] [CrossRef]
- Sun, Z.K.; Yang, H.Q.; Chen, S.D. Traditional Chinese medicine: A promising candidate for the treatment of Alzheimer’s disease. Transl. Neurodegener. 2013, 2, 6. [Google Scholar] [CrossRef]
- Shan, L.; Tong, L.; Hang, L.; Fan, H. Fangchinoline supplementation attenuates inflammatory markers in experimental rheumatoid arthritis-induced rats. Biomed. Pharmacother. 2019, 111, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Liu, E.; Chen, Z.C.; Xu, M.L.; Yu, A.; Wu, Q.Y.; Xia, Y.J.; Duan, R.; Dong, T.; Tsim, K. Synergistic inhibition of acetylcholinesterase by alkaloids derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules 2019, 24, 4567. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.L.; Wu, Z.Z.; Chen, L.X.; Wu, B.X.; Chen, L.L.; Qin, G.C.; Gui, B.; Zhou, J.Y. Neuroprotective effects of tetrandrine against vascular dementia. Neural Regen. Res. 2016, 11, 454–459. [Google Scholar] [PubMed]
- Bao, F.; Tao, L.; Zhang, H. Neuroprotective effect of natural alkaloid fangchinoline against oxidative glutamate toxicity: Involvement of Keap1-Nrf2 axis regulation. Cell. Mol. Neurobiol. 2019, 39, 1177–1186. [Google Scholar] [CrossRef]
- Koh, S.B.; Ban, J.Y.; Lee, B.Y.; Seong, Y.H. Protective effects of fangchinoline and tetrandrine on hydrogen peroxide-induced oxidative neuronal cell damage in cultured rat cerebellar granule cells. Planta Med. 2003, 69, 506–512. [Google Scholar]
- Chalupova, K.; Korabecny, J.; Bartolini, M.; Monti, B.; Lamba, D.; Caliandro, R.; Pesaresi, A.; Brazzolotto, X.; Gastellier, A.J.; Nachon, F.; et al. Novel tacrine-tryptophan hybrids: Multi-target directed ligands as potential treatment for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 168, 491–514. [Google Scholar] [CrossRef]
- Maraković, N.; Knežević, A.; Vinković, V.; Kovarik, Z.; Šinko, G. Design and synthesis of N-substituted-2-hydroxyiminoacetamides and interactions with cholinesterases. Chem. Biol. Interact. 2016, 259, 122–132. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s Disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Xiong, X.J. Fangji Dihuang decoction formula syndrome and Fengyin decoction formula syndrome: Application in stroke and mental disorders. Chin. J. Chin. Mater. Med. 2019, 44, 602–607. [Google Scholar]
- Wang, Q.S.; Ding, S.L.; Mao, H.P.; Cui, Y.L.; Qi, X.J. Antidepressant-like effect of ethanol extract from Zuojin Pill, containing two herbal drugs of Rhizoma Coptidis and Fructus Evodiae, is explained by modulating the monoaminergic neurotransmitter system in mice. J. Ethnopharmacol. 2013, 148, 603–609. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tsim, K.W.; Randall, W.R.; Barnard, E.A. An asymmetric form of muscle acetylcholinesterase contains three subunit types and two enzymic activities in one molecule. Proc. Natl. Acad. Sci. USA 1988, 85, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Luk, W.W.; Cui, W.; Hu, S.; Tsim, K.W.; Han, Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014, 53, 511–516. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Ikemura, N.; Yamaori, S.; Kobayashi, C.; Kamijo, S.; Murayama, N.; Yamazaki, H.; Ohmori, S. Inhibitory effects of antihypertensive drugs on human cytochrome P450 2J2 activity: Potent inhibition by azelnidipine and manidipine. Chem. Biol. Interact. 2019, 306, 1–9. [Google Scholar] [CrossRef]
- Al-Shamary, D.S.; Al-Alshaikh, M.A.; Kheder, N.A.; Mabkhot, Y.N.; Badshah, S.L. Molecular docking and biological evaluation of some thioxoquinazolin-4(3H)-one derivatives as anticancer, antioxidant and anticonvulsant agents. Chem. Cent. J. 2017, 11, 48. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
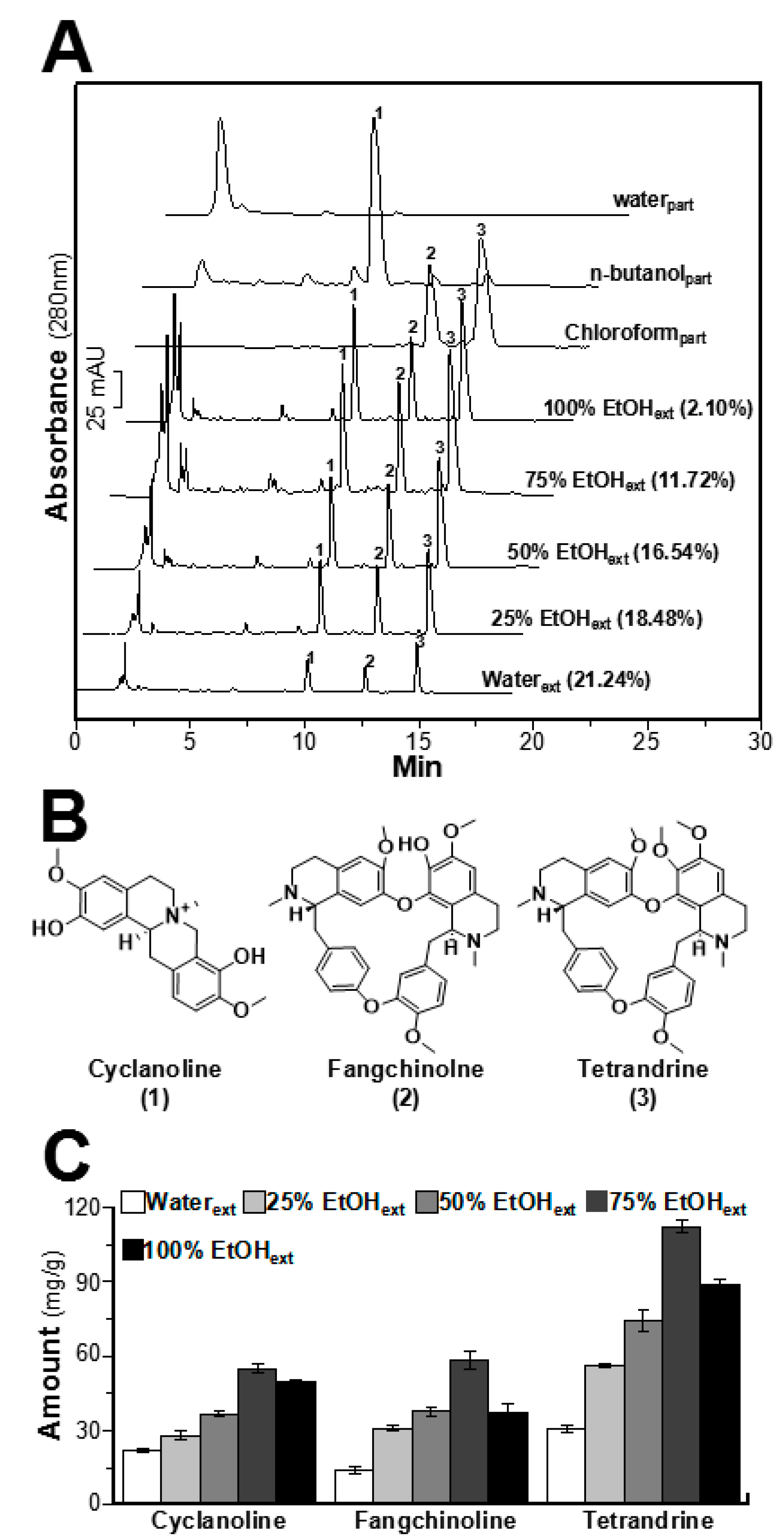


| Alkaloids | Linear Equation | R2 | Linear Range (μg/mL) | Content (mg/g) |
|---|---|---|---|---|
| Cyclanoline | y = 7732.9x + 21862 | 0.9997 | 7.8125–250 | 2.79 ± 0.25 |
| Fangchinoline | y = 7435.7x − 4899.2 | 0.9990 | 3.125–100 | 5.03 ± 0.33 |
| Tetrandrine | y = 6608.7x − 15895 | 0.9996 | 6.25–200 | 11.14 ± 0.73 |
| Name | Alkaloid Binding Interactions with AChE | Scores 1 | ||||
|---|---|---|---|---|---|---|
| Receptor | Ligand | Interaction | Binding Affinity kJ/mol | Active Site | ||
| Fangchinoline | Trp-286 | 6-ring (B, C′) | π–π | −35.14 | PAS 2 | −7.11 ± 0.32 |
| H (1-CH; 3, 9, 11′-CH2; 11, 14-Ar H) | H–π | −35.85 | ||||
| Leu-76 | 6-ring (C′) | π–H | −2.74 | / | ||
| Leu-289 | 6-ring (C) | π–H | −3.34 | / | ||
| Val-294 | 6-ring (C) | π–H | −0.70 | / | ||
| Tyr-341 | 6-ring (C′) | π–H | −2.41 | PAS | ||
| Glu-292 | H (7′-OH) | H–donor | −5.68 | / | ||
| Cyclanoline | Trp-286 | 6-ring (A, D) | π–π | −33.24 | PAS | −7.00 ± 0.01 |
| H (1-Ar H; 5, 8-CH2; 10-OCH3) | H–π | −45.78 | ||||
| Trp-86 | H (4-Ar H; 10-OCH3) | H–π | −4.92 | AS 3 | ||
| Ser-293 | H (6, 8-CH2; 7-NCH3; 9-OH) | H–donor | −10.99 | / | ||
| Tyr-341 | H (3, 10-OCH3) | H–π | −1.95 | PAS | ||
| Asp-74 | N (7) | Metal/ion | −2.64 | PAS | ||
| H (2, 9-OH), H (8-CH2; 14-CH) | H–donor | −8.95 | ||||
| Glu-202 | H (9-OH) | H–donor | −3.73 | / | ||
| His-447 | 6-ring (D) | π–H | −1.65 | CS 4 | ||
| H (2-OH) | H–donor | −1.33 | ||||
Sample Availability: Samples of the compounds, e.g., fangchinoline, tetrandrine and cyclanoline, are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.-P.; Ren, H.-Q.; Liu, E.Y.L.; Leung, K.-W.; Guo, S.-C.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix. Molecules 2020, 25, 5914. https://doi.org/10.3390/molecules25245914
Kong X-P, Ren H-Q, Liu EYL, Leung K-W, Guo S-C, Duan R, Dong TTX, Tsim KWK. The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix. Molecules. 2020; 25(24):5914. https://doi.org/10.3390/molecules25245914
Chicago/Turabian StyleKong, Xiang-Peng, Hai-Qin Ren, Etta Y. L. Liu, Ka-Wing Leung, Shu-Chen Guo, Ran Duan, Tina T. X. Dong, and Karl W. K. Tsim. 2020. "The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix" Molecules 25, no. 24: 5914. https://doi.org/10.3390/molecules25245914
APA StyleKong, X.-P., Ren, H.-Q., Liu, E. Y. L., Leung, K.-W., Guo, S.-C., Duan, R., Dong, T. T. X., & Tsim, K. W. K. (2020). The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix. Molecules, 25(24), 5914. https://doi.org/10.3390/molecules25245914









