CO2 Absorption Mechanism by the Nonaqueous Solvent Consisting of Hindered Amine 2-[(1,1-dimethylethyl)amino]ethanol and Ethylene Glycol
Abstract
1. Introduction
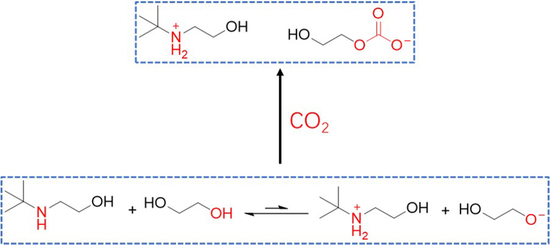
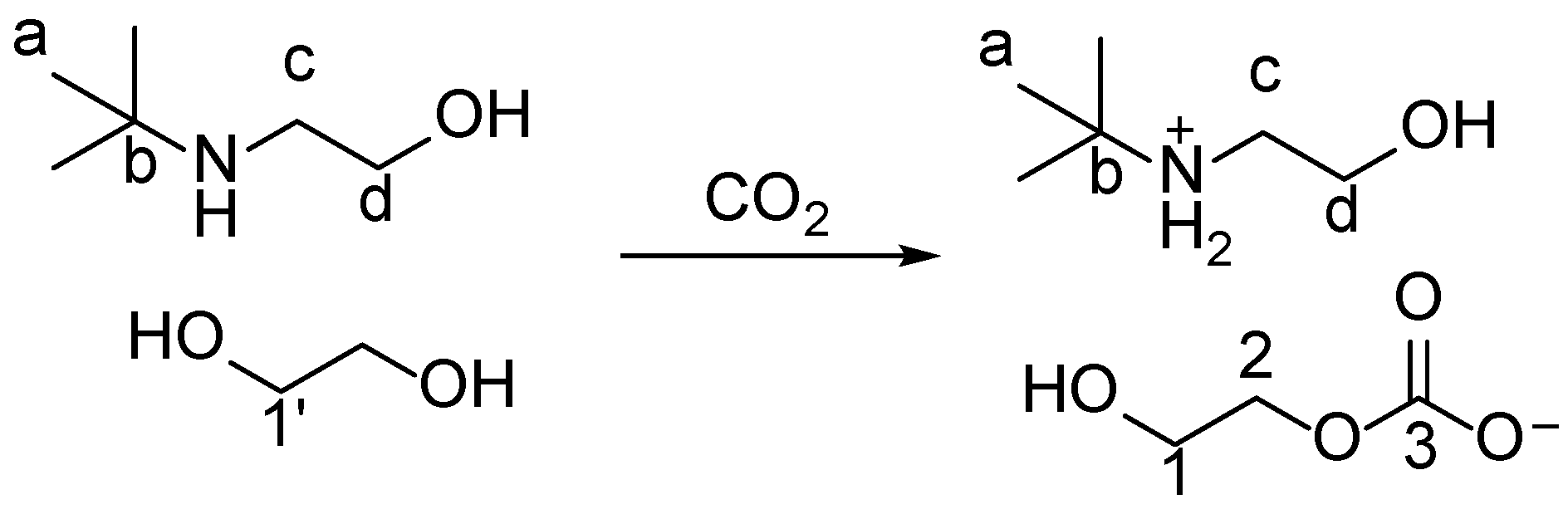
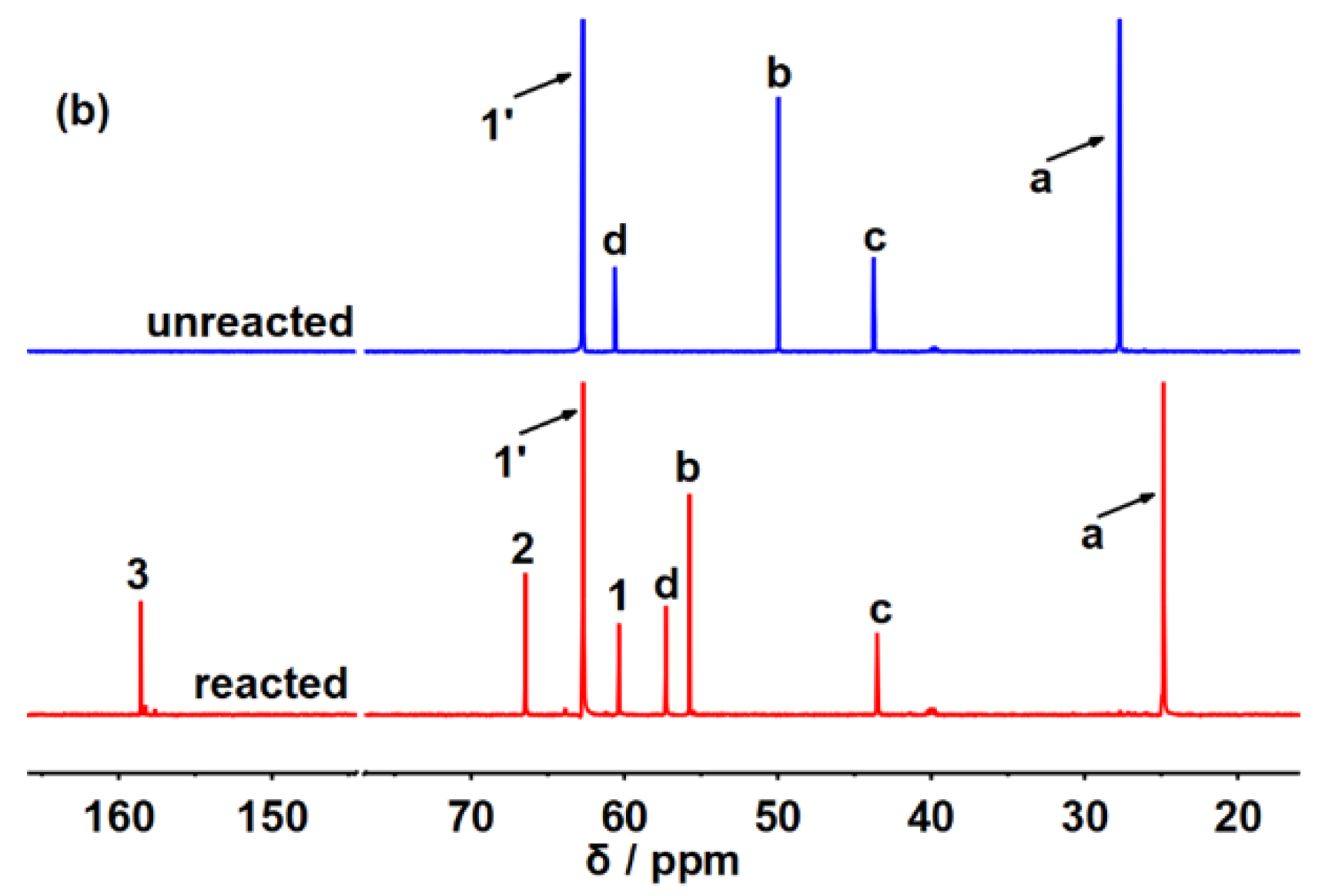
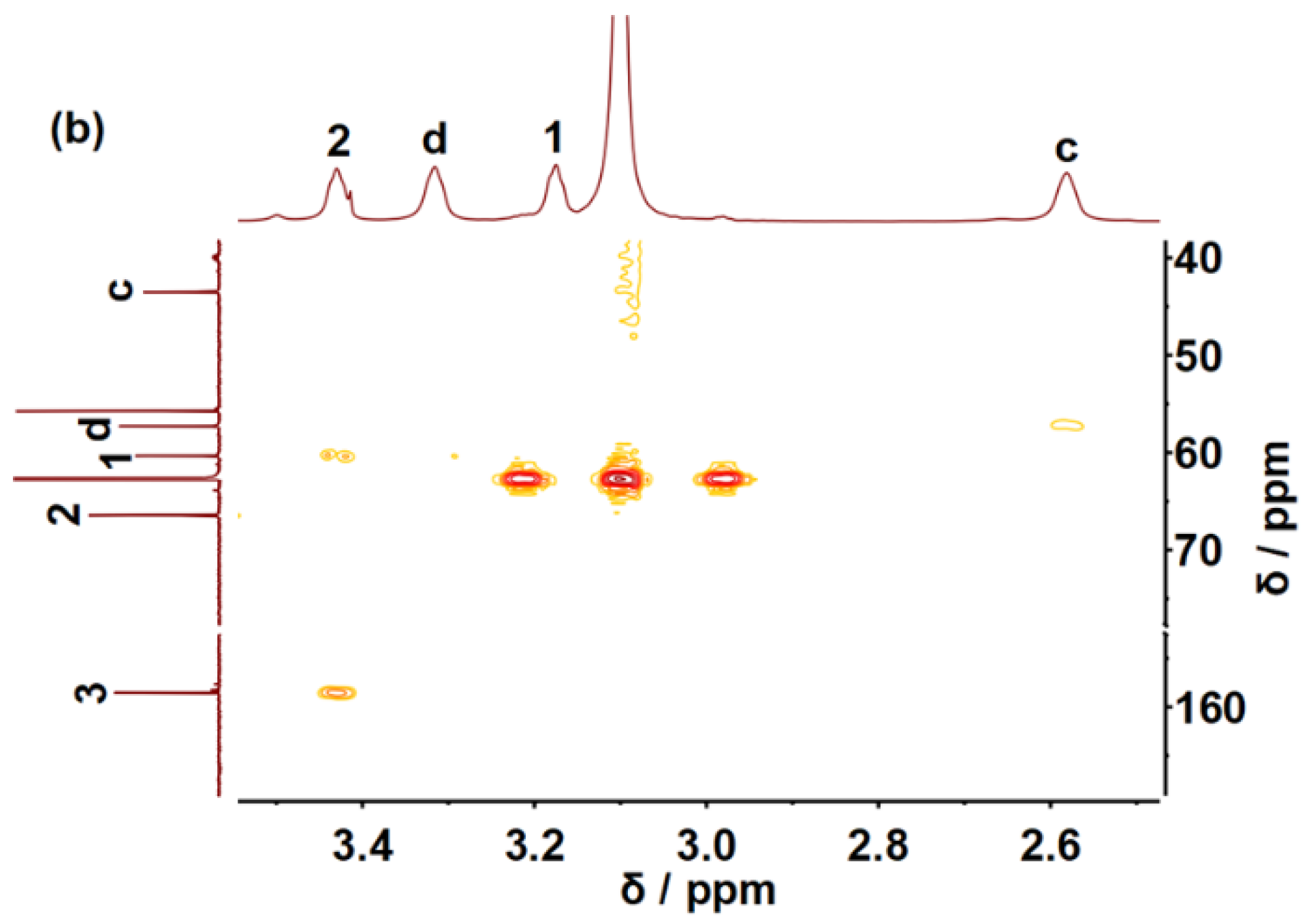
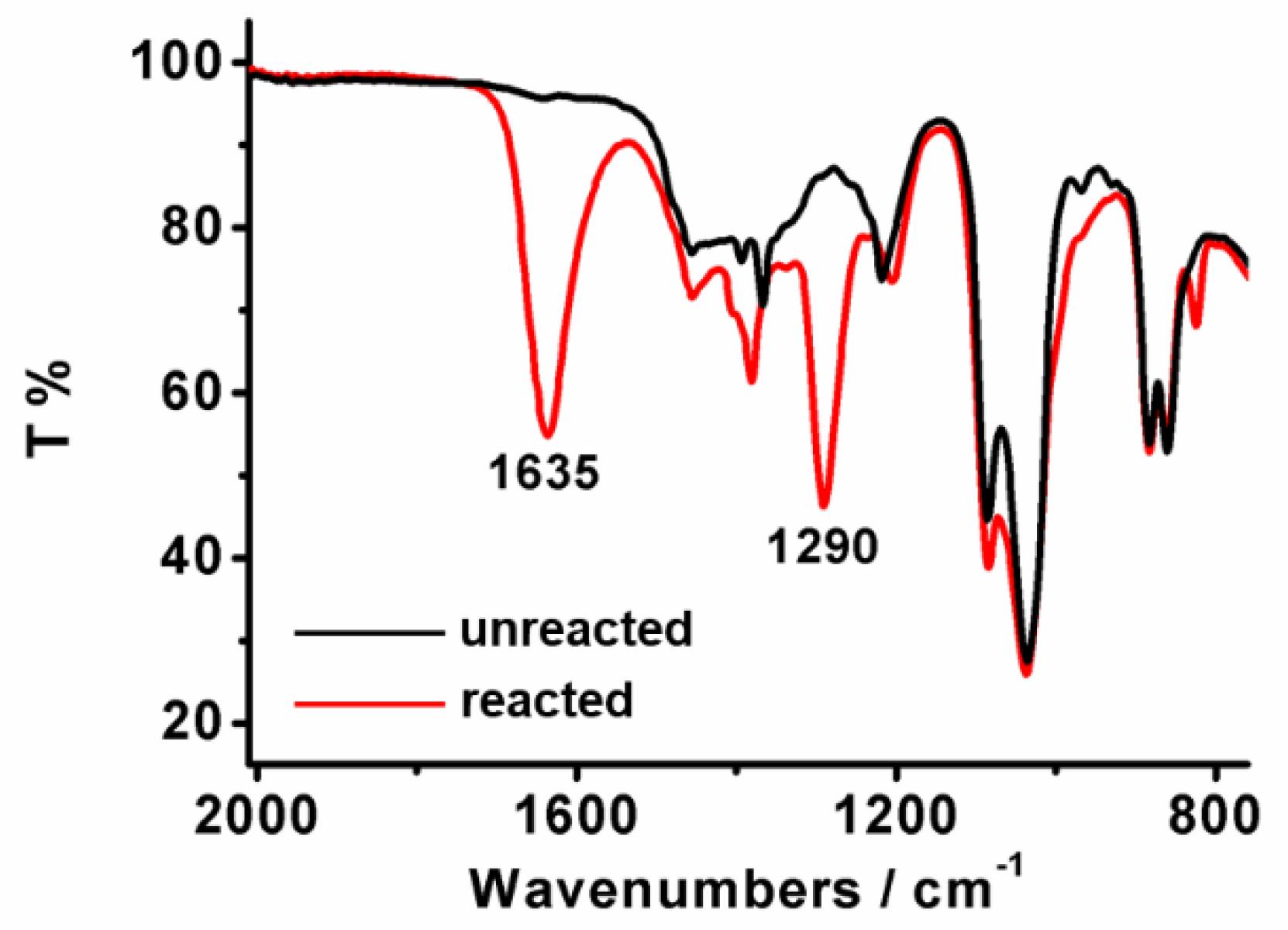
2. Results and Discussions
3. Conclusions
4. Experimental Sections
4.1. Materials and Characterization
4.2. Absorption of CO2
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Hallett, J.P.; Mac Dowell, N. Solvent selection and design for CO2 capture—how we might have been missing the point. Sustain. Energy Fuels 2017, 1, 2078–2090. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-Lean Solvents for Post-Combustion CO2 Capture: Fundamentals, Uncertainties, Opportunities, and Outlook. Chem. Rev. 2017, 117, 9594–9624. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Zhao, Y.-N.; Li, B.; Yu, B. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion. Energy Environ. Sci. 2011, 4, 3971–3975. [Google Scholar] [CrossRef]
- Tan, J.; Shao, H.; Xu, J.; Du, L.; Luo, G. Mixture Absorption System of Monoethanolamine−Triethylene Glycol for CO2 Capture. Ind. Eng. Chem. Res. 2011, 50, 3966–3976. [Google Scholar] [CrossRef]
- Barbarossa, V.; Barzagli, F.; Mani, F.; Lai, S.; Stoppioni, P.; Vanga, G. Efficient CO2 capture by non-aqueous 2-amino-2-methyl-1-propanol (AMP) and low temperature solvent regeneration. RSC Adv. 2013, 3, 12349–12355. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Efficient CO2 absorption and low temperature desorption with non-aqueous solvents based on 2-amino-2-methyl-1-propanol (AMP). Int. J. Greenh. Gas Control. 2013, 16, 217–223. [Google Scholar] [CrossRef]
- Zheng, C.; Tan, J.; Wang, Y.J.; Luo, G.S. CO2 Solubility in a Mixture Absorption System of 2-Amino-2-methyl-1-propanol with Ethylene Glycol. Ind. Eng. Chem. Res. 2013, 52, 12247–12252. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhang, Y.; Qin, L.; Guo, C.; Chen, J. Species distribution of CO2 absorption/desorption in aqueous and non-aqueous N-ethylmonoethanolamine solutions. Int. J. Greenh. Gas Control. 2016, 47, 151–158. [Google Scholar] [CrossRef]
- Kang, M.-K.; Jeon, S.-B.; Cho, J.-H.; Kim, J.-S.; Oh, K.-J. Characterization and comparison of the CO2 absorption performance into aqueous, quasi-aqueous and non-aqueous MEA solutions. Int. J. Greenh. Gas Control. 2017, 63, 281–288. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Comparative Study of CO2 Capture by Aqueous and Nonaqueous 2-Amino-2-methyl-1-propanol Based Absorbents Carried Out by 13C NMR and Enthalpy Analysis. Ind. Eng. Chem. Res. 2019, 58, 4364–4373. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Unusual temperature-promoted carbon dioxide capture in deep-eutectic solvents: The synergistic interactions. Chem. Commun. 2019, 55, 3939–3942. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Sarmad, S.; Xie, Y.; Mikkola, J.-P.; Ji, X. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity. New J. Chem. 2017, 41, 290–301. [Google Scholar] [CrossRef]
- Im, J.; Hong, S.Y.; Cheon, Y.; Lee, J.; Lee, J.S.; Kim, H.S.; Cheong, M.; Park, H. Steric hindrance-induced zwitterionic carbonates from alkanolamines and CO2: Highly efficient CO2 absorbents. Energy Environ. Sci. 2011, 4, 4284–4289. [Google Scholar] [CrossRef]
- Xie, H.-B.; Wei, X.; Wang, P.; He, N.; Chen, J. CO2 Absorption in an Alcoholic Solution of Heavily Hindered Alkanolamine: Reaction Mechanism of 2-(tert-Butylamino)ethanol with CO2 Revisited. J. Phys. Chem. A 2015, 119, 6346–6353. [Google Scholar] [CrossRef]
- Cui, G.; Lv, M.; Yang, D. Efficient CO2 absorption by azolide-based deep eutectic solvents. Chem. Commun. 2019, 55, 1426–1429. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry, 16th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef]



Sample Availability: Samples of all the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wu, C.; Yang, D. CO2 Absorption Mechanism by the Nonaqueous Solvent Consisting of Hindered Amine 2-[(1,1-dimethylethyl)amino]ethanol and Ethylene Glycol. Molecules 2020, 25, 5743. https://doi.org/10.3390/molecules25235743
Li R, Wu C, Yang D. CO2 Absorption Mechanism by the Nonaqueous Solvent Consisting of Hindered Amine 2-[(1,1-dimethylethyl)amino]ethanol and Ethylene Glycol. Molecules. 2020; 25(23):5743. https://doi.org/10.3390/molecules25235743
Chicago/Turabian StyleLi, Ran, Congyi Wu, and Dezhong Yang. 2020. "CO2 Absorption Mechanism by the Nonaqueous Solvent Consisting of Hindered Amine 2-[(1,1-dimethylethyl)amino]ethanol and Ethylene Glycol" Molecules 25, no. 23: 5743. https://doi.org/10.3390/molecules25235743
APA StyleLi, R., Wu, C., & Yang, D. (2020). CO2 Absorption Mechanism by the Nonaqueous Solvent Consisting of Hindered Amine 2-[(1,1-dimethylethyl)amino]ethanol and Ethylene Glycol. Molecules, 25(23), 5743. https://doi.org/10.3390/molecules25235743