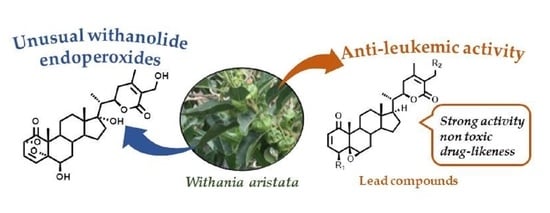
Withanolide-Type Steroids from Withania aristata as Potential Anti-Leukemic Agents
Abstract
1. Introduction
2. Results
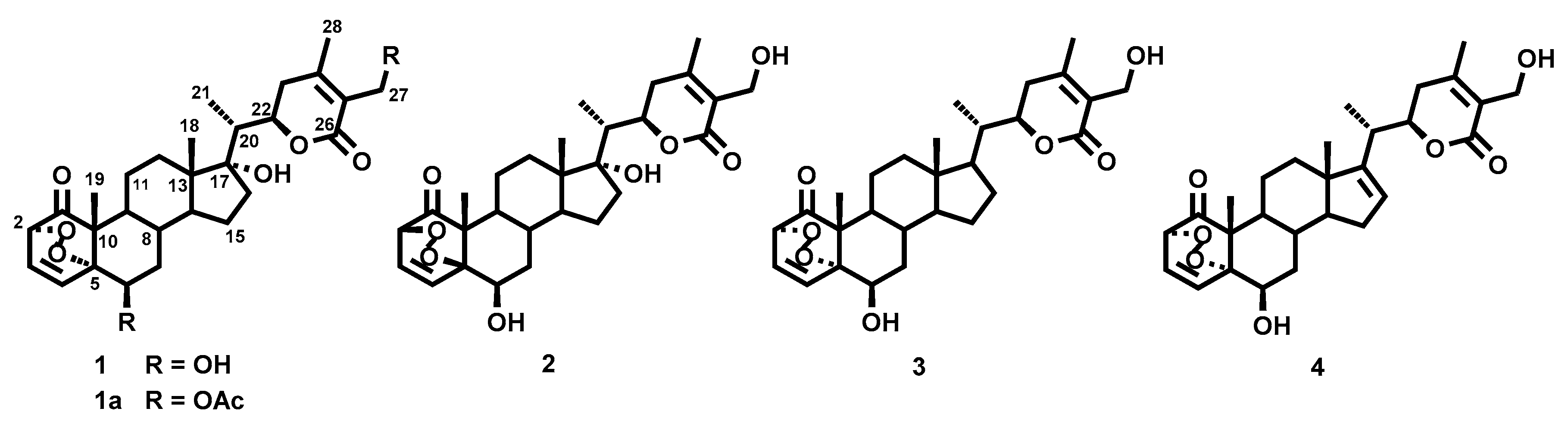
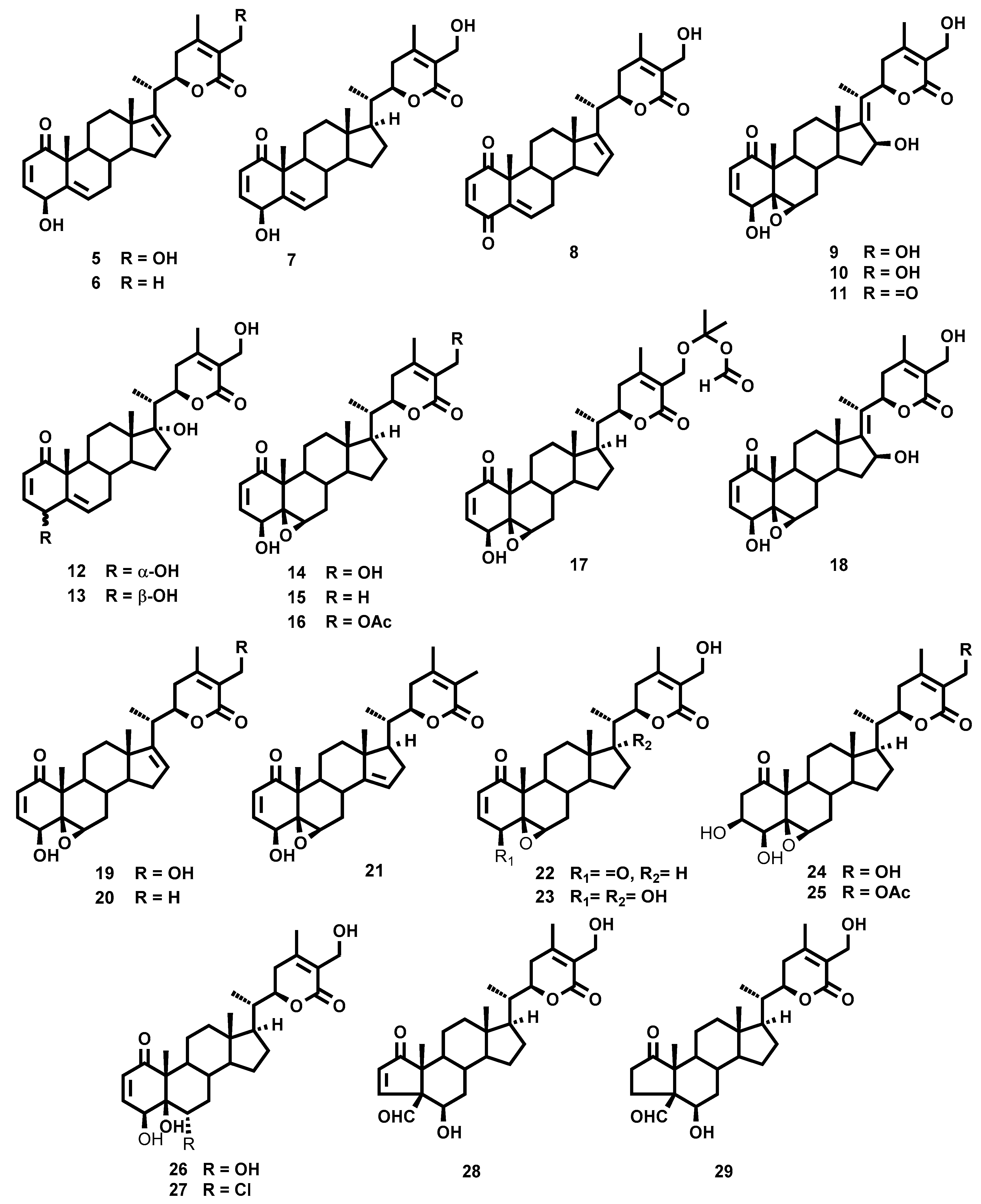
2.1. Chemistry
2.2. Antiproliferative Activity
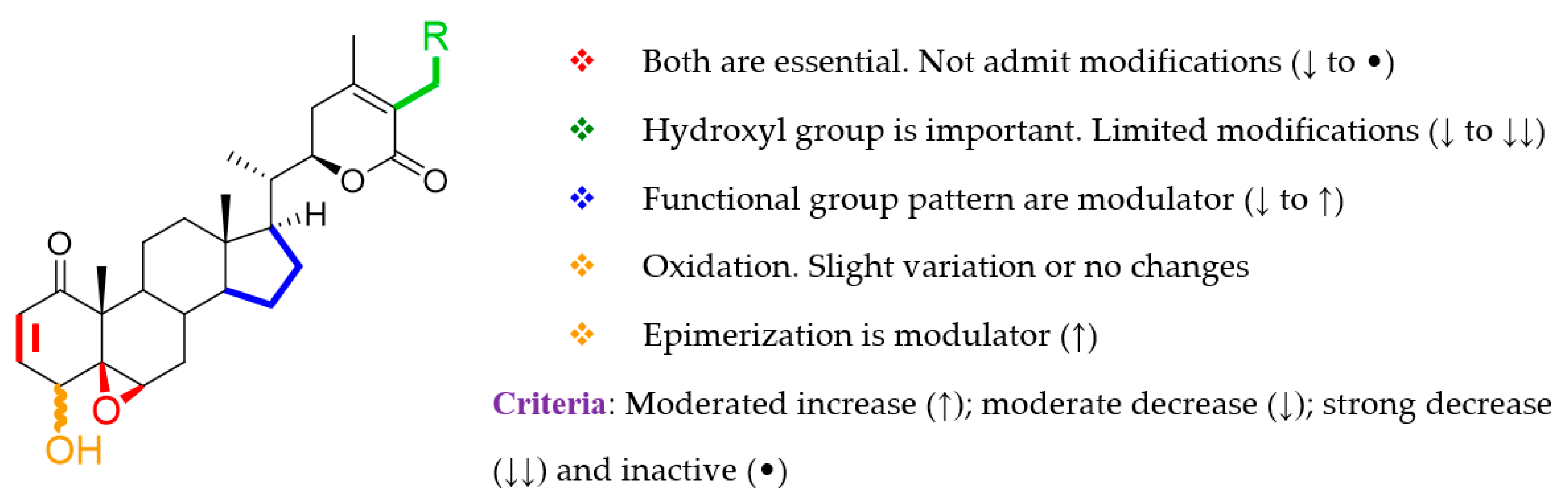
2.3. Structure–Activity Relationship Analysis
2.4. In Silico ADMET Predictions
3. Materials and Methods
3.1. General
3.2. Biological Assays
3.2.1. Cells
3.2.2. Cell Viability
3.3. Plant Material
3.4. Extraction and Isolation
- 6β,17α,27-Trihydroxy-2α,5α-dioxan-1-oxo-witha-3,24-dien-26,22-olide (withaperoxidin A, 1). Colorless lacquer; [α]D20 + 20.4 (c 0.3, CHCl3); UV λmax 216 nm; IR νmax 3740, 2929, 2862, 1700, 1454, 1399, 1258, 1060, 830 cm−1; 1H and 3C-NMR data (see Table 1 and Table 2, respectively); ESIMS m/z % 525 [M + Na]+ (100); HRESIMS m/z 525.2454 (calcd for C28H38O8Na, 525.2464).
- Acetylation of 1. To a solution of 1 (7.0 mg, 0.12 mmol) in dry dichloromethane (2 mL) was added triethylamine (0.1 mL, 0.8 mmol) and acetic anhydride (0.15 mL, 0.12 mmol). The reaction mixture was stirred for 72 h at room temperature until all starting material was consumed. The mixture was evaporated to dryness. The residue was then purified by preparative TLC (dichloromethane/acetone, 9:1) to give 1a (4.5 mg, 56%): Colorless lacquer; [α]D20 + 56.0 (c 0.2, CHCl3); UV λmax 223 nm; IR νmax 2925, 1697, 1454, 1393, 1255, 1063, 834 cm−1; 1H-NMR δ 0.88 (3H, s, Me18), 1.05 (3H, d, J = 6.9 Hz, Me21), 1.14 (3H, s, Me19), 1.21 (1H, H15), 1.39 (1H, H12), 1.52 (1H, H11), 1.61 (1H, H16), 1.63 (1H, H12), 1.68 (1H, H8), 1.69 (1H, H11), 1.71 (1H, H14), 1.72 (1H, H7α), 1.73 (1H, H16), 1.76 (1H, H15), 1.98 (1H, H9), 2.00 (1H, H7β), 2.09 (3H, s, Me28), 2.36 (1H, m, H20), 2.59 (2H, d, J = 7.8 Hz, H23), 4.54 (1H, d, J = 6.2 Hz, H2), 4.68 (1H, dt, J = 3.0, 8.8 Hz, H22), 4.89, 4.93 (2H, dAB, J = 11.8 Hz, H27), 5.38 (1H, br s, H6), 6.62 (1H, t, J = 8.5 Hz, H3), 7.00 (1H, d, J = 8.5 Hz, H4), OAc-27 [2.09 (3H, s)], OAc-6 [2.16 (3H, s)]; 13C-NMR δ 9.4 (CH3-21), 15.0 (CH3-18), 18.0 (CH3-19), 20.6 (CH3-28), 21.8 (CH2-11), 23.6 (CH2-15), 29.8 (CH-8), 31.9 (CH2-16), 32.0 (CH2-12), 33.1 (CH2-23), 36.5 (CH2-7), 42.6 (CH-20), 43.5 (CH-9), 47.1 (C-10), 48.3 (C-13), 49.1 (CH-14), 58.0 (CH2-27), 69.8 (CH-6), 78.7 (CH-2), 79.1 (CH-22), 85.0 (C-5), 85.9 (C-17), 121.3 (C-25), 130.9 (CH-3), 142.9 (CH-4), 158.6 (C-24), 165.5 (C-26), 205.4 (C-1), OAc-6 [20.8 (CH3), 169.6 (COO)], OAc-27 [21.0 (CH3), 171.0 (COO)]; ESI/MS m/z % 609 [M + Na]+ (100); HRESIMS m/z 609.2679 (calcd for C32H42O10Na, 609.2676).
- 6β,17α,27-Trihydroxy-2β,5β-dioxan-1-oxo-witha-3,24-dien-26,22-olide (withaperoxidin B, 2). Colorless lacquer; [α]D20 + 31.9 (c 0.2, CHCl3); UV λmax 214 nm; IR νmax 3430, 2929, 2855, 1689, 1457, 1394, 1013, 754 cm−1; 1H and 3C-NMR data (see Table 1 and Table 2, respectively); ESIMS m/z % 525 [M + Na]+ (100); HRESIMS m/z 525.2465 (calcd for C28H38O8Na, 525.2464).
- 6β,27-Dihydroxy-2α,5α-dioxan-1-oxo-witha-3,24-dien-26,22-olide (withaperoxidin C, 3). Colorless lacquer; [α]D20 − 3.9 (c 0.3, CHCl3); UV λmax 217 nm; IR νmax 3444, 2927, 1700, 1458, 1393, 1130, 1024, 754 cm−1; 1H and 3C-NMR data (see Table 1 and Table 2, respectively); ESI/MS m/z % 509 [M + Na]+ (100); HRESIMS m/z 509.2508 (calcd for C28H38O7Na, 509.2515).
- 6β,27-Dihydroxy-2α,5α-dioxan-1-oxo-witha-3,16,24-trien-26,22-olide (withaperoxidin D, 4). Colorless lacquer; [α]D20 − 4.0 (c 0.5, CHCl3); UV λmax 214 nm; IR νmax 3410, 2930, 1681, 1461, 1380, 1131, 1078, 755 cm−1; 1H and 3C-NMR data (see Table 1 and Table 2, respectively); ESI/MS m/z % 507 [M + Na]+ (100); HRESIMS m/z 507.2355 (calcd for C28H36O7Na, 507.2359).
3.5. ADME Property Predictions of Withanolides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bispo, J.A.B.; Pinheiro, P.S.; Kobetz, E.K. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb. Perspect. Med. 2020, 10, a34819. [Google Scholar] [CrossRef]
- Watts, J.; Nimer, S. Recent advances in the understanding and treatment of acute myeloid leukemia. F1000Research 2018, 7, 1196/1–1196/4. [Google Scholar] [CrossRef]
- Liu, X.-L.; Liu, H.-Q.; Li, J.; Mao, C.-Y.; Hee, J.-T.; Zhao, X. Role of epigenetic in leukemia: From mechanism to therapy. Chem. Biol. Interact. 2020, 317, 108963. [Google Scholar] [CrossRef]
- Conneely, S.E.; Stevens, A.M. Advances in pediatric acute promyelocytic leukemia. Children 2020, 7, 11. [Google Scholar] [CrossRef]
- DiNardo, C.; Lachowiez, C. Acute myeloid leukemia: From mutation profiling to treatment decisions. Curr. Hematol. Malig. Rep. 2019, 14, 386–394. [Google Scholar] [CrossRef]
- Houshmand, M.; Garello, F.; Circosta, P.; Stefania, R.; Aime, S.; Saglio, G.; Giachino, C. Nanocarriers as magic bullets in the treatment of leukemia. Nanomaterials 2020, 10, 276. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Xia, Y.-G.; Pan, J.; Liu, Y.; Wang, Q.-H.; Kuang, H.-X. Phytochemistry and biosynthesis of δ-lactone withanolides. Phytochem. Rev. 2016, 15, 771–797. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Wang, K.-W. Natural bioactive new withanolides. Mini Rev. Med. Chem. 2020, 20, 1101–1117. [Google Scholar] [CrossRef]
- Shohat, B.; Gitter, S.; Abraham, A.; Lavie, D. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother. Rep. 1967, 51, 271–276. [Google Scholar]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Berghe, T.V.; Berghe, W.V. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Dom, M.; Berghe, W.V.; Ostade, X.V. Broad-spectrum antitumor properties of withaferin A: A proteomic perspective. RSC Med. Chem. 2020, 11, 30–50. [Google Scholar] [CrossRef]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar] [CrossRef]
- Okamoto, S.; Tsujioka, T.; Suemori, S.I.; Kida, J.I.; Kondo, T.; Tohyama, Y.; Tohyama, K. Withaferin A suppresses the growth of myelodysplasia and leukemia cell lines by inhibiting cell cycle progression. Cancer Sci. 2016, 107, 1302–1314. [Google Scholar] [CrossRef]
- Mandal, C.; Dutta, A.; Mallick, A.; Chandra, S.; Misra, L.; Sangwan, R.S.; Mandal, C. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis 2008, 13, 1450–1464. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, T.J.; Kim, S.H.; Choi, Y.H.; Lee, S.H.; Lee, J.M.; Kim, Y.H.; Park, J.W.; Kwon, T.K. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008, 13, 1494–1504. [Google Scholar] [CrossRef]
- Mehrotra, A.; Kaul, D.; Joshi, K. LXR-α selectively reprogrammes cancer cells to enter into apoptosis. Mol. Cell Biochem. 2011, 349, 41–55. [Google Scholar] [CrossRef]
- Cruz, S.J. Más de 100 Plantas Medicinales; Medicina Popular Canaria Monografías; Las Palmas de Gran Canaria, Imprenta Pérez Galdós: Las Palmas de Gran Canaria, Spain, 2007; pp. 427–430. [Google Scholar]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Vázquez, J.T.; Bazzochi, I.L. Withanolides from Withania aristata and their cytotoxic activity. Steroids 2010, 75, 974–981. [Google Scholar] [CrossRef]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Bazzochi, I.L. Withaferin A-related steroids from Withania aristata exhibit potent antiproliferative activity by inducing apoptosis in human tumor cells. Eur. J. Med. Chem. 2012, 54, 499–511. [Google Scholar] [CrossRef]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Rodríguez, J.; Jiménez, C.; Bazzochi, I.L. Structure-based design, synthesis, and biological evaluation of withaferin A-analogues as potent apoptotic inducers. Eur. J. Med. Chem. 2017, 140, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Perestelo, N.R.; LLanos, G.G.; Reyes, C.P.; Amesty, A.; Sooda, K.; Afshinjavid, S.; Jiménez, I.A.; Javid, F.; Bazzochi, I.L. Expanding the chemical space of withaferin A by incorporating silicon to improve its clinical potential on human ovarian carcinoma cells. J. Med. Chem. 2019, 62, 4571–4585. [Google Scholar] [CrossRef] [PubMed]
- Riveira, M.J. Studies on biomimetic singlet oxygen oxidations: Application to the synthesis of the alkaloid simulenoline. J. Nat. Prod. 2020, 83, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- PC Model, Version 9.0 with MMX Force Field; Serena Sotware: Bloomington, IN, USA.
- Choudhary, M.I.; Yousuf, S.; Nawaz, S.A.; Ahmed, S.; Rahman, A. Cholinesterase inhibiting withanolides from Withania Somnifera. Chem. Pharm. Bull. 2004, 52, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Gamoh, K.; Ikekawa, N. Synthetic studies of withanolides. Part 6. Stereoselective synthesis of withaferin A and 27-deoxywithaferin A. Tetrahedron Lett. 1982, 23, 4725–4728. [Google Scholar] [CrossRef]
- González, A.G.; Bretón, J.L.; Trujillo, J.M. Withania steroids. III. Steroidal lactones of Withania frutescens. An. Quím. 1974, 70, 69–73. [Google Scholar]
- Misra, L.; Mishra, P.; Pandey, A.; Sangwan, R.S.; Sangwan, N.S.; Tuli, R. Whitanolides from Whitania somnifera roots. Phytochemistry 2008, 69, 1000–1004. [Google Scholar] [CrossRef]
- Kirson, I.; Glotter, E.; Abraham, A.; Lavie, D. Constituents of Withania somnifera dun-XI: The structure of three new withanolides. Tetrahedron 1970, 26, 2209–2219. [Google Scholar] [CrossRef]
- González, A.G.; Darias, V.; Martín-Herrera, D.A.; Suárez, M.C. Cytostatic activity of natural withanolides from Spanish Withanias. Fitoterapia 1982, 53, 85–88. [Google Scholar]
- Misra, L.; Lal, P.; Sangwan, R.S.; Sangwan, N.S.; Uniyal, G.C.; Tuli, R. Unusually sulfated and oxygenated steroids from Withania somnifera. Phytochemistry 2005, 66, 2702–2707. [Google Scholar] [CrossRef]
- González, A.G.; Bretón, J.L.; Trujillo, J.M. Withania steroids. II. Five steroidal lactones of Withania aristata. An. Quím. 1974, 70, 64–68. [Google Scholar]
- Pelletier, S.W.; Mody, N.V.; Nowacki, J.; Bahattacharyya, J. Carbon-13 nuclear magnetic resonance spectral analysis of naturally occurring withanolides and their derivatives. J. Nat. Prod. 1979, 42, 512–521. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Gebeyehu, G.; Nowacki, J.; Mody, N.V. Viscosalactone A and viscosalactone B, two new steroidal lactones from Physalis viscosa. Heterocycles 1981, 15, 317–320. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Abbas, S.; Jamal, S.A.; Atta-ur-Rahman. Withania somniofera a source of exotic whitanolides. Heterocycles 1996, 42, 555–563. [Google Scholar]
- Tamez-Fernández, J.F.; Melchor-Martínez, E.M.; Ibarra-Rivera, T.R.; Rivas-Galindo, V.M. Plant-derived endoperoxides: Structure, occurrence, and bioactivity. Phytochem. Rev. 2020, 19, 827–864. [Google Scholar] [CrossRef]
- Makino, B.; Kawai, M.; Iwata, Y.; Yamamura, H.; Butsugan, Y.; Ogawa, K.; Hayasi, M. Physalins possessing an endoperoxy structure from Physalis alkekengi var. francheti. Structural revision of physalin K. Bull. Chem. Soc. Jpn. 1995, 68, 219–226. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiang, Y.J.; Ding, W.J.; Cheng, L.; Ma, Z.-J. Physalinol A, a 1,10-seco-physalin with an epidioxy from Physalis alkekengi L. var. franchetii (Mast.) Makino. Tetrahedron Lett. 2019, 60, 1330–1332. [Google Scholar] [CrossRef]
- Cirigliano, A.M.; Veleiro, A.S.; Oberti, J.C.; Burton, G. Spiranoid withanolides from Jaborosa odonelliana. J. Nat. Prod. 2002, 65, 1049–1051. [Google Scholar] [CrossRef]
- Fuska, J.; Fusková, A.; Rosazza, J.P.; Nicholas, A.W. Novel cytotoxic and antitumor agents. IV. Withaferin A: Relation of its structure to the in vitro cytotoxic effects on P388 cells. Neoplasma 1984, 31, 31–36. [Google Scholar]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; Volume 6, pp. 71–133. [Google Scholar]
- Tao, S.; Tillotson, J.; Kithsiri-Wijeratne, E.M.; Xu, Y.; Kang, M.J.; Wu, T.; Lau, E.C.; Mesa, C.; Mason, D.J.; Brown, R.V.; et al. Withaferin A analogs that target the AAA+ chaperone p97. ACS Chem. Biol. 2015, 10, 1916–1924. [Google Scholar] [CrossRef]
- Kar, S.; Leszczynski, J. Open access in silico tools to predict the ADMET profiling of drug candidates. Expert Opin. Drug Discov. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Navgeet; Kumar, S. Ganoderic acid targering multiple receptors in cancer: In silico and in vitro study. Tumor Biol. 2016, 37, 14271–14290. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Kumar, S.; Saloni, S.; Singh, H.; Kim, M.-H.; Sharma, P.; Misra, S.; Khan, F. Molecular docking, QSAR and ADMET studies of withanolide analogs against breast cancer. Drug Des. Dev. Ther. 2017, 11, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2020-1: QikProp; Schrödinger, LLC: New York, NY, USA, 2020.
- Zhou, W.; Wang, Y.; Lu, A.; Zhang, G. Systems pharmacology in small molecular drug discovery. Int. J. Mol. Sci. 2016, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Duffy, E.M.; Jorgensen, W.L. Prediction of properties from simulations: Free energies of solvation in hexadecane, octanol, and water. J. Am. Chem. Soc. 2000, 122, 2878–2888. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| H | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | 4.53 d (6.2) | 4.60 d (6.4) | 4.53 d (6.5) | 4.53 d (6.2) |
| 3 | 6.69 dd (6.2, 8.2) | 6.67 dd (6.4, 8.1) | 6.70 dd (6.5, 8.4) | 6.69 dd (6.2, 8.1) |
| 4 | 7.04 d (8.2) | 6.58 d (8.1) | 7.04 d (8.4) | 7.05 d (8.1) |
| 6 | 4.08 s | 4.15 s | 4.08 s | 4.10 s |
| 7 | 1.70 α; 2.01 β, m | 1.45 α; 2.01 β | 1.62 α; 2.14 β, m | 1.95 α; 2.14 β, m |
| 8 | 1.88 | 1.98 | 1.83 | 2.01 |
| 9 | 1.90 | 1.03 | 1.92 | 1.99 |
| 11 | 1.61, 1.90 | 1.69, 2.26 m | 1.60, 1.81 | 1.65, 1.70 |
| 12 | 1.50, 1.73 | 1.38, 1.69 | 1.23, 2.03 | 1.69, 1.73 |
| 14 | 1.75 | 1.59 | 1.19 | 1.55 |
| 15 | 1.25, 1.74 | 1.28, 1.72 | 1.20, 1.67 | 1.43, 2.11 m |
| 16 | 1.63, 1.71 | 1.46, 2.00 | 1.40, 1.69 | 5.55 s |
| 17 | 1.16 | |||
| 18 | 0.88 s | 0.88 s | 0.79 s | 0.88 s |
| 19 | 1.31 s | 1.61 s | 1.31 s | 1.35 s |
| 20 | 2.35 m | 2.35 m | 2.04 | 2.54 m |
| 21 | 1.06 d (7.0) | 1.04 d (6.9) | 1.05 d (6.7) | 1.14 d (7.0) |
| 22 | 4.69 t (8.2) | 4.67 dt (2.9, 11.8) | 4.46 dt (3.3, 13.3) | 4.47 dt (3.9, 12.9) |
| 23 | 2.56 d (8.0) | 2.55 d (8.1) | 2.02 α; 2.54 β, m | 2.19 α, m; 2.56 β, m |
| 27 | 4.37, 4.42 dAB (12.6) | 4.37, 4.41 dAB (11.8) | 4.38, 4.40 dAB (12.2) | 4.38, 4.42 dAB (13.7) |
| 28 | 2.05 s | 2.04 s | 2.08 s | 2.06 s |
| C | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 206.0, C | 205.9, C | 206.0, C | 206.2, C |
| 2 | 78.6, CH | 80.0, CH | 78.6, CH | 78.6, CH |
| 3 | 126.5, CH | 125.9, CH | 126.4, CH | 126.4, CH |
| 4 | 141.4, CH | 142.5, CH | 141.3, CH | 141.5, CH |
| 5 | 84.2, C | 84.6, C | 84.1, C | 84.3, C |
| 6 | 67.1, CH | 69.1, CH | 67.1, CH | 67.2, CH |
| 7 | 36.6, CH2 | 36.4, CH2 | 31.1, CH2 | 31.2, CH2 |
| 8 | 29.6, CH | 30.4, CH | 29.4, CH | 28.0, CH |
| 9 | 42.1, CH | 50.0, CH | 42.4, CH | 43.1, CH |
| 10 | 48.2, C | 48.8, C | 48.2, C | 48.5, C |
| 11 | 22.0, CH2 | 23.1, CH2 | 22.2, CH2 | 22.2, CH2 |
| 12 | 32.0, CH2 | 31.9, CH2 | 39.4, CH2 | 34.8, CH2 |
| 13 | 48.3, C | 48.3, C | 42.9, C | 47.2, C |
| 14 | 49.3, CH | 49.4, CH | 54.8, CH | 56.0, CH |
| 15 | 23.6, CH2 | 23.6, CH2 | 24.3, CH2 | 34.3, CH2 |
| 16 | 34.9, CH2 | 34.0, CH2 | 27.2, CH2 | 124.4, CH |
| 17 | 84.9, C | 85.0, C | 51.9, CH | 155.3, C |
| 18 | 15.0, CH3 | 15.1, CH3 | 12.0, CH3 | 16.5, CH3 |
| 19 | 19.4, CH3 | 16.0, CH3 | 19.5, CH3 | 19.5, CH3 |
| 20 | 42.7, CH | 42.6, CH | 38.8, CH | 35.8, CH |
| 21 | 9.4, CH3 | 9.4; CH3 | 13.4, CH3 | 16.9, CH3 |
| 22 | 79.2, CH | 79.1, CH | 78.8, CH | 79.1, CH |
| 23 | 32.9, CH2 | 32.9, CH2 | 29.8, CH2 | 32.9, CH2 |
| 24 | 154.3, C | 154.1, C | 152.9, C | 152.6, C |
| 25 | 125.1, C | 125.2, C | 125.6, C | 125.7, C |
| 26 | 167.1, C | 167.1, C | 166.7, C | 166.4, C |
| 27 | 57.4, CH2 | 57.5, CH2 | 57.4, CH2 | 57.5, CH2 |
| 28 | 19.9, CH3 | 20.0, CH3 | 19.9, CH3 | 19.9, CH3 |
| Compound | HL 60 | Vero | SI b |
|---|---|---|---|
| 1 | 12.8 ± 0.24 | >40 | >3.1 |
| 2 | 26.7 ± 0.60 | >40 | >1.5 |
| 3 | 3.3 ± 0.45 | 16.0 ± 0.73 | 4.9 |
| 4 | 6.2 ± 0.10 | 31.6 ± 0.08 | 5.1 |
| 5 | 8.2 ± 0.09 | 6.4 ± 0.12 | 0.8 |
| 6 | 7.0 ± 0.55 | 11.5 ± 0.09 | 1.6 |
| 7 | 15.7 ± 0.04 | >40 | >2.6 |
| 8 | 4.2 ± 0.25 | 15.5 ± 0.35 | 3.7 |
| 9 | 14.8 ± 0.68 | 25.7 ± 0.65 | 1.7 |
| 10 | 16.8 ± 0.75 | >40 | >2.4 |
| 11 | 27.8 ± 0.45 | >40 | >1.4 |
| 12 | 6.6 ± 0.14 | >40 | >6.1 |
| 13 | 12.3 ± 0.11 | 23.8 ± 0.41 | 1.9 |
| 14 | 0.2 ± 0.02 | 6.4 ± 0.21 | 33.0 |
| 15 | 0.5 ± 0.07 | 4.6 ± 0.09 | 9.2 |
| 16 | 0.4 ± 0.06 | 5.7 ± 0.13 | 14.8 |
| 17 | 6.8 ± 0.30 | 38.6 ± 0.09 | 5.7 |
| 18 | 8.2 ± 0.43 | 34.7 ± 0.75 | 4.2 |
| 19 | 0.5 ± 0.05 | 1.9 ± 0.08 | 3.8 |
| 20 | 4.6 ± 0.35 | 10.0 ± 0.61 | 2.2 |
| 21 | 0.9 ± 0.05 | 6.2 ± 0.11 | 6.9 |
| 22 | 0.7 ± 0.09 | 16.9 ± 0.7 | 24.1 |
| 23 | 6.5 ± 0.55 | >40 | >6.2 |
| 24 | >40 | >40 | 1.0 |
| 25 | 1.5 ± 0.54 | 11.8 ± 0.1 | 7.9 |
| 26 | 13.4 ± 0.85 | >40 | >3.0 |
| 27 | 1.1 ± 0.2 | 7.8 ± 0.36 | 7.1 |
| 28 | 30.5 ± 0.45 | >40 | >1.3 |
| 29 | 19.2 ± 0.84 | >40 | >2.1 |
| Etoposide c | 2.4 ± 0.1 | 19.5 ± 0.16 | 8.2 |
| Parameters | 14 | 15 | 16 | 19 | 21 | 22 | 25 | 27 | Range b |
|---|---|---|---|---|---|---|---|---|---|
| #stars | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0–5 |
| QPlogBB | −1.391 | −0.825 | −1.626 | −1.358 | −0.806 | −1.397 | −1.958 | −1.63 | −3.0 to 1.2 |
| QPPCaco | 226.10 | 615.13 | 188.31 | 242.52 | 634.31 | 189.69 | 94.33 | 128.79 | <25 poor, >500 great |
| QPPMDCK | 99.18 | 292.58 | 81.39 | 106.99 | 302.46 | 82.04 | 38.55 | 104.92 | <25 poor, >500 great |
| QPlogKhsa | 0.349 | 0.529 | 0.442 | 0.358 | 0.507 | −0.1 | 0.204 | 0.711 | −1.5 to 1.5 |
| QPlogPo/w | 3.054 | 3.55 | 3.341 | 3.123 | 3.537 | 2.501 | 2.456 | 3.833 | −2.0 to 6.5 |
| QPlogKp | −3.96 | −3.302 | −4.114 | −3.824 | −3.186 | −4.204 | −4.857 | −4.355 | −8.0 to −1.0 |
| QPlogS | −5.138 | −5.579 | −6.077 | −5.147 | −5.495 | −4.131 | −5.438 | −6.262 | −6.5 to 0.5 |
| #metab | 4 | 4 | 4 | 6 | 6 | 3 | 6 | 5 | 1 to 8 |
| %HOA | 86.965 | 100 | 74.267 | 87.914 | 100 | 82.361 | 63.711 | 74.192 | >80% high <25% poor |
| PSA | 114.53 | 91.99 | 130.54 | 114.25 | 92.16 | 122.07 | 150.34 | 119.61 | 7.0 to 200.0 |
| SASA | 726.40 | 717.41 | 810.48 | 726.87 | 712.52 | 719.98 | 808.78 | 756.74 | 300.0 to 1000.0 |
| Mol MW | 470.61 | 454.61 | 512.64 | 468.59 | 452.59 | 468.59 | 530.66 | 507.07 | 130.0 to 725.0 |
| #rotor | 5 | 3 | 5 | 5 | 3 | 4 | 6 | 6 | 0 to 15 |
| donorHB | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 0.0 to 6.0 |
| accptHB | 9.4 | 8.7 | 10.7 | 9.4 | 8.7 | 9.7 | 12.4 | 8.15 | 2.0 to 20.0 |
| volume | 1405.7 | 1386.1 | 1553.9 | 1409.1 | 1377.5 | 1393.9 | 1580.1 | 1459.5 | 500.0 to 2000.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moujir, L.M.; Llanos, G.G.; Araujo, L.; Amesty, A.; Bazzocchi, I.L.; Jiménez, I.A. Withanolide-Type Steroids from Withania aristata as Potential Anti-Leukemic Agents. Molecules 2020, 25, 5744. https://doi.org/10.3390/molecules25235744
Moujir LM, Llanos GG, Araujo L, Amesty A, Bazzocchi IL, Jiménez IA. Withanolide-Type Steroids from Withania aristata as Potential Anti-Leukemic Agents. Molecules. 2020; 25(23):5744. https://doi.org/10.3390/molecules25235744
Chicago/Turabian StyleMoujir, Laila M., Gabriel G. Llanos, Liliana Araujo, Angel Amesty, Isabel L. Bazzocchi, and Ignacio A. Jiménez. 2020. "Withanolide-Type Steroids from Withania aristata as Potential Anti-Leukemic Agents" Molecules 25, no. 23: 5744. https://doi.org/10.3390/molecules25235744
APA StyleMoujir, L. M., Llanos, G. G., Araujo, L., Amesty, A., Bazzocchi, I. L., & Jiménez, I. A. (2020). Withanolide-Type Steroids from Withania aristata as Potential Anti-Leukemic Agents. Molecules, 25(23), 5744. https://doi.org/10.3390/molecules25235744