Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Characteristics of Experimental Materials
3.2. Experiment Design, Sample Preparation
3.3. Determination of Phenolic Compounds and Organic Acids
3.4. Determination of Total Phenolic Content (TPC)
3.5. Chemicals
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Tahir, I.; Ahmad, S.S. Physiological and biochemical changes associated with flower development and senescence in Dianthus chinensis L. Ind. J. Plant Physiol. 2014, 19, 215–221. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds, Health Benefits and Potential Applications; Campos, M.R.S., Ed.; Elsevier: Sawston, Cambridge, MA, USA, 2019; pp. 33–50. [Google Scholar]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different cultivars of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.X.; Tan, Q.L.; Zheng, C.S.; Gui, H.P.; Zeng, W.N.; Sun, X.C.; Zhao, X.H. Plant nutrition status yield and quality of satsuma mandarin (Citrus unshiu, Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Silva, A.R.; Fernandes, A.; García, P.A.; Barros, L.; Ferreira, I.C.F.R. Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization. Molecules 2019, 24, 1111. [Google Scholar] [CrossRef]
- Rewald, B. The oil phosphatides content of some petals and stamens. Oil. Soap. 1944, 21, 93–95. [Google Scholar] [CrossRef]
- Lim, T.K. (Ed.) Tulip gesneriana. In Edible Medicinal and Non Medicinal Plants: Volume 8. Flowers; Springer: Dordrecht, Germany, 2014; pp. 221–231. [Google Scholar]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramlhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compost. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Kou, L.; Turner, E.R.; Yaguang, L. Extending the shelf life of edible flowers with controlled release of 1-methylcyclopropene and modified atmosphere packaging. J. Food Sci. 2012, 77, 188–193. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, C.; Beta, T.; Liu, S.; Yang, R. Phenolic profile and antioxidant activity of the edible tree peony flower and underlying mechanisms of preventive effect on H2O2-induced oxidative damage in Caco-2 Cells. Foods 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.-Y. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomerinduced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.Y.; Wang, S.L.; Niu, X.Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Biochem. 2016, 104, 250–256. [Google Scholar] [CrossRef]
- Łysiak, G.; Michalska-Ciechanowska, A.; Wodyło, A. Postharvest changes in phenolic compounds and antioxidant capacity of apples cv. Jonagold growing in different locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Zheng, J.; Meenu, M.; Xu, B. A systematic investigation on free phenolic acids and flavonoids profiles of commonly consumed edible flowers in China. J. Pharm. Biomed. Anal. 2019, 172, 268–277. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Wang, Q.; Du, Z.; Zhang, H.; Zhao, L.; Sun, J.; Zheng, X.; Ren, F. Modulation of gut microbiota by polyphenols from adlay (Coix lacryma-jobi L. var. ma-yuen Stapf.) in rats fed a highcholesterol diet. Int. J. Food Sci. Nutr. 2015, 66, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y. Anti-inflammatory effects of sinapic acid on 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch. Pharmacal Res. 2018, 41, 243–250. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant. Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Pires, T.C.P.S.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R. Nutritional and chemical characterization of edible petals and corresponding infusions: Valorization as new food ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Nutritional composition, antioxidant activity and phenolic compounds of wild Taraxacum sect. Ruderalia. Food Res. Int. 2014, 56, 266–271. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
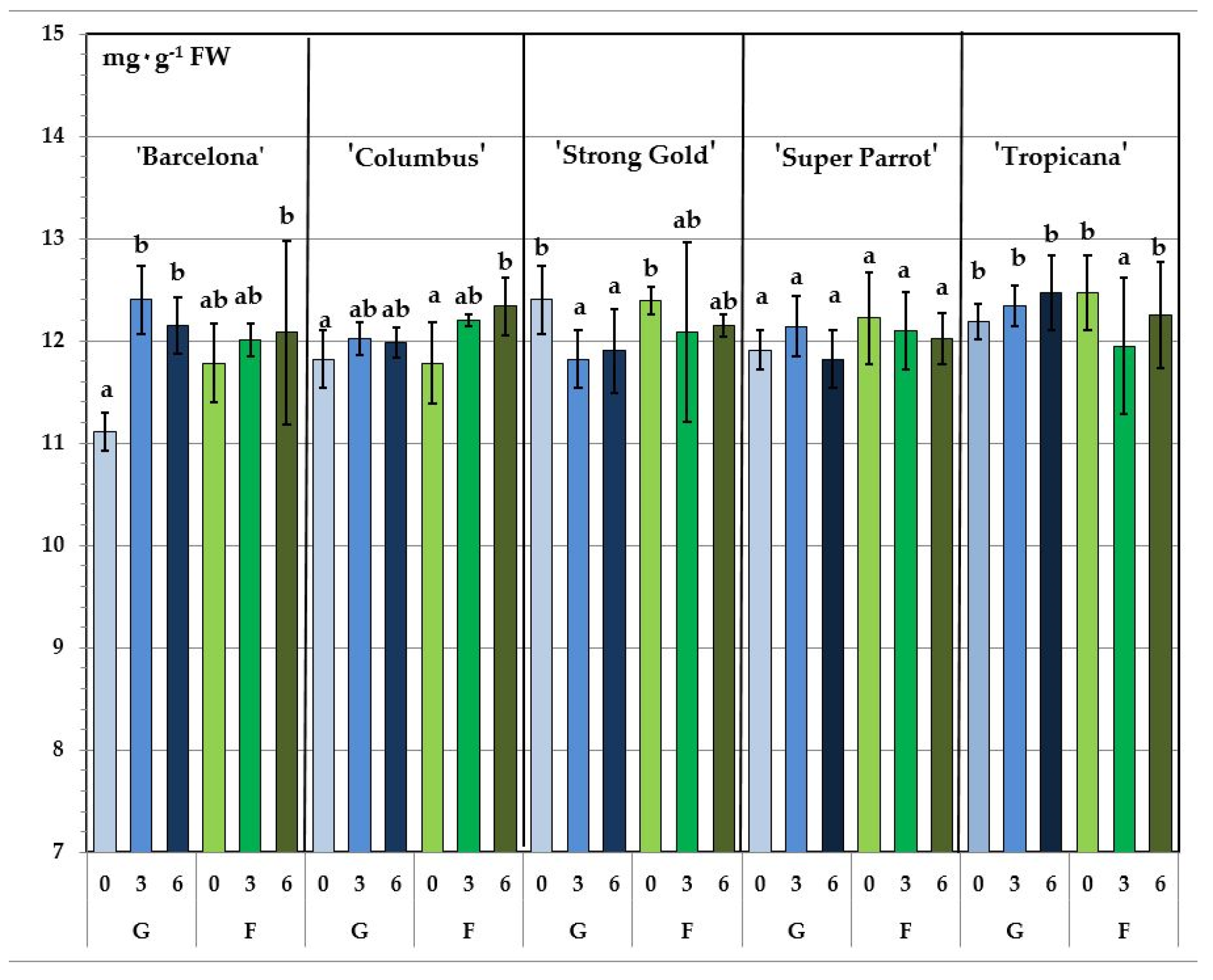
| Cultivar (A) | Type of Cultivation (B) | Storage Length (Days) (C) | TPC | Katechin | Vitexin | Rutin | Quercetin | Luteolin | Sum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg GAE g−1 FW] | [µg g−1 FW] | |||||||||||||||
| Barcelona | greenhouse | 0 | 11.11 | ±0.19 a * | 2.18 | ±0.09 f–i | 1.69 | ±0.24 g,h | 14.63 | ±2.42 i | 0.05 | ±0.01 a,b | 0.1 | ±0.01 c–j | 18.66 | ±2.71 m |
| 3 | 12.4 | ±0.33 b | 1.83 | ±0.22 d–h | 2.25 | ±0.22 i | 15.62 | ±2.26 i | 0.35 | ±0.03 g,h | 0.08 | ±0.01 a–g | 20.13 | ±2.81 m | ||
| 6 | 12.15 | ±0.27 b | 2.82 | ±0.33 j | 1.21 | ±0.22 d–f | 0.92 | ±0.21 a,b | 0.03 | ±0.01 a,b | 0.09 | ±0.01 a–h | 5.07 | ±0.36 b,c | ||
| field | 0 | 11.78 | ±0.38 b | 2.29 | ±0.22 h–j | 5.04 | ±0.14 m | 1.44 | ±0.20 a–d | 0.3 | ±0.071 f–h | 0.11 | ±0.01 i–k | 9.18 | ±0.14 f–i | |
| 3 | 12.01 | ±0.60 b | 3.38 | ±0.44 k | 4.43 | ±0.50 l | 1.42 | ±0.68 a–d | 0.09 | ±0.01 a–c | 0.08 | ±0.01 a–c | 9.39 | ±1.27 g–j | ||
| 6 | 12.08 | ±0.89 b | 8.93 | ±0.59 o | 1.73 | ±0.17 h | 1.48 | ±0.14 a–d | 0.07 | ±0.01 a–c | 0.08 | ±0.01 a–g | 12.29 | ±0.83 k | ||
| Columbus | greenhouse | 0 | 11.82 | ±0.28 b | 1.7 | ±0.18 c–h | 0.29 | ±0.02 a | 5.46 | ±1.29 f | 0.42 | ±0.23 h,i | 0.1 | ±0.01 e–k | 7.96 | ±1.29 d–h |
| 3 | 12.02 | ±0.16 b | 3.37 | ±0.14 k | 1.58 | ±0.20 e–h | 10.24 | ±1.35 h | 0.13 | ±0.03 a–d | 0.14 | ±0.01 l,m | 15.46 | ±1.44 l | ||
| 6 | 11.98 | ±0.15 b | 2.28 | ±0.38 h–j | 0.73 | ±0.10 b,c | 4.88 | ±0.57 e,f | 0.2 | ±0.02 c–f | 0.09 | ±0.01 a–h | 8.17 | ±0.78 e–h | ||
| field | 0 | 11.78 | ±0.40 b | 4.76 | ±0.51 m | 1.28 | ±0.35 d–g | 2.54 | ±0.24 b–d | 0.24 | ±0.03 d–g | 0.08 | ±0.01 a–d | 8.89 | ±0.86 f–i | |
| 3 | 12.2 | ±0.62 b | 1.26 | ±0.36 b–d | 1.75 | ±0.33 h | 2.07 | ±0.49 a–d | 0.12 | ±0.03 a–d | 0.08 | ±0.01 a–e | 5.27 | ±0.90 b,c | ||
| 6 | 12.34 | ±0.28 b | 2.51 | ±0.22 i,j | 0.46 | ±0.03 a,b | 2.66 | ±0.19 b–d | 0.09 | ±0.01 a–c | 0.07 | ±0.01 a | 5.79 | ±0.28 b–d | ||
| Strong Gold | greenhouse | 0 | 12.4 | ±0.33 b | 1.99 | ±0.11 e–i | 0.91 | ±0.04 c,d | 22.03 | ±2.67 j | 0.13 | ±0.01 a–d | 0.09 | ±0.01 b–i | 25.14 | ±2.73 n |
| 3 | 11.82 | ±0.28 b | 0.84 | ±0.15 b | 1.29 | ±0.11 d–g | 42.39 | ±0.59 k | 0.14 | ±0.03 a–e | 0.08 | ±0.01 a–f | 44.75 | ±0.69 o | ||
| 6 | 11.9 | ±0.41 b | 1.63 | ±0.27 c–f | 1.26 | ±0.18 d–g | 47.68 | ±2.09 l | 0.13 | ±0.02 a–e | 0.12 | ±0.05 j–l | 50.82 | ±2.27 p | ||
| field | 0 | 12.39 | ±0.36 b | 5.62 | ±0.47 n | 1.34 | ±0.18 e–h | 2.53 | ±0.41 b–d | 0.25 | ±0.02 d–g | 0.1 | ±0.01 f–k | 9.84 | ±0.92 h–j | |
| 3 | 12.09 | ±0.88 b | 1.48 | ±0.40 c–e | 0.7 | ±0.09 b,c | 2.61 | ±0.32 b–d | 0.02 | ±0.00 a | 0.07 | ±0.02 a–c | 4.89 | ±0.65 b,c | ||
| 6 | 12.15 | ±0.11 b | 4.17 | ±0.27 l | 1.19 | ±0.24 d,e | 2.67 | ±0.04 b–d | 0.12 | ±0.16 a–d | 0.07 | ±0.01 a,b | 8.23 | ±0.85 e–h | ||
| Super Parrot | greenhouse | 0 | 11.91 | ±0.19 b | 1.27 | ±0.28 b–d | 1.4 | ±0.41 e–h | 3.4 | ±0.42 d,e | 0.37 | ±0.03 g,h | 0.12 | ±0.01 kl | 6.56 | ±0.36 b–e |
| 3 | 12.14 | ±0.30 b | 0.71 | ±0.11 b | 0.1 | ±0.03 a | 1.01 | ±0.05 a–c | 0.03 | ±0.01 a | 0.1 | ±0.01 h–k | 1.96 | ±0.07 a | ||
| 6 | 11.82 | ±0.28 b | 0.19 | ±0.03 a | 0.35 | ±0.05 a,b | 1.29 | ±0.20 a–c | 0.14 | ±0.01 a–e | 0.11 | ±0.01 i–k | 2.09 | ±0.14 a | ||
| field | 0 | 12.22 | ±0.44 b | 2.25 | ±0.39 g–j | 2.32 | ±0.28 i | 2.58 | ±0.38 b–d | 0.17 | ±0.03 b–f | 0.08 | ±0.01 a–e | 7.4 | ±0.30 d–g | |
| 3 | 12.1 | ±0.37 b | 3.5 | ±0.28 k | 1.17 | ±0.16 d,e | 3 | ±0.12 c,d | 0.08 | ±0.01 a–c | 0.09 | ±0.01 c–i | 7.84 | ±0.28 d–h | ||
| 6 | 12.02 | ±0.25 b | 1.16 | ±0.10 b,c | 1.63 | ±0.10 f–h | 1.78 | ±0.15 a–d | 0.13 | ±0.01 a–e | 0.08 | ±0.01 a–e | 4.79 | ±0.10 b | ||
| Tropicana | greenhouse | 0 | 12.19 | ±0.18 b | 1.67 | ±0.27 c–g | 0.28 | ±0.06 a | 8.66 | ±1.62 g,h | 0.26 | ±0.23 e–g | 0.1 | ±0.01 d–k | 10.96 | ±1.80 i–k |
| 3 | 12.34 | ±0.20 b | 1.81 | ±0.33 d–h | 0.38 | ±0.20 a,b | 8.48 | ±0.64 g | 0.52 | ±0.01 i | 0.14 | ±0.02 m | 11.34 | ±0.90 j,k | ||
| 6 | 12.47 | ±0.36 b | 2.44 | ±0.18 i,j | 3.09 | ±0.15 j | 2.9 | ±0.17 b–d | 0.34 | ±0.04 g,h | 0.11 | ±0.01 g–k | 8.87 | ±0.46 f–i | ||
| field | 0 | 12.47 | ±0.36 b | 4.13 | ±0.16 l | 2.34 | ±0.2 i | 0.21 | ±0.02 a | 0.26 | ±0.01 e–g | 0.09 | ±0.01 a–h | 7.04 | ±0.28 c–f | |
| 3 | 11.95 | ±0.66 b | 1.82 | ±0.58 d–h | 3.94 | ±0.53 k | 6.06 | ±0.22 f | 0.54 | ±0.05 i | 0.08 | ±0.01 a–g | 12.44 | ±0.93 k | ||
| 6 | 12.25 | ±0.52 b | 3.77 | ±0.19 k,l | 1.42 | ±0.11 e–h | 2.47 | ±0.33 b–d | 0.14 | ±0.01 a–e | 0.08 | ±0.01 a–d | 7.87 | ±0.15 d–h | ||
| Mean for A | Barcelona | 11.93 | ±0.55 a | 3.57 | ±2.53 c | 2.73 | ±1.52 c | 5.92 | ±5.81 c | 0.15 | ±0.13 a | 0.09 | ±0.02 a | 12.45 | ±5.67 d | |
| Columbus | 12.02 | ±0.29 a,b | 2.65 | ±1.21 b | 1.01 | ±0.60 a | 4.64 | ±2.96 b | 0.2 | ±0.14 b | 0.09 | ±0.03 a,b | 8.59 | ±3.53 b | ||
| Strong Gold | 12.12 | ±0.43 a,b | 2.62 | ±1.76 b | 1.12 | ±0.27 a | 19.99 | ±19.56 d | 0.13 | ±0.09 a | 0.09 | ±0.16 a | 23.94 | ±18.67 e | ||
| Super Parrot | 12.03 | ±0.30 a,b | 1.52 | ±1.13 a | 1.16 | ±0.79 a | 2.17 | ±0.93 a | 0.16 | ±0.11 a,b | 0.1 | ±0.02 b | 5.11 | ±2.45 a | ||
| Tropicana | 12.28 | ±0.40 b | 2.61 | ±1.05 b | 1.91 | ±1.41 b | 4.8 | ±3.32 b | 0.35 | ±0.17 c | 0.1 | ±0.03 b | 9.76 | ±2.16 c | ||
| Mean for B | greenhouse | 12.03 | ±0.39 a | 1.78 | ±0.82 a | 1.12 | ±0.82 a | 12.64 | ±12.22 b | 0.22 | ±0.17 b | 0.11 | ±0.02 b | 15.86 | ±14.28 b | |
| field | 12.12 | ±0.43 a | 3.4 | ±2.01 b | 2.05 | ±1.35 b | 2.37 | ±1.25 a | 0.17 | ±0.13 a | 0.08 | ±0.01 a | 8.08 | ±2.39 a | ||
| Mean for C | 0 | 12.02 | ±0.46 a | 2.78 | ±1.46 b | 1.69 | ±1.34 b | 6.35 | ±6.20 a | 0.25 | ±0.14 c | 0.1 | ±0.02 b | 11.16 | ±5.93 a | |
| 3 | 12.11 | ±0.38 a | 2 | ±1.05 a | 1.76 | ±1.40 b | 9.29 | ±9.10 b | 0.2 | ±0.19 b | 0.1 | ±0.03 b | 13.35 | ±11.87 b | ||
| 6 | 12.12 | ±0.39 a | 2.99 | ±2.32 c | 1.31 | ±0.76 a | 6.87 | ±9.00 a | 0.14 | ±0.09 a | 0.09 | ±0.02 a | 11.4 | ±11.30 a | ||
| Cultivar (A) | Type of Cultivation (B) | Storage Length (Days) (C) | Gallic | Protocatechiuc | 4-HBA | Vanilic | Syringic | Transcinnamin | 2.5-DHBA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barcelona | greenhouse | 0 | 0.49 | ±0.04 b–e * | 2.99 | ±0.63 f | 2.74 | ±0.41 i–l | 2.15 | ±0.07 g–i | 2.16 | ±0.33 b–e | 22.71 | ±1.25 l | 5.01 | ±0.36 q |
| 3 | 0.7 | ±0.09 d–f | 0.71 | ±0.03 a,b | 3.83 | ±0.40 no | 1.5 | ±0.35 d–f | 3.46 | ±0.74 f,g | 29.17 | ±1.84 n | 2.68 | ±0.44 l–n | ||
| 6 | 1.38 | ±0.18 j,k | 2.07 | ±0.12 c–f | 2.11 | ±0.18 e–h | 1.06 | ±0.09 b–e | 4.16 | ±0.36 g–i | 4.56 | ±0.56 d–g | 1.88 | ±0.38 j,k | ||
| field | 0 | 1.12 | ±0.20 i,j | 1.6 | ±0.18 b–d | 4.28 | ±0.49 o | 2.64 | ±0.38 i–k | 1.06 | ±0.19 a | 6.36 | ±0.35 f–i | 2.44 | ±0.25 l,m | |
| 3 | 1.12 | ±0.14 i,j | 7.38 | ±0.45 i,j | 6.69 | ±0.47 q | 1.53 | ±0.40 d–f | 5.82 | ±0.91 k | 3.02 | ±0.27 b–d | 1.51 | ±0.45 e–j | ||
| 6 | 0.82 | ±0.03 f–h | 8.51 | ±0.36 j | 1.3 | ±0.27 b–d | 1.67 | ±0.11 f,g | 3.96 | ±0.09 g–i | 3.7 | ±0.33 c,d | 3.35 | ±0.22 o | ||
| Columbus | greenhouse | 0 | 2.55 | ±0.30 m | 7 | ±1.55 i | 2.35 | ±0.39 f–j | 2.09 | ±0.50 g,h | 2.24 | ±0.62 c–e | 26.9 | ±3.54 m | 1.33 | ±0.15 d–i |
| 3 | 1.64 | ±0.06 k | 5.01 | ±0.45 h | 2.95 | ±0.29 j–m | 3.72 | ±0.40 l | 7.35 | ±0.96 l | 22.05 | ±1.75 l | 2.33 | ±0.45 k,l | ||
| 6 | 0.49 | ±0.08 b–e | 7.03 | ±0.19 i | 2.92 | ±0.19 j–l | 3.1 | ±0.28 k | 7.47 | ±0.45 l | 22.34 | ±2.11 l | 0.61 | ±0.01 a,b | ||
| field | 0 | 0.78 | ±0.06 e–g | 7.5 | ±1.48 i,j | 1.81 | ±0.15 d–f | 5.04 | ±0.50 m | 4.07 | ±0.11 g–i | 1.13 | ±0.19 a,b | 1.38 | ±0.14 d–j | |
| 3 | 0.74 | ±0.04 e–g | 0.51 | ±0.15 a | 1.34 | ±0.18 b–d | 1.48 | ±0.33 d–f | 1.28 | ±0.39 a,b | 1.31 | ±0.22 a,b | 1.86 | ±0.60 i,j | ||
| 6 | 0.32 | ±0.02 a–c | 1.29 | ±0.28 a–c | 2.2 | ±0.40 e–i | 3.85 | ±0.32 l | 3.69 | ±0.59 g–i | 1.5 | ±0.22 a–c | 0.76 | ±0.12 a–c | ||
| Strong Gold | greenhouse | 0 | 2.14 | ±0.10 l | 4.95 | ±0.52 h | 1.91 | ±0.24 d–g | 4.07 | ±0.25 l | 2.56 | ±0.29 d,e | 12.38 | ±1.33 k | 2.83 | ±0.33 m,n |
| 3 | 0.42 | ±0.08 a–d | 1.42 | ±0.24 a–c | 0.88 | ±0.15 a,b | 1.65 | ±0.10 f,g | 5.66 | ±0.40 k | 7.14 | ±0.59 h,i | 0.91 | ±0.28 b–d | ||
| 6 | 3.06 | ±0.27 n | 2.04 | ±0.37 c–e | 1.47 | ±0.34 b–d | 0.32 | ±0.03 a | 5.29 | ±0.66 j,k | 3.02 | ±0.16 b–d | 1.41 | ±0.29 d–j | ||
| field | 0 | 2.84 | ±0.20 n | 5.03 | ±0.32 h | 3.13 | ±0.78 k–m | 2.71 | ±0.51 j,k | 3.6 | ±0.23 g,h | 4.34 | ±0.16 d–g | 4.1 | ±0.18 p | |
| 3 | 2.94 | ±0.06 n | 1.33 | ±0.16 a–c | 1.12 | ±0.18 a–c | 2.49 | ±0.35 h–j | 1.64 | ±0.12 a–d | 1.58 | ±0.33 a–c | 1.68 | ±0.08 g–j | ||
| 6 | 0.31 | ±0.04 a–c | 1.68 | ±0.24 b–d | 1.71 | ±0.08 c–e | 1.49 | ±0.18 d–f | 4.47 | ±0.41 h–j | 2.62 | ±0.25 a–d | 1.63 | ±0.19 f–j | ||
| Super Parrot | greenhouse | 0 | 0.37 | ±0.05 a–c | 0.92 | ±0.25 a,b | 0.6 | ±0.07 a | 1.01 | ±0.30 b–d | 1.41 | ±0.08 a–c | 3.14 | ±0.52 b–d | 0.41 | ±0.04 a |
| 3 | 5.07 | ±0.21 o | 2.9 | ±0.34 e,f | 0.54 | ±0.05 a | 0.54 | ±0.12 a,b | 4.02 | ±0.66 g–i | 1.66 | ±0.25 a–c | 1.69 | ±0.22 g–j | ||
| 6 | 2.19 | ±0.41 l | 0.86 | ±0.36 a,b | 1.14 | ±0.17 a–c | 2.09 | ±0.14 g,h | 1.83 | ±0.08 a–e | 0.67 | ±0.08 a | 1.11 | ±0.28 c–f | ||
| field | 0 | 0.21 | ±0.01 a,b | 2.4 | ±0.32 d–f | 1.6 | ±0.26 c–e | 0.79 | ±0.25 a–c | 2.65 | ±0.55 e,f | 10.02 | ±2.52 j | 2.37 | ±0.09 l,m | |
| 3 | 0.53 | ±0.02 c–f | 1.19 | ±0.17 a–c | 3.33 | ±0.31 l–n | 1.13 | ±0.11 c–f | 2.39 | ±0.38 d,e | 4.57 | ±0.23 d–g | 1.32 | ±0.23 d–h | ||
| 6 | 0.14 | ±0.01 a | 1.24 | ±0.16 a–c | 0.96 | ±0.11 a,b | 0.92 | ±0.07 b,c | 4.35 | ±0.17 g–i | 8.36 | ±1.04 i,j | 1.73 | ±0.09 h–j | ||
| Tropicana | greenhouse | 0 | 0.34 | ±0.06 a–c | 9.15 | ±0.70 k | 3.53 | ±0.39 m,n | 1.28 | ±0.13 c–f | 4.29 | ±0.72 g–i | 35.75 | ±2.94 o | 0.35 | ±0.12 a |
| 3 | 0.55 | ±0.02 c–f | 4.97 | ±0.17 h | 0.98 | ±0.22 a,b | 1.54 | ±0.35 d–f | 4.58 | ±0.27 i,j | 4.1 | ±0.38 d–f | 1.18 | ±0.24 c–g | ||
| 6 | 1 | ±0.08 g–i | 7.13 | ±0.29 i | 2.53 | ±0.35 h–k | 3.11 | ±0.41 k | 6.9 | ±0.32 l | 6.01 | ±0.35 e–h | 0.54 | ±0.02 a,b | ||
| field | 0 | 1.1 | ±0.18 h–j | 3.97 | ±0.29 g | 6.75 | ±0.36 q | 8.38 | ±0.51 n | 7.36 | ±0.33 l | 6.44 | ±0.36 g–i | 9.35 | ±0.38 r | |
| 3 | 2.98 | ±0.47 n | 2.44 | ±0.40 d–f | 5.06 | ±0.51 p | 4.05 | ±0.14 l | 9.24 | ±0.63 m | 2.57 | ±0.20 a–d | 2.93 | ±0.11 n,o | ||
| 6 | 0.47 | ±0.04 b–e | 1.63 | ±0.46 b–d | 2.49 | ±0.44 g–j | 1.6 | ±0.16 e–g | 11.61 | ±0.89 n | 3.77 | ±0.57 c–e | 1 | ±0.28 b–e | ||
| Mean for A | Barcelona | 0.94 | ±0.33 a | 3.83 | ±2.99 c | 3.49 | ±1.82 d | 1.76 | ±0.57 b | 3.44 | ±1.62 b | 11.59 | ±10.70 c | 2.81 | ±1.22 d | |
| Columbus | 1.09 | ±0.81 b | 4.72 | ±3.00 d | 2.26 | ±0.64 c | 3.21 | ±1.25 d | 4.35 | ±2.47 d | 12.54 | ±11.80 d | 1.38 | ±0.67 a | ||
| Strong Gold | 1.95 | ±1.20 d | 2.74 | ±1.68 b | 1.7 | ±0.81 b | 2.12 | ±1.22 c | 3.87 | ±1.51 c | 5.18 | ±3.81 a | 2.09 | ±1.12 b | ||
| Super Parrot | 1.42 | ±1.34 c | 1.59 | ±2.70 d | 1.36 | ±0.99 a | 1.08 | ±0.53 a | 2.77 | ±1.16 a | 4.74 | ±3.64 a | 1.44 | ±0.64 a | ||
| Tropicana | 1.07 | ±0.94 b | 4.88 | ±0.83 a | 3.56 | ±1.97 d | 3.33 | ±2.55 d | 7.32 | ±2.66 e | 9.78 | ±9.10 b | 2.56 | ±2.25 c | ||
| Mean for B | greenhouse | 1.49 | ±1.31 b | 3.94 | ±2.70 b | 2.03 | ±1.06 a | 1.95 | ±1.12 a | 4.23 | ±2.00 a | 13.44 | ±11.59 b | 1.62 | ±1.22 a | |
| field | 1.09 | ±0.98 a | 3.16 | ±2.59 a | 2.92 | ±1.93 b | 2.65 | ±1.99 b | 4.48 | ±2.92 b | 4.09 | ±2.68 a | 2.49 | ±2.06 b | ||
| Mean for C | 0 | 1.19 | ±0.95 b | 4.55 | ±2.68 c | 2.87 | ±1.69 c | 3.01 | ±2.25 b | 3.14 | ±1.79 a | 12.92 | ±11.31 c | 2.96 | ±2.61 c | |
| 3 | 1.67 | ±1.49 c | 2.79 | ±2.22 a | 2.67 | ±2.01 b | 1.96 | ±1.11 a | 4.54 | ±2.50 b | 7.72 | ±7.42 b | 1.81 | ±0.70 b | ||
| 6 | 1.02 | ±0.92 a | 3.32 | ±2.80 b | 1.88 | ±0.68 a | 1.92 | ±1.09 a | 5.37 | ±2.65 c | 5.66 | ±5.08 a | 1.4 | ±0.82 a | ||
| Cultivar (A) | Type of Cultivation (B) | Storage Length (Days) (C) | Caffeic | p–Cumaric | Chlorogenic | Ferulic | Sinapic | Salicylic | Sum | |||||||
| Barcelona | greenhouse | 0 | 4.24 | ±0.20 j | 0.62 | ±0.07 d–h | 1.77 | ±0.25 a–c | 2.24 | ±0.35 h | 13.26 | ±1.88 k | 0.14 | ±0.15 a | 60.53 | ±0.50 k |
| 3 | 1.42 | ±0.19 d–f | 2.06 | ±0.14 n | 0.17 | ±0.01 a | 1.68 | ±0.33 f,g | 7.02 | ±0.28 j | 0.15 | ±0.04 a | 54.55 | ±3.69 j | ||
| 6 | 0.64 | ±0.02 a,b | 0.67 | ±0.08 e–h | 1.35 | ±0.24 a,b | 1.49 | ±0.06 d–f | 13.74 | ±1.44 k | 0.73 | ±0.19 a–c | 35.84 | ±1.49 g | ||
| field | 0 | 3.47 | ±0.47 i | 0.4 | ±0.06 b–e | 0.97 | ±0.13 a,b | 1.15 | ±0.20 c–f | 4.79 | ±0.23 g,h | 0.62 | ±0.12 a–c | 30.92 | ±1.41 e,f | |
| 3 | 0.55 | ±0.12 a | 1.55 | ±0.47 m | 1.44 | ±0.18 a,b | 0.34 | ±0.04 a,b | 16.92 | ±1.50 l | 0.1 | ±0.01 a | 47.97 | ±2.18 i | ||
| 6 | 2.35 | ±0.33 g | 1.28 | ±0.27 k–m | 0.17 | ±0.02 a | 2 | ±0.37 g,h | 8.16 | ±0.54 j | 0.63 | ±0.04 a–c | 37.61 | ±0.78 g | ||
| Columbus | greenhouse | 0 | 0.81 | ±0.14 a–c | 0.37 | ±0.06 a–e | 32.98 | ±5.25 e | 0.74 | ±0.23 b,c | 3.23 | ±0.60 c–g | 0.29 | ±0.02 a,b | 82.89 | ±5.48 m |
| 3 | 1.21 | ±0.38 c–f | 1.24 | ±0.13 j–k | 1.56 | ±0.48 a,b | 1.42 | ±0.34 d–f | 4.64 | ±0.27 g,h | 0.45 | ±0.03 a,b | 55.58 | ±2.56 j | ||
| 6 | 0.62 | ±0.03 a,b | 0.34 | ±0.06 a–d | 1.62 | ±0.21 a,b | 1.25 | ±0.15 c–f | 3.98 | ±0.28 e–h | 8.78 | ±0.78 i | 60.55 | ±3.08 k | ||
| field | 0 | 0.62 | ±0.06 a,b | 0.91 | ±0.03 h,i | 3.51 | ±0.44 c | 0.76 | ±0.10 b,c | 13.39 | ±2.12 k | 2.18 | ±0.15 e | 43.08 | ±4.25 h | |
| 3 | 0.55 | ±0.13 a | 0.16 | ±0.02 a,b | 0.38 | ±0.13 a,b | 1.03 | ±0.22 c–e | 15.42 | ±1.65 l | 0.67 | ±0.12 a–c | 26.73 | ±1.92 c–e | ||
| 6 | 1.62 | ±0.23 f | 1.45 | ±0.33 l,m | 0.46 | ±0.03 a,b | 3.32 | ±0.10 i | 4.8 | ±0.78 g,h | 0.79 | ±0.03 a–c | 26.05 | ±1.21 b–d | ||
| Strong Gold | greenhouse | 0 | 5.17 | ±0.36 k | 0.57 | ±0.05 c–f | 0.92 | ±0.06 a,b | 0.94 | ±0.03 c,d | 4.96 | ±0.06 g–i | 0.2 | ±0.03 a | 43.61 | ±2.83 h |
| 3 | 1.56 | ±0.38 e,f | 0.41 | ±0.08 b–e | 0.17 | ±0.00 a | 0.97 | ±0.10 c,d | 4.08 | ±0.43 e–h | 0.23 | ±0.06 a | 25.49 | ±0.22 b–d | ||
| 6 | 0.39 | ±0.04 a | 3.56 | ±0.30 p | 0.15 | ±0.11 a | 1.12 | ±0.08 c–e | 3.86 | ±0.43 e–h | 1.17 | ±0.14 c,d | 26.85 | ±0.71 c–e | ||
| field | 0 | 1.03 | ±0.22 b–d | 0.99 | ±0.21 i,j | 0.92 | ±0.15 a,b | 1.21 | ±0.13 c–f | 4.61 | ±0.3 g,h | 0.61 | ±0.04 a–c | 35.11 | ±2.28 f,g | |
| 3 | 2.6 | ±0.43 g,h | 0.8 | ±0.07 f–i | 3.38 | ±0.35 c | 1.17 | ±0.10 c–f | 2.42 | ±0.45 b–e | 0.96 | ±0.06 b–d | 24.12 | ±1.63 b,c | ||
| 6 | 2.85 | ±0.10 h | 0.18 | ±0.02 a,b | 1.56 | ±0.24 ab | 1.56 | ±0.40 e–g | 3.39 | ±0.37 c–h | 0.39 | ±0.04 a,b | 23.83 | ±0.94 b,c | ||
| Super Parrot | greenhouse | 0 | 0.51 | ±0.09 a | 3.05 | ±0.18 o | 0.14 | ±0.03 a | 1.46 | ±0.37 d–f | 0.97 | ±0.2 a,b | 3.56 | ±0.31 g | 17.54 | ±1.37 a |
| 3 | 2.26 | ±0.55 g | 0.09 | ±0.82 a | 1.08 | ±0.62 a,b | 1.19 | ±2.68 c–f | 51 | ±3.63 a | 0.26 | ±1.38 a | 21.81 | ±1.52 b | ||
| 6 | 2.5 | ±0.30 g,h | 0.53 | ±0.07 c–f | 0.82 | ±0.24 a,b | 0.15 | ±0.05 a | 0.29 | ±0.01 a | 1.31 | ±0.15 c,d | 15.5 | ±0.60 a | ||
| field | 0 | 0.52 | ±0.06 a | 0.16 | ±0.03 a,b | 0.54 | ±0.05 a,b | 0.41 | ±0.07 a,b | 5.31 | ±0.14 h,i | 1.3 | ±0.31 c,d | 28.28 | ±2.42 c–e | |
| 3 | 1.35 | ±0.10 d–f | 1.36 | ±0.11 l,m | 2.29 | ±0.28 b,c | 1.21 | ±0.15 c–f | 1.67 | ±0.59 a–c | 2.23 | ±0.39 e | 24.55 | ±2.00 b–d | ||
| 6 | 0.85 | ±0.10 a–c | 0.88 | ±0.07 g–i | 0.91 | ±0.07 a,b | 0.72 | ±0.03 b,c | 4.38 | ±0.26 f–h | 1.52 | ±0.13 d | 26.95 | ±1.15 c–e | ||
| Tropicana | greenhouse | 0 | 1.13 | ±0.08 c–e | 0.53 | ±0.04 c–f | 0.14 | ±0.01 a | 7.35 | ±0.81 j | 2.67 | ±0.28 b–f | 0.19 | ±0.00 a | 66.69 | ±5.17 l |
| 3 | 0.55 | ±0.12 a | 0.3 | ±0.02 a–c | 0.13 | ±0.01 a | 8.13 | ±0.61 k | 1.9 | ±0.53 a–d | 5.81 | ±0.25 h | 34.72 | ±1.45 f,g | ||
| 6 | 1.39 | ±0.18 d–f | 0.55 | ±0.10 c–f | 6.81 | ±0.48 d | 1.15 | ±0.15 c–f | 3.7 | ±0.58 d–h | 10.8 | ±1.65 j | 51.64 | ±3.74 i,j | ||
| field | 0 | 3.5 | ±0.25 i | 0.61 | ±0.03 d–g | 1.35 | ±0.07 a,b | 1.09 | ±0.17 c–e | 16.48 | ±2.91 l | 2.83 | ±0.17 f | 69.2 | ±4.05 l | |
| 3 | 3.86 | ±0.32 i,j | 1.04 | ±0.27 i–k | 1.4 | ±0.20 a,b | 1.18 | ±0.18 c–f | 6.64 | ±1.63 i,j | 0.25 | ±0.02 a | 43.65 | ±1.04 h | ||
| 6 | 1.51 | ±0.24 e,f | 1.01 | ±0.09 i–j | 1.49 | ±0.40 a,b | 1.22 | ±0.14 c–f | 1.02 | ±0.18 a,b | 0.33 | ±0.02 a,b | 29.16 | ±1.41 d,e | ||
| Mean for A | Barcelona | 2.11 | ±1.44 c,d | 1.1 | ±0.63 b | 0.98 | ±0.65 a | 1.48 | ±0.68 c | 10.65 | ±4.50 e | 0.39 | ±0.29 a | 44.56 | ±11.10 c | |
| Columbus | 0.9 | ±0.42 a | 0.75 | ±0.52 a | 6.75 | ±12.3 c | 1.42 | ±0.93 c | 7.58 | ±5.13 d | 2.19 | ±2.11 c | 49.15 | ±20.70 d | ||
| Strong Gold | 2.27 | ±1.67 d | 1.09 | ±1.10 b | 1.18 | ±1.14 a | 1.16 | ±0.26 b | 3.89 | ±0.91 b | 0.59 | ±0.38 a | 29.84 | ±7.58 b | ||
| Super Parrot | 1.33 | ±0.85 b | 1.01 | ±1.00 b | 0.96 | ±0.70 a | 0.86 | ±0.52 a | 2.19 | ±2.02 a | 1.7 | ±1.07 b | 22.44 | ±5.01 a | ||
| Tropicana | 1.99 | ±1.29 c | 0.68 | ±0.29 b | 1.89 | ±1.35 b | 3.35 | ±3.22 d | 5.4 | ±5.30 c | 3.37 | ±3.04 d | 49.18 | ±15.70 d | ||
| Mean for B | greenhouse | 1.63 | ±1.39 a | 0.99 | ±1.03 b | 3.32 | ±3.26 b | 2.09 | ±2.01 b | 4.59 | ±3.98 a | 2.27 | ±2.20 b | 43.59 | ±19.80 b | |
| field | 1.81 | ±0.74 b | 0.53 | ±0.48 a | 1.38 | ±0.99 a | 1.23 | ±0.71 a | 7.29 | ±5.47 b | 1.03 | ±0.81 a | 34.48 | ±32.22 a | ||
| Mean for C | 0 | 2.1 | ±1.74 b | 0.82 | ±0.80 a | 4.33 | ±4.26 b | 1.74 | ±1.70 b | 6.97 | ±5.27 c | 1.19 | ±1.15 a | 47.79 | ±20.51 c | |
| 3 | 1.59 | ±1.05 a | 0.9 | ±0.66 a | 1.2 | ±1.04 a | 1.83 | ±1.73 b | 6.12 | ±5.56 b | 1.11 | ±1.01 a | 35.92 | ±12.97 b | ||
| 6 | 1.47 | ±0.85 a | 1.05 | ±0.95 b | 1.53 | ±1.44 a | 1.4 | ±0.83 a | 4.73 | ±3.71 a | 2.64 | ±2.51 b | 33.4 | ±13.20 a | ||
| Cultivar (A) | Type of Cultivation (B) | Storage Length (Days) (C) | Oxalic | Quinic | Malic | Malonic | Citric | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barcelona | greenhouse | 0 | 0.02 | ±0.05 a * | 0.19 | ±0.04 a,b | 0 | ±0.00 a | 7.53 | ±1.48 b–e | 0 | ±0.00 a |
| 3 | 0.01 | ±0.00 a | 0.28 | ±0.10 a–d | 0 | ±0.00 a | 3.92 | ±0.61 a–d | 0 | ±0.00 a | ||
| 6 | 0.12 | ±0.16 a–c | 0.34 | ±0.15 b–e | 0 | ±0.00 a | 6.01 | ±0.30 a–e | 0.2 | ±0.04 a | ||
| field | 0 | 0.04 | ±0.00 a,b | 0.3 | ±0.09 a–d | 0 | ±0.00 a | 11.61 | ±2.40 e–g | 19.99 | ±4.38 e | |
| 3 | 0.08 | ±0.01 a,b | 2.8 | ±0.41 i | 0 | ±0.00 a | 73.96 | ±8.89 i | 0.27 | ±0.01 a | ||
| 6 | 0.04 | ±0.01 a,b | 0.52 | ±0.02 c–f | 0.42 | ±0.02 c | 8.13 | ±0.70 c–e | 0.06 | ±0.00 a | ||
| Columbus | greenhouse | 0 | 0.01 | ±0.00 a | 0.32 | ±0.15 b–e | 0 | ±0.00 a | 7.38 | ±2.13 b–e | 0 | ±0.00 a |
| 3 | 0.02 | ±0.01 a | 0.37 | ±0.06 b–e | 0 | ±0.00 a | 8.91 | ±2.77 d–f | 7.76 | ±0.82 c | ||
| 6 | 0.02 | ±0.00 a | 0.29 | ±0.09 a–d | 0 | ±0.00 a | 7.46 | ±3.05 b–e | 0.15 | ±0.04 a | ||
| field | 0 | 0.05 | ±0.02 a,b | 0.67 | ±0.36 f | 0 | ±0.00 a | 10.14 | ±3.33 d–f | 0 | ±0.00 a | |
| 3 | 0.05 | ±0.01 a,b | 1.73 | ±0.25 h | 0 | ±0.00 a | 1.86 | ±0.22 a–c | 12.55 | ±1.62 d | ||
| 6 | 0.24 | ±0.30 c | 0.3 | ±0.03 a–d | 0 | ±0.00 a | 1.5 | ±0.26 a,b | 0.19 | ±0.14 a | ||
| Strong Gold | greenhouse | 0 | 0.02 | ±0.01 a | 0.22 | ±0.04 a–c | 0 | ±0.00 a | 15.26 | ±3.33 f–h | 0.13 | ±0.04 a |
| 3 | 0.02 | ±0.01 a | 0.18 | ±0.07 a,b | 0 | ±0.00 a | 8.92 | ±0.52 d–f | 0.11 | ±0.02 a | ||
| 6 | 0.01 | ±0.00 a | 0.47 | ±0.25 b–f | 0 | ±0.00 a | 19.52 | ±8.21 h | 1.06 | ±0.21 a,b | ||
| field | 0 | 0.12 | ±0.15 a–c | 0.35 | ±0.08 b–e | 0 | ±0.00 a | 18.95 | ±2.54 h | 13.92 | ±3.22 d | |
| 3 | 0.03 | ±0.00 a | 0.28 | ±0.08 a–d | 0.2 | ±0.01 b | 1.73 | ±0.18 a–c | 24.31 | ±2.31 f | ||
| 6 | 0.01 | ±0.00 a | 0.26 | ±0.01 a–d | 0 | ±0.00 a | 0.27 | ±0.04 a | 0 | ±0.00 a | ||
| Super Parrot | greenhouse | 0 | 0.05 | ±0.01 a,b | 0.22 | ±0.03 a–c | 0 | ±0.00 a | 9.09 | ±4. d–f 07 | 1.46 | ±0.67 a,b |
| 3 | 0.04 | ±0.03 a,b | 0.28 | ±0.11 a–d | 0 | ±0.00 a | 9.44 | ±4.43 d–f | 1.17 | ±1.11 a,b | ||
| 6 | 0.04 | ±0.14 a,b | 0.24 | ±0.08 a–c | 0 | ±0.00 a | 7.44 | ±4.50 b–e | 3.67 | ±4.24 b | ||
| field | 0 | 0.08 | ±0.01 a,b | 1.3 | ±0.14 g | 1.07 | ±0.05 d | 8.27 | ±2.79 c–e | 14.44 | ±1.85 d | |
| 3 | 0.03 | ±0.01 a,b | 0.56 | ±0.04 d–f | 0 | ±0.00 a | 14.96 | ±1.65 f–h | 0.1 | ±0.08 a | ||
| 6 | 0.03 | ±0.01 a,b | 1.32 | ±0.34 g | 0 | ±0.00 a | 2.19 | ±0.10 a–c | 21.99 | ±1.06 e,f | ||
| Tropicana | greenhouse | 0 | 0.02 | ±0.01 a | 0 | ±0.00 a | 0 | ±0.00 a | 5.16 | ±4.71 a–e | 2.48 | ±0.73 a,b |
| 3 | 0.02 | ±0.01 a | 0.24 | ±0.06 a–c | 0 | ±0.00 a | 4.85 | ±2.68 a–d | 0.19 | ±0.06 a | ||
| 6 | 0.02 | ±0.00 a | 0.32 | ±0.02 b–e | 0 | ±0.00 a | 100.03 | ±6.24 j | 0 | ±0.00 a | ||
| field | 0 | 0.17 | ±0.02 b,c | 1.57 | ±0.29 g,h | 0 | ±0.00 a | 17.13 | ±3.50 g,h | 0 | ±0.00 a | |
| 3 | 0.06 | ±0.01 a,b | 0.31 | ±0.04 a–e | 0 | ±0.00 a | 0.15 | ±0.01 a | 0 | ±0.00 a | ||
| 6 | 0.08 | ±0.01 a,b | 0.62 | ±0.02 e,f | 0 | ±0.00 a | 1.67 | ±0.32 a–c | 8.6 | ±0.52 c | ||
| Mean for A | Barcelona | 0.05 | ±0.04 a | 0.74 | ±0.64 d | 0.07 | ±0.01 c | 18.53 | ±12.58 c | 3.42 | ±2.77 b | |
| Columbus | 0.06 | ±0.03 a | 0.61 | ±0.56 b,c | 0 | ±0.00 a | 6.21 | ±3.96 a | 3.44 | ±3.14 b | ||
| Strong Gold | 0.04 | ±0.02 a | 0.29 | ±0.14 a | 0.03 | ±0.00 b | 10.77 | ±8.57 b | 6.59 | ±5.73 c | ||
| Super Parrot | 0.05 | ±0.02 a | 0.65 | ±0.51 c,d | 0.18 | ±0.02 d | 8.57 | ±4.77 b | 7.14 | ±6.16 c | ||
| Tropicana | 0.06 | ±0.05 a | 0.51 | ±0.48 b | 0 | ±0.00 a | 21.5 | ±20.10 d | 1.88 | ±3.24 a | ||
| Mean for B | greenhouse | 0.03 | ±0.05 a | 0.26 | ±0.13 a | 0 | ±0.00 a | 14.73 | ±13.59 b | 1.23 | ±1.28 a | |
| field | 0.07 | ±0.09 b | 0.86 | ±0.74 b | 0.11 | ±0.00 b | 11.5 | ±11.16 a | 7.76 | ±7.17 b | ||
| Mean for C | 0 | 0.06 | ±0.06 a | 0.51 | ±0.52 a | 0.11 | ±0.02 c | 11.05 | ±5.06 a | 5.24 | ±5.20 b | |
| 3 | 0.04 | ±0.02 a | 0.7 | ±0.85 b | 0.02 | ±0.00 a | 12.87 | ±11.36 b | 4.65 | ±4.01 b | ||
| 6 | 0.06 | ±0.11 a | 0.47 | ±0.34 a | 0.04 | ±0.01 b | 15.42 | ±19.34 c | 3.59 | ±3.30 a | ||
| Cultivar (A) | Type of Cultivation (B) | Storage Length (Days) (C) | Acetic | Fumaric | Succinic | Sum | ||||||
| Barcelona | greenhouse | 0 | 19.44 | ±7.06 c–e | 0.09 | ±0.03 a–d | 23.46 | ±2.91 e–i | 50.73 | ±11.43 e–j | ||
| 3 | 23.03 | ±11.94 d–f | 0.18 | ±0.13 a–d | 11.34 | ±1.90 a–f | 38.77 | ±9.65 b–f | ||||
| 6 | 13.12 | ±1.52 b–d | 0.16 | ±0.05 a–d | 14.98 | ±0.83 a–h | 34.94 | ±2.61 a–f | ||||
| field | 0 | 1.01 | ±0.14 a | 0.22 | ±0.07 a–e | 30.97 | ±6.55 g–i | 64.14 | ±4.84 h–j | |||
| 3 | 136.41 | ±24.6 j | 0.06 | ±0.04 a–d | 65.7 | ±3.29 j | 279.29 | ±19.80 l | ||||
| 6 | 52.86 | ±2.70 h | 0.26 | ±0.05 d,e | 0.26 | ±0.05 a,b | 62.55 | ±1.97 g–j | ||||
| Columbus | greenhouse | 0 | 8.19 | ±5.58 a,b | 0 | ±0.00 a | 18.67 | ±10.05 c–h | 34.56 | ±6.39 a–f | ||
| 3 | 0 | ±0.00 a | 0 | ±0.00 a | 18.77 | ±14.72 c–h | 35.83 | ±16.62 a–f | ||||
| 6 | 5.12 | ±0.89 a,b | 0.01 | ±0.00 a–c | 7.85 | ±2.81 a–e | 20.9 | ±1.62 a,b | ||||
| field | 0 | 33.86 | ±4.81 g | 0.14 | ±0.04 a–d | 23.75 | ±10.10 e–i | 68.6 | ±12.20 j | |||
| 3 | 0 | ±0.00 a | 0.24 | ±0.04 c–e | 26.53 | ±2.31 f–i | 42.96 | ±1.14 b–h | ||||
| 6 | 28.17 | ±2.21 e–g | 0.1 | ±0.00 a–d | 0 | ±0.00 a | 30.5 | ±2.59 a–f | ||||
| Strong Gold | greenhouse | 0 | 30.25 | ±4.49 f,g | 0.2 | ±0.03 a–e | 20.78 | ±4.08 d–h | 66.87 | ±11.66 j | ||
| 3 | 10.72 | ±2.86 a–c | 0.23 | ±0.05 b–e | 12.53 | ±3.12 a–f | 32.71 | ±5.41 a–f | ||||
| 6 | 9.62 | ±2.02 a–c | 0.17 | ±0.15 a–d | 13.63 | ±1.23 a–g | 44.48 | ±9.41 c–i | ||||
| field | 0 | 4.4 | ±0.61 a,b | 0.22 | ±0.03 a–e | 27.7 | ±4.18 f–i | 65.65 | ±4.56 i,j | |||
| 3 | 0 | ±0.00 a | 0.14 | ±0.01 a–d | 22.33 | ±2.14 d–h | 49.03 | ±4.22 e–j | ||||
| 6 | 21.84 | ±0.98 d–f | 0.4 | ±0.51 e | 0.3 | ±0.04 a,b | 23.09 | ±1.44 a–c | ||||
| Super Parrot | greenhouse | 0 | 3.9 | ±3.37 a,b | 0 | ±0.00 a | 1.68 | ±0.18 a–c | 16.4 | ±7.35 a | ||
| 3 | 2.2 | ±1.55 a,b | 0.02 | ±0.01 a–c | 23.1 | ±21.20 d–h | 36.26 | ±32.76 a–f | ||||
| 6 | 1.2 | ±0.66 a | 0.02 | ±0.01 a–c | 39.77 | ±25.20 i | 52.38 | ±29.70 f–j | ||||
| field | 0 | 1.28 | ±0.32 a | 0.06 | ±0.04 a–d | 13.65 | ±2.05 a–g | 40.16 | ±2.88 b–f | |||
| 3 | 0 | ±0.00 a | 0.01 | ±0.00 a,b | 32.18 | ±5.65 h,i | 47.84 | ±7.12 d–j | ||||
| 6 | 0 | ±0.00 a | 0 | ±0.00 a | 90.47 | ±5.92 k | 116 | ±5.65 k | ||||
| Tropicana | greenhouse | 0 | 6.62 | ±2.53 a,b | 0.08 | ±0.05 a–d | 14.93 | ±9.00 a–h | 29.29 | ±10.81 a–e | ||
| 3 | 3.31 | ±1.66 a,b | 0.07 | ±0.02 a–d | 6.66 | ±0.62 a–e | 15.35 | ±4.74 a | ||||
| 6 | 3.09 | ±0.18 a,b | 0.03 | ±0.00 a–c | 5.96 | ±0.30 a–d | 109.44 | ±6.69 k | ||||
| field | 0 | 77.06 | ±7.26 i | 0.15 | ±0.06 a–d | 0.88 | ±0.06 a,b | 96.95 | ±11.1 k | |||
| 3 | 23.46 | ±1.02 d–f | 0.12 | ±0.03 a–d | 17.57 | ±1.37 b–h | 41.67 | ±1.50 b–g | ||||
| 6 | 0 | ±0.00 a | 0.13 | ±0.02 a–d | 15.11 | ±0.57 a–h | 26.2 | ±0.84 a–d | ||||
| Mean for A | Barcelona | 40.98 | ±30.8 d | 0.16 | ±0.09 c,d | 24.45 | ±21.6 b | 88.4 | ±80.00 c | |||
| Columbus | 12.56 | ±12.10 b | 0.08 | ±0.05 a,b | 15.93 | ±11.9 a | 38.89 | ±17.00 a | ||||
| Strong Gold | 12.81 | ±0.21 b | 0.23 | ±0.21 d | 16.21 | ±9.36 a | 46.97 | ±17.50 b | ||||
| Super Parrot | 1.43 | ±10.80 a | 0.02 | ±0.01 a | 33.48 | ±32.3 c | 51.51 | ±35.60 b | ||||
| Tropicana | 18.92 | ±16.00 c | 0.1 | ±0.07 b,c | 10.18 | ±6.95 a | 53.15 | ±37.90 b | ||||
| Mean for B | greenhouse | 9.32 | ±8.30 a | 0.09 | ±0.10 a | 15.61 | ±13.26 a | 41.26 | ±19.80 a | |||
| field | 25.36 | ±12.22 b | 0.15 | ±0.15 b | 24.49 | ±24.22 b | 70.31 | ±12.22 b | ||||
| Mean for C | 0 | 18.61 | ±15.19 b | 0.12 | ±0.09 a | 17.65 | ±11.00 a | 53.34 | ±20.51 a | |||
| 3 | 19.91 | ±20.01 b | 0.11 | ±0.10 a | 23.67 | ±18.52 b | 61.97 | ±12.97 b | ||||
| 6 | 13.5 | ±12.19 a | 0.13 | ±0.11 a | 18.83 | ±17.69 a | 52.05 | ±13.20 a | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzymińska, A.; Gąsecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules 2020, 25, 5627. https://doi.org/10.3390/molecules25235627
Krzymińska A, Gąsecka M, Magdziak Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules. 2020; 25(23):5627. https://doi.org/10.3390/molecules25235627
Chicago/Turabian StyleKrzymińska, Agnieszka, Monika Gąsecka, and Zuzanna Magdziak. 2020. "Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars" Molecules 25, no. 23: 5627. https://doi.org/10.3390/molecules25235627
APA StyleKrzymińska, A., Gąsecka, M., & Magdziak, Z. (2020). Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules, 25(23), 5627. https://doi.org/10.3390/molecules25235627








