Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
3. Methodology
3.1. Materials
3.2. Fluorescence Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lucarini, M.; Durazzo, A.; Nicoli, S.F.; Raffo, A.; Santini, A.; Novellino, E.; Souto, E.B.; Romani, A.; Belcaro, M.F.; Vita, C. Chapter 41—Cold pressed argan (Argania spinose) oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 459–465. [Google Scholar]
- Zielińska, A.; Wójcicki, K.; Klensporf-Pawlik, D.; Dias-Ferreira, J.; Lucarini, M.; Durazzo, A.; Lucariello, G.; Capasso, R.; Santini, A.; Souto, E.B.; et al. Chemical and Physical Properties of Meadowfoam Seed Oil and Extra Virgin Olive Oil: Focus on Vibrational Spectroscopy. J. Spectrosc. 2020, 2020, 8870170. [Google Scholar] [CrossRef]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil—Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Ghannadi, A.; Asadi, M.; Etemad, M.; Mahzouni, P. Anti-inflammatory effect of Prunus armeniaca L.(Apricot) extracts ameliorates TNBS-induced ulcerative colitis in rats. Res. Pharm. Sci. 2014, 9, 225. [Google Scholar] [PubMed]
- Turan, S.; Topcu, A.; Karabulut, I.; Vural, H.; Hayaloglu, A.A. Fatty acid, triacylglycerol, phytosterol, and tocopherol variations in kernel oil of Malatya apricots from Turkey. J. Agric. food Chem. 2007, 55, 10787–10794. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M.P.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Kraujalytė, V.; Venskutonis, P.R.; Pukalskas, A.; Česonienė, L.; Daubaras, R. Antioxidant properties, phenolic composition and potentiometric sensor array evaluation of commercial and new blueberry (Vaccinium corymbosum) and bog blueberry (Vaccinium uliginosum) genotypes. Food Chem. 2015, 188, 583–590. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Lizard, G.; Filali-Zegzouti, Y.; Midaoui, A.E. Benefits of Argan Oil on Human Health-May 4-6 2017, Errachidia, Morocco. Int. J. Mol. Sci. 2017, 18, 1383. [Google Scholar] [CrossRef]
- Goik, U.; Goik, T.; Załęska, I. The properties and application of argan oil in cosmetology. Eur. J. Lipid Sci. Technol. 2019, 121, 1800313. [Google Scholar] [CrossRef]
- Berrougui, H.; Cloutier, M.; Isabelle, M.; Khalil, A. Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis 2006, 184, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.Y.; Jozwik, A.; Grzybek, W.; Echeverría, J.; Wang, D.; et al. Natural products in diabetes research: Quantitative literature analysis. Curr. Med. Chem. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Skrzypczak-Zielińska, M.; Zielińska, A.; Eder, P.; Souto, E.B.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int. J. Mol. Sci. 2020, 21, 5229. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, Q.; Zhang, Q.; Tuo, X.; Fan, D.; Deng, J.; Yang, H. Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 2019, 125, 85–94. [Google Scholar] [CrossRef]
- Irandoost, P.; Ebrahimi-Mameghani, M.; Pirouzpanah, S. Does grape seed oil improve inflammation and insulin resistance in overweight or obese women? Int. J. Food Sci. Nutr. 2013, 64, 706–710. [Google Scholar] [CrossRef]
- La Paz, S.M.-D.; Fernández-Arche, M.; Ángel-Martín, M.; García-Giménez, M. Phytochemical characterization of potential nutraceutical ingredients from Evening Primrose oil (Oenothera biennis L.). Phytochem. Lett. 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Fernández-Arche, Á.; Ángel-Martín, M.; García-Giménez, M.D. The sterols isolated from Evening Primrose oil modulate the release of proinflammatory mediators. Phytomedicine 2012, 19, 1072–1076. [Google Scholar] [CrossRef]
- Mahboubi, M. Evening Primrose (Oenothera biennis) oil in management of female ailments. J. Menopausal. Med. 2019, 25, 74–82. [Google Scholar] [CrossRef]
- Majdinasab, N.; Namjoyan, F.; Taghizadeh, M.; Saki, H. The effect of evening primrose oil on fatigue and quality of life in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2018, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Erşahin, Y.Ş.; Weiland, J.E.; Zasada, I.A.; Reed, R.L.; Stevens, J.F. Identifying rates of meadowfoam (Limnanthes alba) seed meal needed for suppression of Meloidogyne hapla and Pythium irregulare in soil. Plant Dis. 2014, 98, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. Chem. Nauka Tech. Rynek 2014, 68, 103–110. [Google Scholar]
- Undersander, D.; Oelke, E.; Kaminski, A.; Doll, J.; Putnam, D.; Combs, S.; Hanson, C. Alternative Field Crops Manual; University of Wisconsin-Madison and Minnesota: St. Paul, MI, USA, 1990; 48p. [Google Scholar]
- Alander, J.; Andersson, A.; Lindstrom, C. Cosmetic emollients with high stability against photo-oxidation. Lipid Technol. 2006, 18, 226. [Google Scholar]
- Slabaugh, M.B.; Cooper, L.D.; Kishore, V.K.; Knapp, S.J.; Kling, J.G. Genes affecting novel seed constituents in Limnanthes alba Benth: Transcriptome analysis of developing embryos and a new genetic map of meadowfoam. PeerJ 2015, 3, e915. [Google Scholar] [CrossRef]
- Zielińska, A.; Dąbrowska, M.; Nowak, I. Olej z nasion meadowfoam–„perła” wśród olejów roślinnych. Pol. J. Cosmetol. 2015, 18, 113–116. [Google Scholar]
- Zandomeneghi, M.; Carbonaro, L.; Caffarata, C. Fluorescence of vegetable oils: Olive oils. J. Agric. Food Chem. 2005, 53, 759–766. [Google Scholar] [CrossRef]
- Díaz, T.G.; Merás, I.D.; Correa, C.A.; Roldán, B.; Cáceres, M.I.R. Simultaneous fluorometric determination of chlorophylls a and b and pheophytins a and b in olive oil by partial least-squares calibration. J. Agric. Food Chem. 2003, 51, 6934–6940. [Google Scholar] [CrossRef]
- Sikorska, E.; Górecki, T.; Khmelinskii, I.V.; Sikorski, M.; Kozioł, J. Classification of edible oils using synchronous scanning fluorescence spectroscopy. Food Chem. 2005, 89, 217–225. [Google Scholar] [CrossRef]
- Carpenter, E.L.; Le, M.N.; Miranda, C.L.; Reed, R.L.; Stevens, J.F.; Indra, A.K.; Ganguli-Indra, G. Photoprotective Properties of Isothiocyanate and Nitrile Glucosinolate Derivatives From Meadowfoam (Limnanthes alba) Against UVB Irradiation in Human Skin Equivalent. Front. Pharmacol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Sikorska, E. Spectroscopic techniques and chemometrics in analysis of blends of extra virgin with refined and mild deodorized olive oils. Eur. J. Lipid Sci. Technol. 2015, 117, 92–102. [Google Scholar] [CrossRef]
- Wójcicki, K.; Grzechowiak, S.; Sikorska, E. Detection of extra virgin olive oil adulteration using fluorescence spectroscopy. Towarozn. Probl. Jakości 2013, 95, 100–107. [Google Scholar]
- Liu, Y.; Lan, X.; Shen, Z.; Lu, J.; Ni, X. Influence of excitation light wavelength on the fluorescence spectra of ethanol solutions. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2005, 25, 242–245. [Google Scholar] [PubMed]
- Song, C.; Li, R.; Ge, L.; Liu, Y. Study on the fluorescence characteristic and mechanism of ether-water solution. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2007, 27, 534–538. [Google Scholar]
- Lan, X.-F.; Luo, X.-S.; Shen, Z.-H.; Lu, J.; Liu, Y.; Ni, X.-W.; Peng, C.-D. Fluorescence spectrum characteristics of ethanol-water clusters. Acta Phys. Sin. 2005, 54, 5455–5461. [Google Scholar]
- Sv, B.N.K.; Tk, R.L.C.; Nr, K.S.B. Alcohol (Ethanol and Diethyl Ethyl Ether)-Diesel Blended Fuels for Diesel Engine Applications-A Feasible Solution. Adv. Automob. Eng. 2015, 4, 1–8. [Google Scholar]
- Nikolova, K.; Zlatanov, M.; Eftimov, T.; Brabant, D.; Yosifova, S.; Halil, E.; Antova, G.; Angelova, M. Fluoresence Spectra From Vegetable Oils Using Violet And Blue Ld/Led Exitation And An Optical Fiber Spectrometer. Int. J. Food Prop. 2014, 17, 1211–1223. [Google Scholar] [CrossRef]
- Sikorska, E.; Wójcicki, K.; Kozak, W.; Gliszczyńska-Świgło, A.; Khmelinskii, I.; Górecki, T.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A. Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage. Foods 2019, 8, 665. [Google Scholar] [CrossRef]
- Górnaś, P.; Radziejewska-Kubzdela, E.; Mišina, I.; Biegańska-Marecik, R.; Grygier, A.; Rudzińska, M. Tocopherols, Tocotrienols and Carotenoids in Kernel Oils Recovered from 15 Apricot (Prunus armeniaca L.). Genotypes 2017, 94, 693–699. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Khallouki, F.; Eddouks, M.; Mourad, A.; Breuer, A.; Owen, R.W. Ethnobotanic, Ethnopharmacologic Aspects and New Phytochemical Insights into Moroccan Argan Fruits. Int. J. Mol. Sci. 2017, 18, 2277. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.R.; Knothe, G.; Cermak, S.C. Biodiesel from meadowfoam (Limnanthes alba L.) seed oil: Oxidative stability and unusual fatty acid composition. Energy Environ. Sci. 2010, 3, 318–327. [Google Scholar] [CrossRef]
- Khan, M.A.; Shahidi, F. Photooxidative stability of stripped and non-stripped borage and evening primrose oils and their emulsions in water. Food Chem. 2002, 79, 47–53. [Google Scholar] [CrossRef]

| Sample | 2D | 3D |
|---|---|---|
| A | 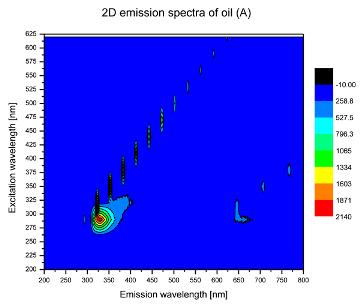 | 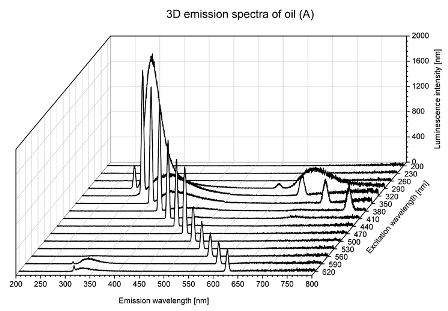 |
| B | 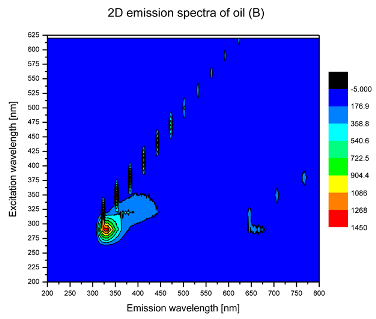 | 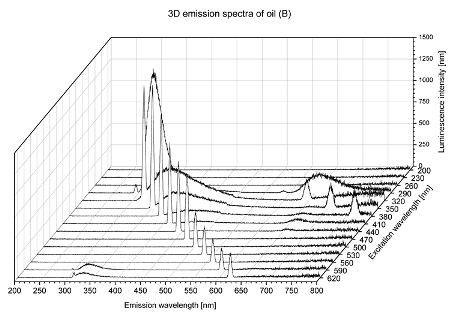 |
| C | 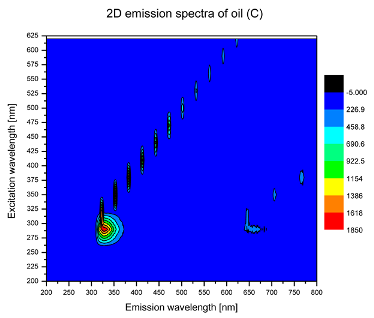 |  |
| D | 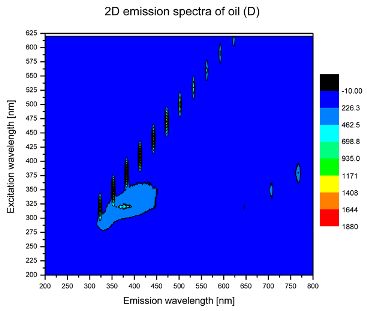 | 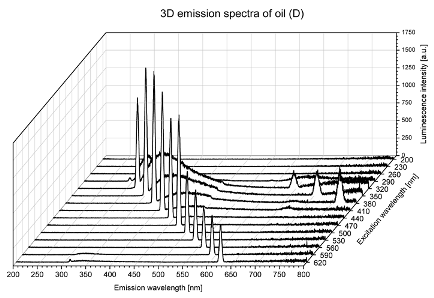 |
| E |  |  |
| F | 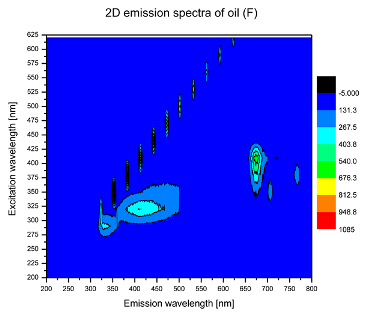 | 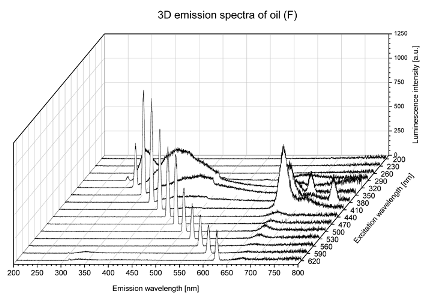 |
| G | 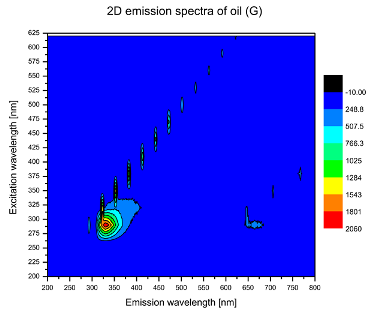 |  |
| Oil | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Plant | Apricot | Blueberry | Argan | Kiwi | Grape | Primrose | Meadowfoam |
| tocopherols | x | x | x | x | x | x | |
| (excitation range of 270–310 nm and the emission range of 300–360 nm) | |||||||
| chlorophylls and pheophytins | x | ||||||
| (excitation range of 330–450 nm and emission range between 660 to 700 nm) | |||||||
| oxidation products | x | x | x | ||||
| (excitation range at 300–350 nm and emission range 370–460 nm) |
| Sample | Oil Name | Fatty Acids and Other Ingredients | Health Benefits Briefly |
|---|---|---|---|
| A | apricot (Prunus armeniaca) kernel oil | PA (5); LA (30); OA (65); tocopherols; phytosterols | improving balance of destructive cytokines and reduction of toxic stress in the bowel cells; antioxidant and antimicrobial activities |
| B | blueberry (Vaccinium spp.) oil | PA (5.7); SA (2.8); ALA (25.1); LA (43.5); OA (22.9); anthocyanins; polyphenols; tocopherols; tocotrienols; carotenoids | improves inflammatory markers; promotes cardiovascular health; support healthy aging and gut health; radical scavenging activity |
| C | argan (Argania spinosa) nut oil | PA (12.8); SA (5.8); ALA (0.5); LA (33); OA (46.6); polyphenols, tocopherols; antioxidants; sterols; carotenoids; xanthophylls; squalene | cardioprotective properties; used in the treatment of skin infections; cures skin pimples, juvenile acne, and chicken pox pustules; reduces the rate of appearance of wrinkles; fights dry skin and dry hair; choleretic, hepatoprotective, useful to treat hypercholesterolemia and atherosclerosis |
| D | kiwi (Actinidia deliciosa) seed oil | PA; SA; ALA (67); LA (14–57); OA (12); tocopherols; tocotrienols | aids in the relief of itchy, scaly, irritated skin conditions, e.g., eczema/psoriasis; improves skin elasticity, reduces skin lines, dryness, wrinkles, enhances regeneration of skin cells |
| E | grape (Vitis vinifera) seed oil | PA; SA; ALA (0.5); LA (72–85); OA (10); tocopherols; tocotrienols; phenolic compounds [flavonoids, carotenoids, phenolic acids, tannins, stilbenes]; resveratrol; quercetin; procyanidins; carotenoids; phytosterols; gallic acid; catechin; epicatechin; procyanidins; proanthocyanidins | maintenances the ratio between anti and pro-inflammatory cytokines on serum (TNF-α/IL-10); reduces oxidative stress, decreases low-density lipoprotein (LDL) levels; inhibits lipid oxidation; anti-inflammatory and antioxidant capabilities; has a toxicity effect on some pathogens, suggesting an antimicrobial feature; cardioprotective and anticancer effects; |
| F | evening primrose (Oenothera biennis) oil | PA (6.2); SA (1.8); ALA (<2); LA (75); GLA (9–10); OA (5.4); phytosterols [4-desmethylsterols, erythrodiol and uvaol]; phenols [mainly ferulic acid]; tocopherols | widely used as a dietary supplement; helps in rheumatic and arthritic conditions, atopic dermatitis, psoriasis, premenstrual and menopausal syndrome - although there is little evidence to support these uses; inhibitory effect on leukotriene synthesis; implicates various inflammatory and immunologic pathogeneses |
| G | meadowfoam (Limnanthes alba) seed oil | EA (63); EU (16–24); C22:1 (17); glucolimnanthin (3–4), methoxylated benzyl glucosinolate (a phenylalanine-derived); | anti-microbial properties; its exceptional oxidative stability and lubricity; ameliorates abnormal skin conditions |
Sample Availability: Not available. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Kubasiewicz, K.; Wójcicki, K.; Silva, A.M.; Nunes, F.M.; Szalata, M.; Słomski, R.; Eder, P.; Souto, E.B. Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications. Molecules 2020, 25, 5608. https://doi.org/10.3390/molecules25235608
Zielińska A, Kubasiewicz K, Wójcicki K, Silva AM, Nunes FM, Szalata M, Słomski R, Eder P, Souto EB. Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications. Molecules. 2020; 25(23):5608. https://doi.org/10.3390/molecules25235608
Chicago/Turabian StyleZielińska, Aleksandra, Konrad Kubasiewicz, Krzysztof Wójcicki, Amélia M. Silva, Fernando M. Nunes, Marlena Szalata, Ryszard Słomski, Piotr Eder, and Eliana B. Souto. 2020. "Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications" Molecules 25, no. 23: 5608. https://doi.org/10.3390/molecules25235608
APA StyleZielińska, A., Kubasiewicz, K., Wójcicki, K., Silva, A. M., Nunes, F. M., Szalata, M., Słomski, R., Eder, P., & Souto, E. B. (2020). Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications. Molecules, 25(23), 5608. https://doi.org/10.3390/molecules25235608







