Sensory Quality Evaluation of Korla Pear from Different Orchards and Analysis of Their Primary and Volatile Metabolites
Abstract
1. Introduction
2. Results
2.1. Sensory Evaluation of Korla Pear
2.2. Primary Metabolites in Korla Pear
2.3. Volatiles in Korla Pear
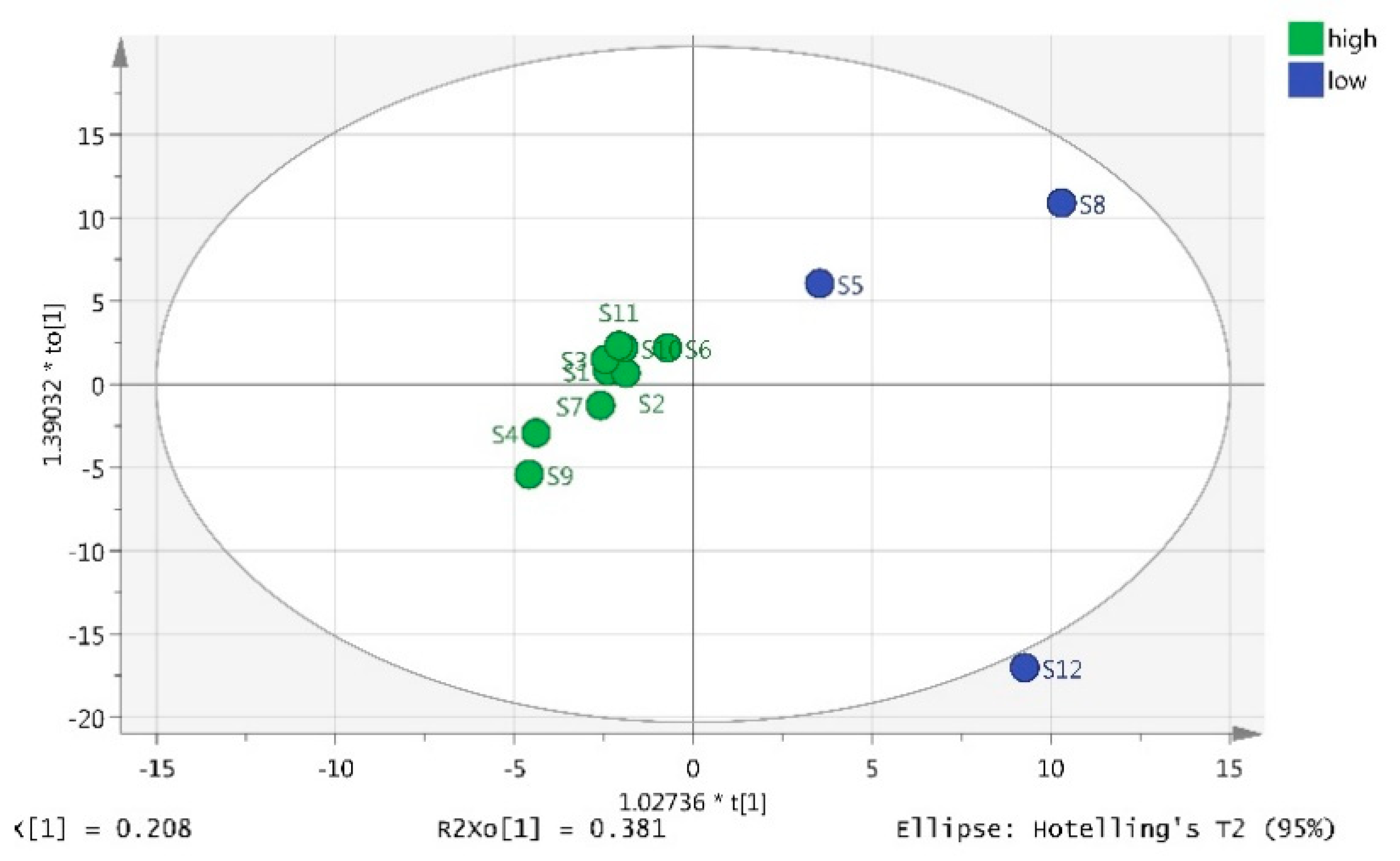
2.4. OPLS-DA Analysis on Korla Pear Fruit from 12 Orchards
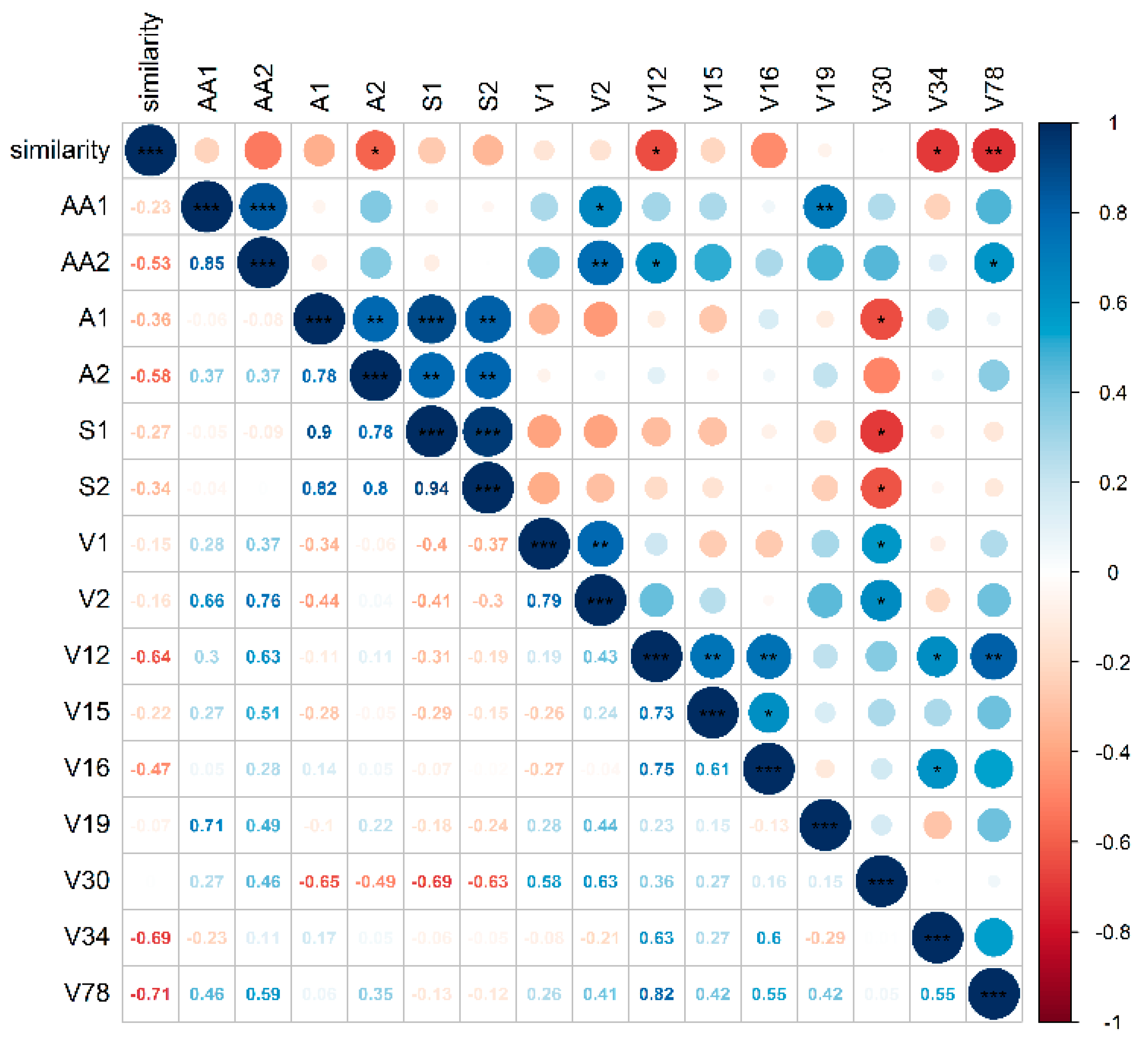
2.5. Correlation Analysis between Sensory Scores and Metabolites
3. Discussion
3.1. Important Sensory Quality Attributes Defined in the Study
3.2. Metabolites Detected in Korla Pear
3.3. Association between Metabolites and the Sensory Quality of Korla Pear
4. Materials and Methods
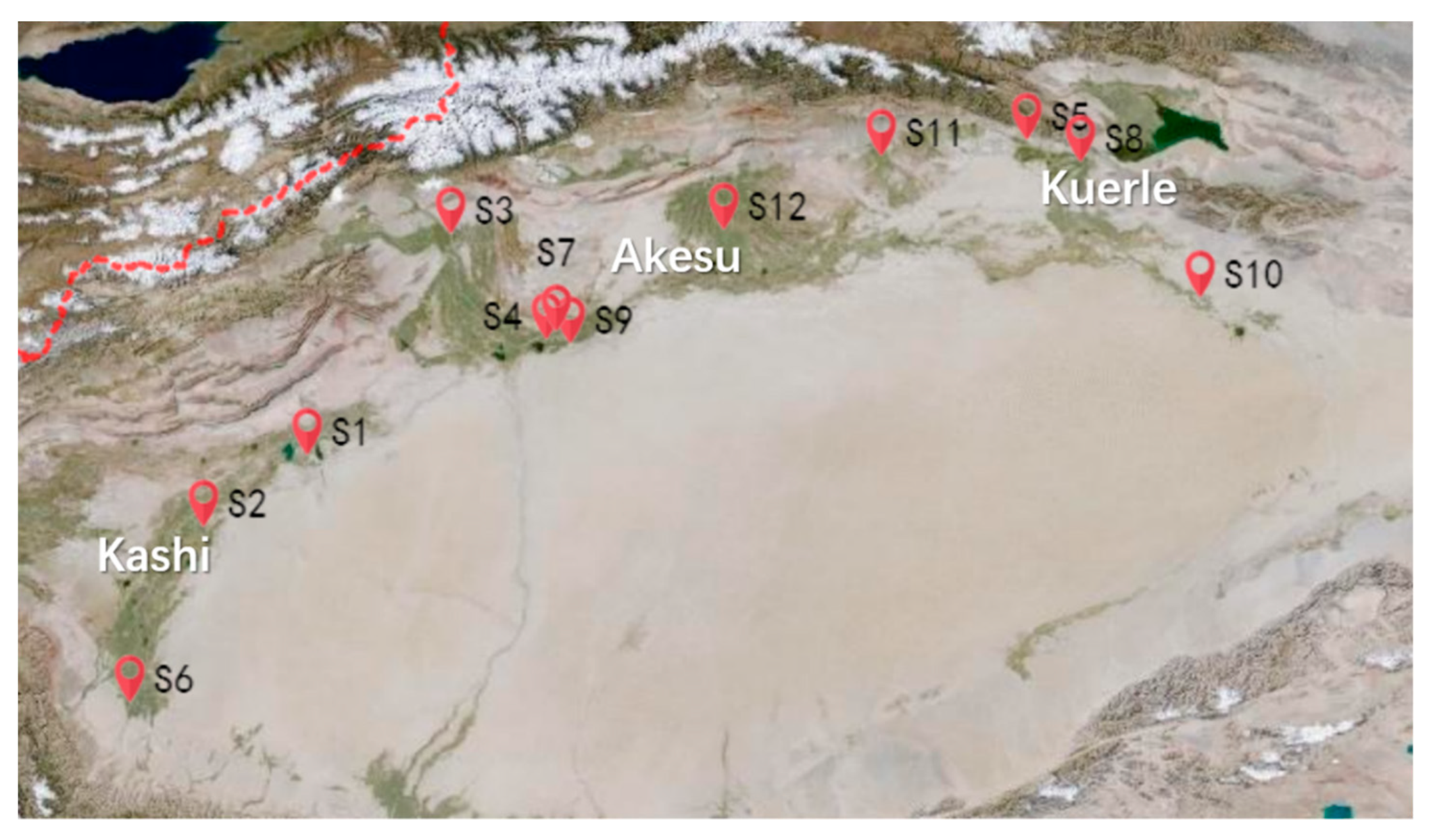
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Sensory Evaluation of Korla Pear Fruit
4.3.1. Calculation of the Overall Quality Scores
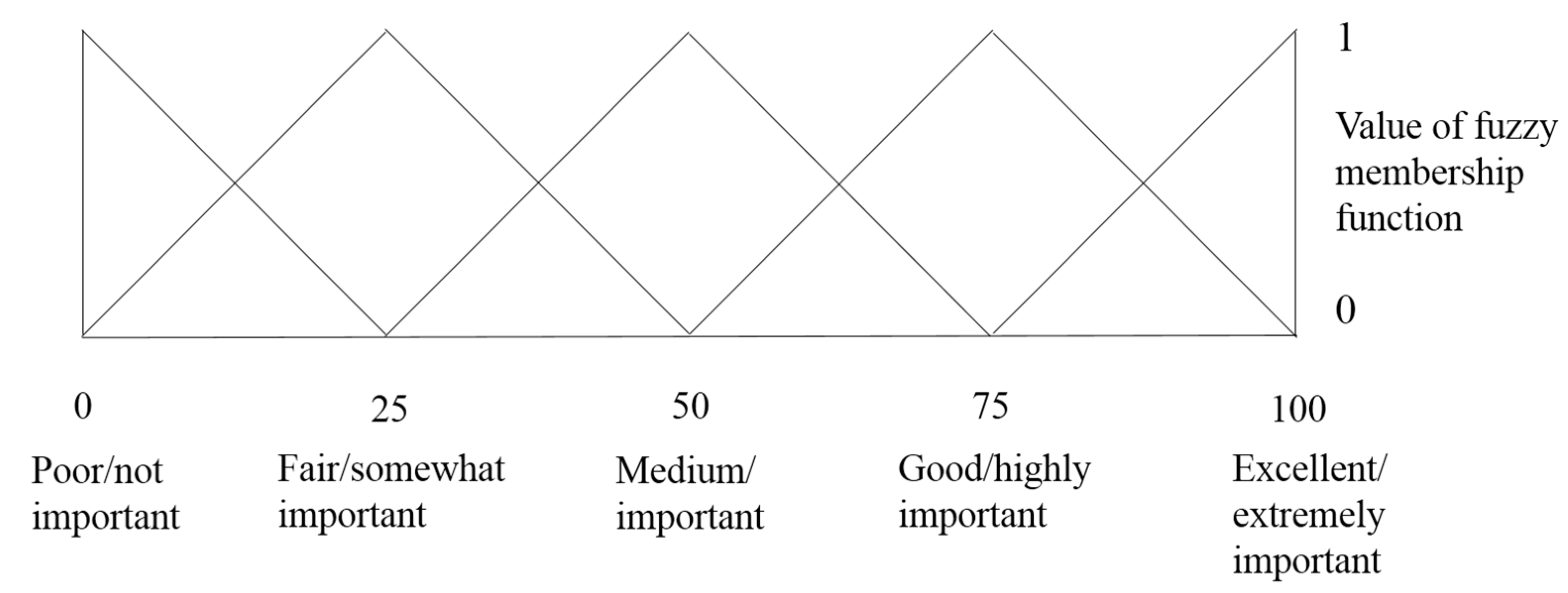
4.3.2. Calculation of Membership Function on the Standard Fuzzy Scale
F4 = (0 0 0 0 0.5 1 1 0.5 0 0), F5 = (0 0 0 0 0 0 0.5 1 1 0.5) and F6 = (0 0 0 0 0 0 0 0 0.5 1)
if x = a, Bx = 1; if x < (a − b) or x > (a + c), Bx = 0.
4.3.3. Calculation of Similarity Values and Ranking of the Korla Pear Fruit from Different Orchards
4.4. Analysis of Primary Metabolites by Using GC–MS
4.5. Volatile Determination by Headspace Solid Phase Microextraction-GC-MS
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W.; Zhang, S. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yang, Z.; Zhang, S.; Cao, Y.; Liu, J.; Wu, J. Characteristics of components and contents of organic acid in pear fruits from different cultivated species. Sci. Agric. Sin. 2014, 41, 755–764. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Wu, X.; Shi, X.; Qi, K.; Zhang, S. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Yao, G.; Zhang, S.; Cao, Y.; Liu, J.; Wu, J.; Yuan, J.; Zhang, H.; Xiao, C. Characteristics of Components and Contents of Soluble Sugars in Pear Fruits from Different Species. Sci. Agric. Sin. 2010, 43, 4229–4237. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, Y.; Xu, Z. Quality evaluation of mixed brewed perries based on PCA and sensory evaluation. Front. Agric. China 2011, 5, 529–533. [Google Scholar] [CrossRef]
- Hudina, M.; Colarič, M.; Štampar, F. Primary metabolites in the leaves and fruits of three pear cultivars during the growing season. Can. J. Plant Sci. 2007, 87, 327–332. [Google Scholar] [CrossRef]
- Chen, J.; Lü, J.; He, Z.; Zhang, F.; Zhang, S.; Zhang, H. Investigations into the production of volatile compounds in Korla fragrant pears (Pyrus sinkiangensis Yu). Food Chem. 2020, 302, 125337. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; He, Z.; Tao, S.; Li, G.; Zhang, S. Effects of bagging on soluble sugars, organic acids, and aroma compounds in Pyrus sinkiangensis ‘Korla Xiangli’ fruit. Acta Hortic. Sin. 2016, 41, 1443–1450. [Google Scholar] [CrossRef]
- Ji, L.C.; Ji, H.W.; Wang, Q.; Deng, H.; Xiao, S.H. Changes in the volatile compounds and chemical and physical properties of Kuerle fragrant pear (Pyrus serotina Reld) during storage. J. Agric. Food Chem. 2006, 54, 8842–8847. [Google Scholar] [CrossRef]
- Pan, Y.; Meng, X.; Che, F.; Xue, S.; Zhang, T.; Zhao, S.; Liao, K. Metabolic Profiles of Sugar Metabolism and Respiratory Metabolism of Korla Pear (Pyrus sinkiangensis Yu) Throughout Fruit Development and Ripening. Sci. Agric. Sin. 2016, 49, 3391–3412. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Wang, Q.; Hu, X. Analysis of aroma components of Xinjiang kuerle fragrant pear. Food Sci. Technol. 2007, 6, 114–117. [Google Scholar] [CrossRef]
- Tu, J.; Qin, Z.; Li, X.; Liu, X.; Yang, F.; Zhu, H. Analysis of Fruit Aromatic Components of Pyrus pyrifolia Nakai and P. sinkiangensis Yu by GC-MS. Hubei Agric. Sci. 2011, 50, 3186–3190. [Google Scholar] [CrossRef]
- Debjani, C.; Das, S.; Das, H. Aggregation of sensory data using fuzzy logic for sensory quality evaluation of food. J. Food Sci. Technol. 2013, 50, 1088–1096. [Google Scholar] [CrossRef]
- Vivek, K.; Subbarao, K.V.; Routray, W.; Kamini, N.R.; Dash, K.K. Application of Fuzzy Logic in Sensory Evaluation of Food Products: A Comprehensive Study. Food Bioprocess Technol. 2020, 13, 1–29. [Google Scholar] [CrossRef]
- Zare, D.; Ghazali, H.M. Assessing the quality of sardine based on biogenic amines using a fuzzy logic model. Food Chem. 2017, 221, 936–943. [Google Scholar] [CrossRef]
- Routray, W.; Mishra, H.N. Sensory evaluation of different drinks formulated from dahi (Indian Yogurt) powder using fuzzy logic. J. Food Process. Preserv. 2012, 36, 1–10. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Niakousari, M.; Khoshnoudi-Nia, S.; Hosseini, S.M.H. Sensory evaluation of selected formulated milk barberry drinks using the fuzzy approach. Food Sci. Nutr. 2017, 5, 739–749. [Google Scholar] [CrossRef]
- Sinija, V.R.; Mishra, H.N. Fuzzy Analysis of Sensory Data for Quality Evaluation and Ranking of Instant Green Tea Powder and Granules. Food Bioprocess Technol. 2011, 4, 408–416. [Google Scholar] [CrossRef]
- Du, X.F.; Kurnianta, A.; McDaniel, M.; Finn, C.E.; Qian, M.C. Flavour profiling of “Marion” and thornless blackberries by instrumental and sensory analysis. Food Chem. 2010, 121, 1080–1088. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J. Application of Sensory Evaluation, HS-SPME GC-MS, E-Nose, and E-Tongue for Quality Detection in Citrus Fruits. J. Food Sci. 2015, 80, S2296–S2304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Wang, L.Y.; Xiao, Z.B.; Niu, Y.W. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography–olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC–MS) and flame photometric detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Favati, F.; Condelli, N.; Galgano, F.; Caruso, M.C. Extra virgin olive oil bitterness evaluation by sensory and chemical analyses. Food Chem. 2013, 139, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Tokitomo, Y.; Steinhaus, M.; Büttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Fastowski, O.; Poplacean, I.; Engel, K.H. Analysis and Sensory Evaluation of Volatile Constituents of Fresh Blackcurrant (Ribes nigrum L.) Fruits. J. Agric. Food Chem. 2017, 65, 9475–9487. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, S.; Wang, J.; Wu, C.; Tang, Z.; Gong, H.; Zhang, X.; Xu, J. GC-MS analyses of volatiles and primary metabolites in Korla Pear fruit. J. Huazhong Agric. Univ. 2020, 39, 44–52. [Google Scholar] [CrossRef]
- Kim, J.L.; Elfman, L.; Mi, Y.; Wieslander, G.; Smedje, G.; Norbäck, D. Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools—Associations with asthma and respiratory symptoms in pupils. Indoor Air 2007, 17, 153–163. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Yang, W.; Zhang, M.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. GABA Pathway Rate-Limit Citrate Degradation in Postharvest Citrus Fruit Evidence from HB Pumelo (Citrus grandis) × Fairchild (Citrus reticulata) Hybrid Population. J. Agric. Food Chem. 2017, 65, 1669–1676. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pear peels and compounds are available from the authors. |
| Sample | F1/Not Satisfactory | F2/Fair | F3/Satisfactory | F4/Good | F5/Very Good | F6/Excellent | Rank |
|---|---|---|---|---|---|---|---|
| 2018 | |||||||
| S1 | 0.014 | 0.171 | 0.465 | 0.683 | 0.544 | 0.169 | 9 |
| S2 | 0.024 | 0.226 | 0.534 | 0.687 | 0.452 | 0.119 | 7 |
| S3 | 0.025 | 0.237 | 0.564 | 0.705 | 0.430 | 0.096 | 1 |
| S4 | 0.016 | 0.186 | 0.488 | 0.690 | 0.513 | 0.151 | 5 |
| S5 | 0.008 | 0.136 | 0.409 | 0.659 | 0.597 | 0.199 | 12 |
| S6 | 0.021 | 0.212 | 0.525 | 0.694 | 0.463 | 0.122 | 3 |
| S7 | 0.018 | 0.193 | 0.492 | 0.688 | 0.507 | 0.147 | 6 |
| S8 | 0.048 | 0.310 | 0.652 | 0.675 | 0.317 | 0.037 | 11 |
| S9 | 0.026 | 0.238 | 0.550 | 0.685 | 0.441 | 0.114 | 8 |
| S10 | 0.020 | 0.207 | 0.517 | 0.691 | 0.469 | 0.127 | 4 |
| S11 | 0.014 | 0.180 | 0.494 | 0.704 | 0.512 | 0.143 | 2 |
| S12 | 0.010 | 0.151 | 0.437 | 0.675 | 0.575 | 0.185 | 10 |
| 2019 | |||||||
| S1 | 0.024 | 0.254 | 0.630 | 0.721 | 0.373 | 0.057 | 5 |
| S2 | 0.005 | 0.139 | 0.462 | 0.710 | 0.559 | 0.167 | 6 |
| S3 | 0.024 | 0.244 | 0.594 | 0.709 | 0.411 | 0.087 | 7 |
| S4 | 0.030 | 0.296 | 0.693 | 0.698 | 0.279 | 0.023 | 9 |
| S5 | 0.022 | 0.241 | 0.610 | 0.722 | 0.399 | 0.074 | 4 |
| S6 | 0.033 | 0.307 | 0.683 | 0.663 | 0.302 | 0.090 | 11 |
| S7 | 0.038 | 0.311 | 0.682 | 0.675 | 0.28 | 0.026 | 12 |
| S8 | 0.039 | 0.318 | 0.686 | 0.666 | 0.279 | 0.027 | 10 |
| S9 | 0.019 | 0.221 | 0.578 | 0.726 | 0.430 | 0.09 | 3 |
| S10 | 0.026 | 0.264 | 0.635 | 0.708 | 0.365 | 0.058 | 8 |
| S11 | 0.007 | 0.162 | 0.518 | 0.741 | 0.486 | 0.112 | 1 |
| S12 | 0.009 | 0.165 | 0.508 | 0.733 | 0.502 | 0.125 | 2 |
| Compounds | RT | Concentration (μg/g FW) | |||
|---|---|---|---|---|---|
| Maximum | Sample | Minimum | Sample | ||
| Amino acid | |||||
| l-Valine | 6.11 | 5.36 ± 0.29 | S12 | 0.30 ± 0.25 | S6 |
| l-Isoleucine | 8.12 | 1.01 ± 0.07 | S12 | - | S6 |
| l-Proline | 9.21 | 37.26 ± 0.70 | S12 | 11.93 ± 0.98 | S6 |
| Serine | 10.18 | 4.75 ± 0.21 | S12 | 0.05 ± 0.07 | S5 |
| l-Threonine | 11.00 | 2.81 ± 0.03 | S12 | 0.02 ± 0.00 | S3 |
| l-Aspartic acid | 15.67 | 52.88 ± 5.89 | S12 | 1.32 ± 0.70 | S5 |
| l-Glutamic acid | 19.24 | 4.73 ± 0.10 | S12 | 0.09 ± 0.05 | S6 |
| Organic acids | |||||
| Malic acid | 14.61 | 1809.13 ± 420.52 | S8 | 600.20 ± 25.76 | S9 |
| l-(+)-Tartaric acid | 18.51 | 7.79 ± 1.81 | S8 | 2.36 ± 0.24 | S2 |
| Citric acid | 26.21 | 31.40 ± 2.27 | S11 | 12.35 ± 1.10 | S9 |
| Quininic acid | 27.46 | 212.43 ± 162.28 | S10 | 105.39 ± 20.03 | S11 |
| Fatty acids | |||||
| Palmitic Acid | 34.41 | 14.20 ± 8.54 | S11 | 3.64 ± 2.93 | S6 |
| Stearic acid | 44.07 | 15.05 ± 9.92 | S11 | 1.93 ± 1.71 | S6 |
| Sugars | |||||
| d-(−)-Fructofuranose | 25.77 | 18492.86 ± 4309.41 | S8 | 11285.72 ± 359.93 | S4 |
| Fructose | 26.07 | 20999.04 ± 4646.51 | S8 | 12012.71 ± 22.17 | S4 |
| Psicopyranose | 27.11 | 11453.29 ± 2590.96 | S8 | 6820.89 ± 394.51 | S4 |
| Glucose | 28.81 | 13910.25 ± 3120.71 | S8 | 8145.27 ± 138.45 | S2 |
| d-Galactose | 29.31 | 303.88 ± 47.85 | S8 | 139.70 ± 96.73 | S11 |
| d-Glucitol | 30.26 | 24335.64 ± 5912.74 | S8 | 12171.03 ± 8458.33 | S11 |
| Sucrose | 48.42 | 2968.10 ± 246.14 | S6 | 1900.73 ± 168.91 | S12 |
| Total Amino acid | 108.82 ± 5.90 | S12 | 14.65 ± 2.93 | S6 | |
| Total acid | 1099.01 ± 41.92 | S10 | 806.63 ± 60.89 | S9 | |
| Total sugar | 91705.26 ± 20996.43 | S8 | 56878.51 ± 2251.72 | S4 | |
| Sugar/Acid | 74.03 | S9 | 42.06 | S11 | |
| Compounds | Retention Index (RI) | Concentration Range (μg/kg FW) | Average Concentration (μg/kg FW)/Percentage of Total Volatiles (%) |
|---|---|---|---|
| Aldehydes | 10222.35–75589.04 | 26133.94/85.84 | |
| Hexanal | 800 | 3472.75–35617.72 | 12954.25/42.55 |
| (E)-2-Hexenal | 854 | 2782.32–39086.40 | 12666.51/41.60 |
| Octanal | 1003 | 0.00–318.75 | 39.06/0.13 |
| Nonanal | 1104 | 211.58–798.32 | 416.57/1.37 |
| Decanal | 1206 | 7.91–144.89 | 28.74/0.09 |
| 2,6,10-trimethyl-9-Undecenal | 1416 | 7.74–66.02 | 17.15/0.06 |
| Tetradecanal | 1613 | 0.16–41.36 | 6.50/0.02 |
| 1-Pentadecanal | 1715 | 0.00–23.41 | 2.82/0.01 |
| Hexadecanal | 1817 | 0.00–24.99 | 2.35/0.01 |
| Esters | 361.82–3206.45 | 1314.47/4.32 | |
| Methyl hexanoate | 925 | 0.00–11.27 | 1.62/0.01 |
| Acrylic acid isoamyl ester | 940 | 3.61–24.71 | 10.80/0.04 |
| Ethyl hexanoate | 1000 | 0.00–5.12 | 0.85/- |
| (Z)-3-Hexen-1-yl acetate | 1005 | 152.86–2501.62 | 780.49/2.56 |
| hexyl acetate | 1011 | 82.43–818.52 | 345.54/1.13 |
| (2Z)-2-Hexen-1-yl acetate | 1016 | 0.00–275.32 | 72.59/0.24 |
| Heptyl acetate | 1113 | 0.00–22.30 | 8.38/0.03 |
| Hexyl butyrate | 1192 | 0.00–54.56 | 17.26/0.06 |
| (E)-Butanoic acid-2-hexenyl ester | 1195 | 1.16–31.02 | 9.32/0.03 |
| Ethyl octoate | 1196 | 0.00–0.54 | 0.07/- |
| Octyl acetate | 1210 | 0.00–4.61 | 1.39/- |
| Acetic acid-2-phenylethyl ester | 1258 | 0.00–22.06 | 5.85/0.02 |
| n-Butyric acid 2-ethylhexyl ester | 1317 | 0.00–21.71 | 7.95/0.03 |
| Lavandulyl propionate | 1375 | 0.00–14.75 | 1.74/0.01 |
| Hexanoic acid hexyl ester | 1384 | 0.00–36.33 | 9.35/0.03 |
| (E)-Hexanoic acid-2-hexenyl ester | 1391 | 0.00–5.13 | 1.47/- |
| Formic acid undecyl ester | 1441 | 12.32–61.90 | 25.53/0.08 |
| Benzoic acid hexyl ester | 1580 | 0.00–13.77 | 1.60/0.01 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 1588 | 3.05–33.60 | 12.06/0.04 |
| Propanoic acid 2-methyl-decyl ester | 1590 | 0.00–2.92 | 0.63/- |
| Alcohols | 46.52–1214.99 | 315.82/1.04 | |
| 1-Hexanol | 868 | 0.00–632.22 | 166.87/0.55 |
| (E)-2-Octen-1-ol | 1067 | 0.00–2.60 | 0.55/- |
| 1-Octanol | 1071 | 12.73–97.24 | 31.76/0.10 |
| (Z)-3-Nonen-1-ol | 1156 | 0.00–5.42 | 0.62/- |
| 1-Nonanol | 1173 | 13.23–147.34 | 45.37/0.15 |
| 1-methyl-4-(1-methylethyl)-Cyclohexanol | 1178 | 0.00–35.01 | 2.92/0.01 |
| 9-Decen-1-ol | 1262 | 0.00–25.53 | 4.39/0.01 |
| 1-Decanol | 1273 | 0.00–38.34 | 10.15/0.03 |
| 1-Dodecanol | 1473 | 7.35–52.17 | 16.38/0.05 |
| 1-Tetradecanol | 1676 | 7.66–144.20 | 29.42/0.10 |
| 1-Hexadecanol | 1880 | 0.00–54.61 | 8.18/0.03 |
| 1-Octadecanol | 2082 | 0.00–17.92 | 2.12/0.01 |
| Terpenes | 74.90–1220.67 | 482.98/1.59 | |
| α-Thujene | 929 | 1.93–15.79 | 5.98/0.02 |
| d-Limonene | 34.16–937.00 | 357.05/1.17 | |
| γ-Terpinene | 1060 | 0.00–13.83 | 5.24/0.02 |
| Linalool | 1099 | 2.03–9.05 | 4.75/0.02 |
| Isophorone | 1124 | 0.00–67.36 | 6.49/0.02 |
| trans-Limonene oxide | 1138 | 0.00–7.14 | 0.79/- |
| l-α-Terpineol | 1190 | 0.00–18.46 | 4.62/0.02 |
| Geraniol | 1255 | 0.00–4.36 | 0.96/- |
| cis-Geranylacetone | 1435 | 0.00–65.67 | 17.84/0.06 |
| cis-β-Farnesene | 1444 | 0.00–0.15 | 0.01/- |
| Humulene | 1454 | 0.00–31.44 | 9.76/0.03 |
| (E)-2-Dodecenal | 1468 | 0.00–8.26 | 1.02/- |
| (Z,E)-α-Farnesene | 1491 | 0.00–2.96 | 0.25/- |
| α-Farnesene | 1508 | 0.00–277.18 | 54.17/0.18 |
| β-Curcumene | 1514 | 0.00–3.80 | 0.97/- |
| d-Nerolidol | 1544 | 0.00–29.25 | 4.73/0.02 |
| Nerolidol | 1554 | 0.00–14.44 | 3.04/0.01 |
| Viridiflorol | 1591 | 0.00–23.10 | 3.79/0.01 |
| α-Bulnesene | 0.00–6.57 | 1.51/- | |
| Others | 502.16–15082.46 | 2197.67/7.22 | |
| 1,2-dimethyl-Benzene | 887 | 0.00–108.07 | 31.80/0.10 |
| methoxy-phenyl-Oxime | 3.12–374.30 | 120.12/0.39 | |
| (E,E)-2,4-Hexadienal | 911 | 0.00–1045.21 | 206.89/0.68 |
| (E)-4-Oxohex-2-enal | 958 | 55.12–1815.23 | 309.67/1.02 |
| Phenol | 980 | 0.00–0.41 | 0.07/- |
| Hexanoic acid | 990 | 0.00–202.81 | 56.68/0.19 |
| p-Cymene | 1025 | 0.00–60.38 | 11.66/0.04 |
| Benzeneacetaldehyde | 1045 | 2.11–22.32 | 7.34/0.02 |
| (Z)-1-ethoxy-4-methyl-2-Pentene | 3.52–1941.80 | 354.24/1.16 | |
| 1-methyl-4-(1-methylethylidene)-Cyclohexene | 1088 | 0.00–2.22 | 0.23/- |
| 2-ethenyl-1,4-dimethyl-Benzene | 1090 | 0.00–0.37 | 0.03/- |
| 2-Nonanone | 1092 | 0.00–156.99 | 31.20/0.10 |
| Undecane | 1100 | 0.00–8.23 | 3.08/0.01 |
| 1,2,4,5-tetramethyl-Benzene | 1116 | 0.00–35.93 | 12.78/0.04 |
| Benzoic acid | 1170 | 0.00–103.50 | 12.65/0.04 |
| Naphthalene | 1182 | 2.04–26.36 | 7.28/0.02 |
| Dodecane | 1200 | 9.03–77.73 | 34.46/0.11 |
| (Z)-3,7-dimethyl-2,6-Octadienal | 1240 | 0.00–4.56 | 2.05/0.01 |
| Nonanoic acid | 1273 | 0.00–26.95 | 4.73/0.02 |
| 2,6,11-trimethyl-Dodecane | 1275 | 0.00–36.70 | 10.34/0.03 |
| Tridecane | 1300 | 7.44–58.40 | 18.34/0.06 |
| 2,3,5,8-tetramethyl-Decane | 1318 | 0.00–27.36 | 7.97/0.03 |
| Tetradecane | 1400 | 18.28–91.39 | 35.06/0.12 |
| Pentadecane | 1500 | 0.00–231.63 | 60.09/0.20 |
| 2,6,10-trimethyl-Tetradecane | 1539 | 0.00–19.23 | 6.37/0.02 |
| 5-methyl-Pentadecane | 1547 | 2.21–45.84 | 7.54/0.02 |
| 5,8-Diethyldodecane | 1572 | 3.79–34.53 | 9.61/0.03 |
| Hexadecane | 1600 | 5.84–64.97 | 15.45/0.05 |
| 6,9-Heptadecadiene | 1667 | 0.00–2.30 | 0.56/- |
| 1-Hydroxycyclohexyl phenyl ketone | 1687 | 9.28–199.96 | 39.17/0.13 |
| Tetradecanoic acid | 1768 | 0.00–1225.67 | 102.14/0.34 |
| 2,6,11,15-tetramethyl-Hexadecane | 1792 | 0.19–33.98 | 5.64/0.02 |
| Pentadecanoic acid | 1867 | 0.00–1175.82 | 98.01/0.32 |
| Nonadecane | 1900 | 3.85–94.82 | 18.62/0.06 |
| 9-Hexadecenoic acid | 1942 | 0.00–1610.17 | 135.11/0.44 |
| n-Hexadecanoic acid | 1968 | 0.00–3839.76 | 319.98/1.05 |
| Eicosane | 2000 | 0.00–52.30 | 6.24/0.02 |
| Heptadecanoic acid | 2071 | 0.00–129.25 | 10.77/0.04 |
| Heneicosane | 2100 | 8.04–295.40 | 47.09/0.15 |
| Oleic Acid | 2141 | 0.00–404.28 | 33.69/0.11 |
| Total | 13098.35–79188.69 | 30444.88/100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xiang, S.; Zhang, H.; Zhang, H.; Wu, C.; Tang, Z.; Wang, J.; Xu, J. Sensory Quality Evaluation of Korla Pear from Different Orchards and Analysis of Their Primary and Volatile Metabolites. Molecules 2020, 25, 5567. https://doi.org/10.3390/molecules25235567
Liu Y, Xiang S, Zhang H, Zhang H, Wu C, Tang Z, Wang J, Xu J. Sensory Quality Evaluation of Korla Pear from Different Orchards and Analysis of Their Primary and Volatile Metabolites. Molecules. 2020; 25(23):5567. https://doi.org/10.3390/molecules25235567
Chicago/Turabian StyleLiu, Yuan, Simin Xiang, Haipeng Zhang, Hongyan Zhang, Cuiyun Wu, Zhanghu Tang, Jiangbo Wang, and Juan Xu. 2020. "Sensory Quality Evaluation of Korla Pear from Different Orchards and Analysis of Their Primary and Volatile Metabolites" Molecules 25, no. 23: 5567. https://doi.org/10.3390/molecules25235567
APA StyleLiu, Y., Xiang, S., Zhang, H., Zhang, H., Wu, C., Tang, Z., Wang, J., & Xu, J. (2020). Sensory Quality Evaluation of Korla Pear from Different Orchards and Analysis of Their Primary and Volatile Metabolites. Molecules, 25(23), 5567. https://doi.org/10.3390/molecules25235567







