Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury
Abstract
1. Introduction
2. Results and Discussion
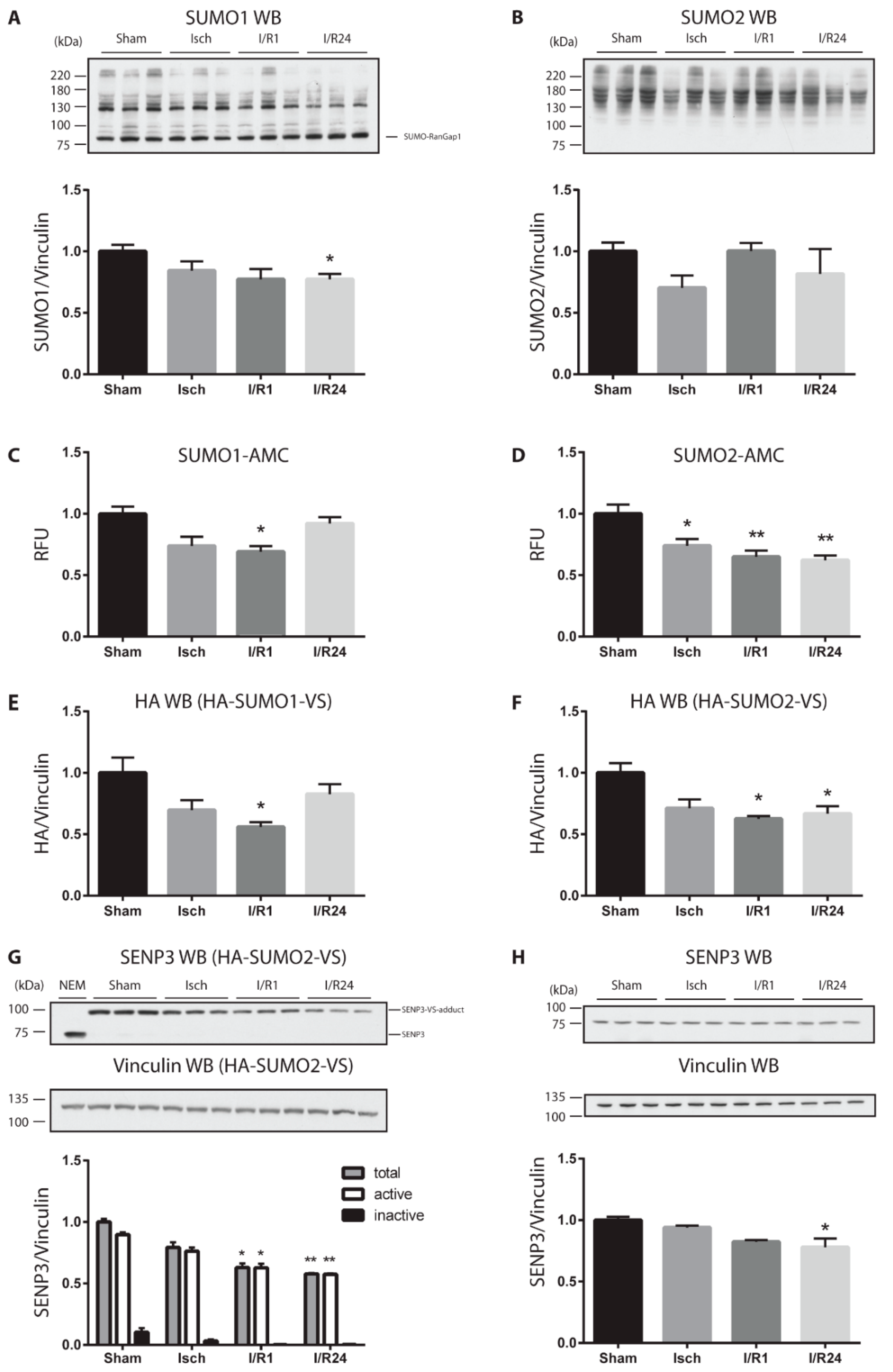
2.1. Cardiac Ischemia Reduces Global Protein SUMOylation and Decreases the Amount and Catalytic Activity of deSUMOylating Enzymes
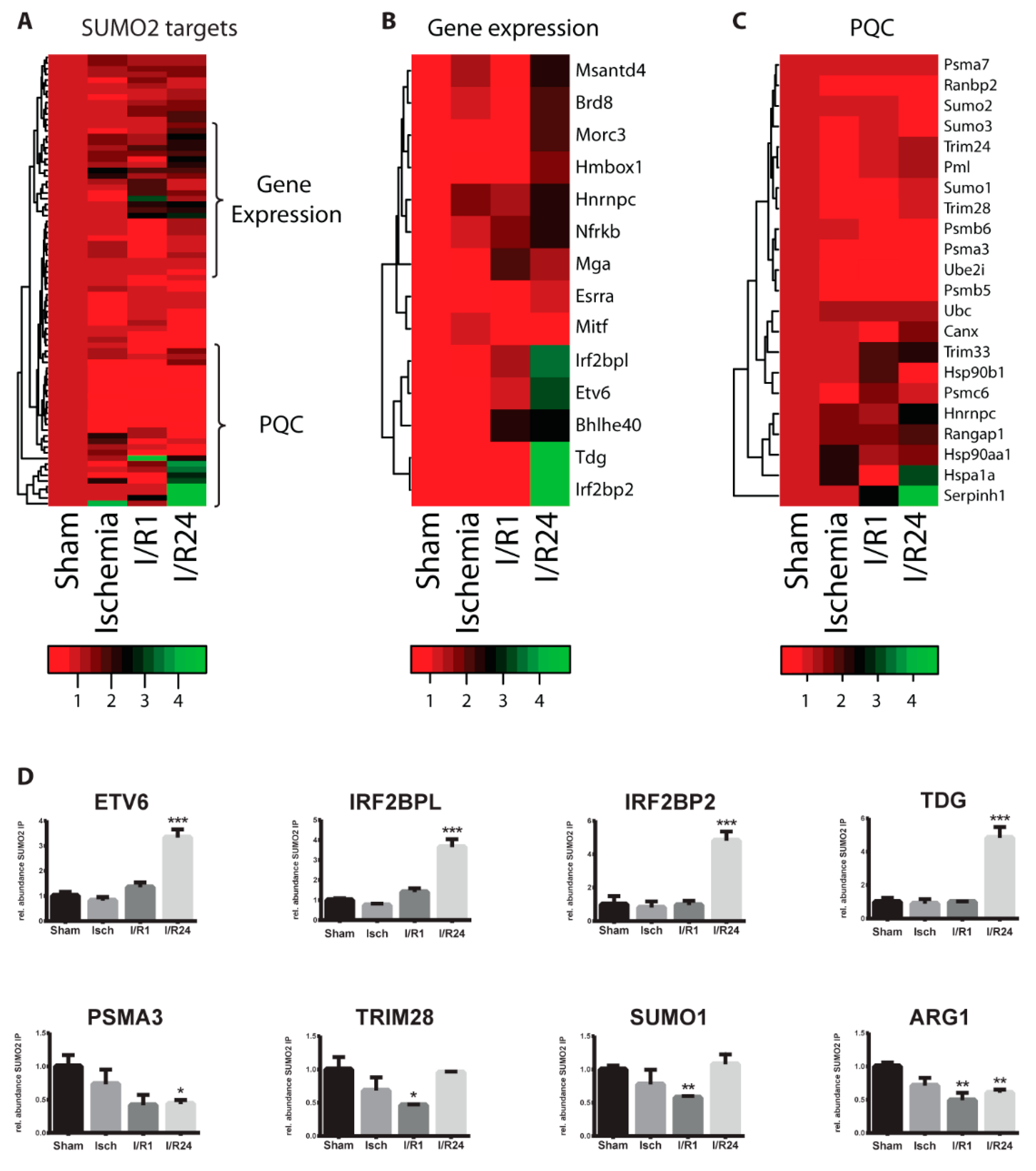
2.2. Identification of SUMO Targets in Murine Heart
2.3. Dynamic Changes of SUMO Target Proteins During Cardiac I/R Injury
3. Conclusions
4. Materials and Methods
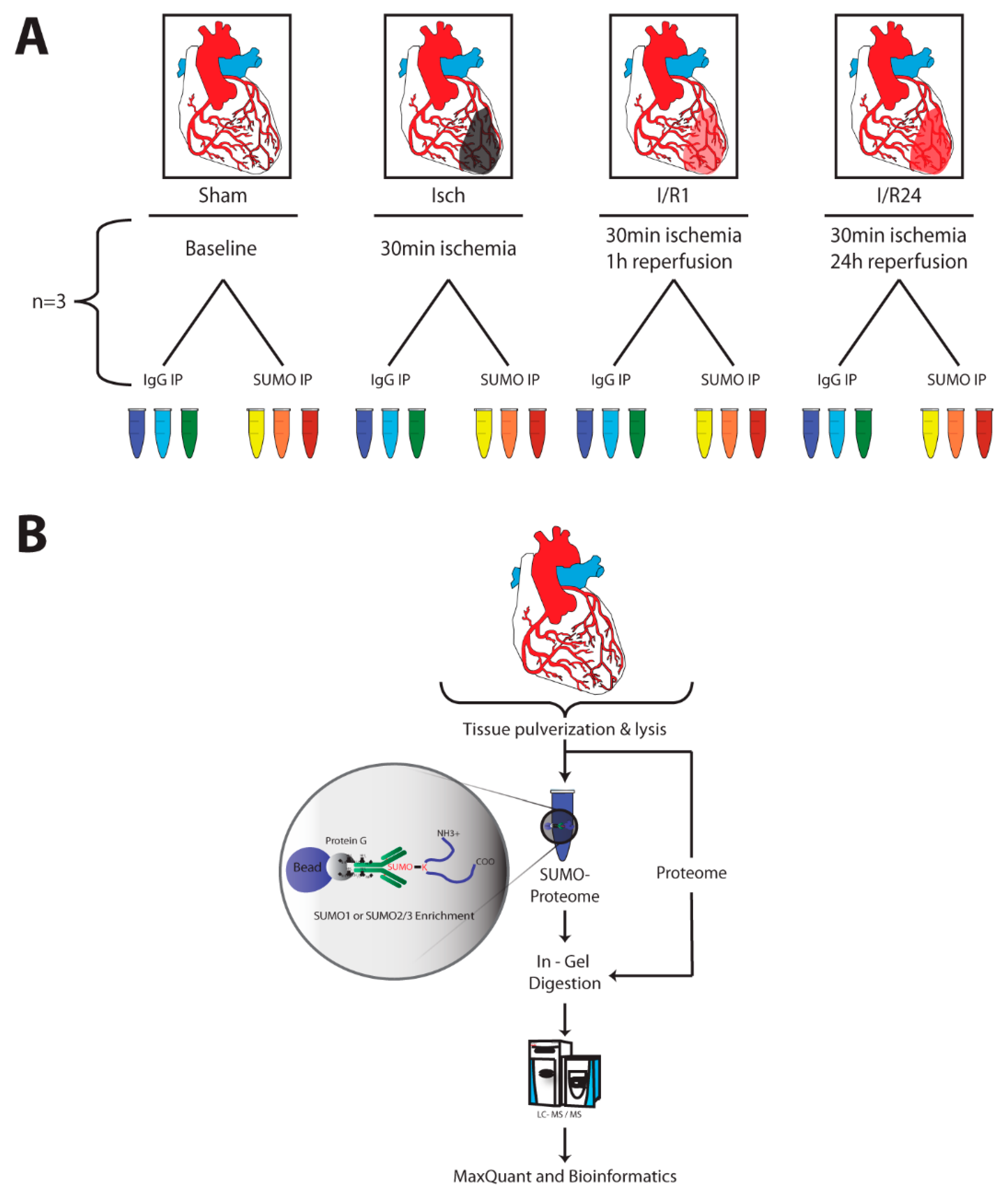
4.1. Animal Model for Cardiac Ischemia and Reperfusion
4.2. Cardiac Troponin-I ELISA
4.3. Tissue Homogenization
4.4. SUMO1/2-AMC Cleavage Assays for SUMO Protease Activity Measurements
4.5. SUMO1/2-VS Adduct Formation Assays for SUMO Protease Activity Measurements
4.6. SDS-PAGE and Western Blot Analysis
4.7. SUMO Immunoprecipitation (IP)
4.8. Sample Preparation, Liquid Chromatography and Mass Spectrometry (MS)
4.9. Quantification and Statistical Analysis
4.10. Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, Y.; Sen, S.; Anway, R.; Shuros, A.; Anand, I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J. Am. Coll. Cardiol. 2004, 43, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kairouz, V.; Lipskaia, L.; Hajjar, R.J.; Chemaly, E.R. Molecular targets in heart failure gene therapy: Current controversies and translational perspectives. Ann. N. Y. Acad. Sci. 2012, 1254, 42–50. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Keaney, J.F.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Hear. J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef]
- Flotho, A.; Melchior, F. Sumoylation: A Regulatory Protein Modification in Health and Disease. Annu. Rev. Biochem. 2013, 82, 357–385. [Google Scholar] [CrossRef]
- Mendler, L.; Braun, T.; Müller, S. The Ubiquitin-Like SUMO System and Heart Function. Circ. Res. 2016, 118, 132–144. [Google Scholar] [CrossRef]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef]
- Kunz, K.; Piller, T.; Müller, S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J. Cell Sci. 2018, 131, jcs211904. [Google Scholar] [CrossRef]
- Nayak, A.; Müller, S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. 2014, 15, 422. [Google Scholar] [CrossRef]
- Müller, S.; Hoege, C.; Pyrowolakis, G.; Jentsch, S. Sumo, ubiquitin’s mysterious cousin. Nat. Rev. Mol. Cell Biol. 2001, 2, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.D. The Fast-Growing Business of SUMO Chains. Mol. Cell 2008, 32, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.S.; Vertegaal, A.C. A Chain of Events: Regulating Target Proteins by SUMO Polymers. Trends Biochem. Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sriramachandran, A.M.; Dohmen, R.J. SUMO-targeted ubiquitin ligases. Biochim. et Biophys. Acta (BBA) Bioenerg. 2014, 1843, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Dasso, M. Modification in reverse: The SUMO proteases. Trends Biochem. Sci. 2007, 32, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sheng, H.; Thompson, J.W.; Zhao, S.; Wang, L.; Miao, P.; Liu, X.; Moseley, M.A.; Paschen, W. Small Ubiquitin-Like Modifier 3–Modified Proteome Regulated by Brain Ischemia in Novel Small Ubiquitin-Like Modifier Transgenic Mice. Stroke 2014, 45, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, Y.; He, J.; Yan, Y.; Xu, L.; Lin, N.; Ji, Q.; Tong, R.; Fu, Y.; Gao, Y.; et al. The desumoylating enzyme sentrin-specific protease 3 contributes to myocardial ischemia reperfusion injury. J. Genet. Genom. 2018, 45, 125–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.-M.; Wang, C.-X.; Gu, J.-M.; Xue, S. SENP3 protects H9C2 cells from apoptosis triggered by H/R via STAT3 pathway. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 2778–2786. [Google Scholar]
- Bian, X.; Xu, J.; Zhao, H.; Zheng, Q.; Xiaozhi, L.; Ma, X.; Li, Y.; Du, X.; Liu, X. Zinc-Induced SUMOylation of Dynamin-Related Protein 1 Protects the Heart against Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Rawlings, N.; Lee, L.; Nakamura, Y.; Wilkinson, K.A.; Henley, J.M. Protective role of the deSUMOylating enzyme SENP3 in myocardial ischemia-reperfusion injury. PLoS ONE 2019, 14, e0213331. [Google Scholar] [CrossRef]
- Engle, S.K.; Jordan, W.H.; Pritt, M.L.; Chiang, A.Y.; Davis, M.A.; Zimmermann, J.L.; Rudmann, D.G.; Heinz-Taheny, K.M.; Irizarry, A.R.; Yamamoto, Y.; et al. Qualification of Cardiac Troponin I Concentration in Mouse Serum Using Isoproterenol and Implementation in Pharmacology Studies to Accelerate Drug Development. Toxicol. Pathol. 2009, 37, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Frobert, A.; Valentin, J.; Magnin, J.-L.; Riedo, E.; Cook, S.; Giraud, M.-N. Prognostic Value of Troponin I for Infarct Size to Improve Preclinical Myocardial Infarction Small Animal Models. Front. Physiol. 2015, 6, 353. [Google Scholar] [CrossRef] [PubMed]
- Kunz, K.; Wagner, K.; Mendler, L.; Hölper, S.; Dehne, N.; Muller, S.C. SUMO Signaling by Hypoxic Inactivation of SUMO-Specific Isopeptidases. Cell Rep. 2016, 16, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Kunz, K.; Muller, S.C.; Mendler, L. Assays of SUMO protease/isopeptidase activity and function in mammalian cells and tissues. Methods Enzym. 2019, 618, 389–410. [Google Scholar] [CrossRef]
- Madu, I.G.; Chen, Y. Assays for Investigating deSUMOylation Enzymes. Curr. Protoc. Mol. Biol. 2012, 99, 10.30.1–10.30.13. [Google Scholar] [CrossRef] [PubMed]
- Barysch, S.V.; Dittner, C.; Flotho, A.; Becker, J.; Melchior, F. Identification and analysis of endogenous SUMO1 and SUMO2/3 targets in mammalian cells and tissues using monoclonal antibodies. Nat. Protoc. 2014, 9, 896–909. [Google Scholar] [CrossRef]
- Gärtner, A.; Muller, S.C. PML, SUMO, and RNF4: Guardians of nuclear protein quality. Mol. Cell 2014, 55, 1–3. [Google Scholar] [CrossRef]
- Keiten-Schmitz, J.; Wagner, K.; Piller, T.; Kaulich, M.; Alberti, S.; Müller, S. The Nuclear SUMO-Targeted Ubiquitin Quality Control Network Regulates the Dynamics of Cytoplasmic Stress Granules. Mol. Cell 2020, 79, 54–67. [Google Scholar] [CrossRef]
- Han, Y.; Huang, C.; Sun, X.; Xiang, B.; Wang, M.; Yeh, E.T.H.; Chen, Y.; Li, H.; Shi, G.; Cang, H.; et al. SENP3-mediated De-conjugation of SUMO2/3 from Promyelocytic Leukemia Is Correlated with Accelerated Cell Proliferation under Mild Oxidative Stress. J. Biol. Chem. 2010, 285, 12906–12915. [Google Scholar] [CrossRef]
- Bernardi, R.; Papa, A.; Pandolfi, P.P. Regulation of apoptosis by PML and the PML-NBs. Oncogene 2008, 27, 6299–6312. [Google Scholar] [CrossRef]
- Palibrk, V.; Suganthan, R.; Scheffler, K.; Wang, W.; Bjørås, M.; Bøe, S.O. PML regulates neuroprotective innate immunity and neuroblast commitment in a hypoxic–ischemic encephalopathy model. Cell Death Dis. 2016, 7, e2320. [Google Scholar] [CrossRef] [PubMed]
- Lallemand-Breitenbach, V.; Jeanne, M.; Benhenda, S.; Nasr, R.; Lei, M.; Peres, L.; Zhou, J.; Raught, B.; De Thé, H. Arsenic degrades PML or PML–RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 2008, 10, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Agbor, T.A.; Cheong, A.; Comerford, K.M.; Scholz, C.C.; Bruning, U.; Clarke, A.; Cummins, E.P.; Cagney, G.; Taylor, C.T. Small Ubiquitin-related Modifier (SUMO)-1 Promotes Glycolysis in Hypoxia. J. Biol. Chem. 2010, 286, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Hajnóczky, G. VDAC2-specific cellular functions and the underlying structure. Biochim. et Biophys. Acta (BBA) Bioenerg. 2016, 1863, 2503–2514. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Shteinfer-Kuzmine, A.; Verma, A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomol. 2020, 10, 1485. [Google Scholar] [CrossRef]
- Huang, L.; Wu, K.; Zhang, L.; Wang, Q.; Tang, S.; Wu, Q.; Jiang, P.; Lin, J.J.; Guo, J.; Wang, L.; et al. Critical Roles of Xirp Proteins in Cardiac Conduction and Their Rare Variants Identified in Sudden Unexplained Nocturnal Death Syndrome and Brugada Syndrome in Chinese Han Population. J. Am. Hear. Assoc. 2018, 7, e006320. [Google Scholar] [CrossRef]
- Cai, Y.; Ying, F.; Liu, H.; Ge, L.; Song, E.; Wang, L.; Zhang, D.; Tang, E.H.C.; Xia, Z.; Irwin, M.G. Deletion of Rap1 protects against myocardial ischemia/reperfusion injury through suppressing cell apoptosis via activation of STAT3 signaling. FASEB J. 2020, 34, 4482–4496. [Google Scholar] [CrossRef]
- Barysch , S.V.; Stankovic-Valentin, N.; Karaca, S.; Doppel, J.; Achour, T.N.; Sticht, C.; Urlaub, H.; Melchior, F. Transient deSUMOylation of IRF2BP proteins controls early transcription in EGFR signaling. bioRxiv 2019, 11, 201–227. [Google Scholar] [CrossRef]
- Teng, A.C.T.; Kuraitis, D.; Deeke, S.A.; Ahmadi, A.; Dugan, S.G.; Cheng, B.L.M.; Crowson, M.G.; Burgon, P.G.; Suuronen, E.J.; Chen, H.-H.; et al. IRF2BP2 is a skeletal and cardiac muscle-enriched ischemia-inducible activator of VEGFA expression. FASEB J. 2010, 24, 4825–4834. [Google Scholar] [CrossRef]
- Schlüter, K.-D.; Schulz, R.; Schreckenberg, R. Arginase induction and activation during ischemia and reperfusion and functional consequences for the heart. Front. Physiol. 2015, 6, 65. [Google Scholar] [CrossRef]
- Taniguchi, T.; Maruyama, N.; Ogata, T.; Kasahara, T.; Nakanishi, N.; Miyagawa, K.; Naito, D.; Hamaoka, T.; Nishi, M.; Matoba, S.; et al. PTRF/Cavin-1 Deficiency Causes Cardiac Dysfunction Accompanied by Cardiomyocyte Hypertrophy and Cardiac Fibrosis. PLoS ONE 2016, 11, e0162513. [Google Scholar] [CrossRef] [PubMed]
- Jansa, P.; Burek, C.; Sander, E.E.; Grummt, I. The transcript release factor PTRF augments ribosomal gene transcription by facilitating reinitiation of RNA polymerase I. Nucleic Acids Res. 2001, 29, 423–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haase, H. Ahnak, a new player in β-adrenergic regulation of the cardiac L-type Ca2+ channel. Cardiovasc. Res. 2007, 73, 19–25. [Google Scholar] [CrossRef]
- Ponnalagu, D.; Gururaja-Rao, S.; Farber, J.; Xin, W.; Hussain, A.T.; Shah, K.; Tanda, S.; Berryman, M.; Edwards, J.C.; Singh, H. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 2016, 27, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, R.J.; Gu, H.; Zhu, Y.; Leonard, M.; Ahmad, A.S.; Clauser, K.R.; Meyer, J.G.; Bennett, E.J.; Komives, E.A. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.A.; Lyon, D.; Su, D.; Skotte, N.H.; Daniel, J.A.; Jensen, L.J.; Nielsen, M.L. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hildick, K.L.; Luo, J.; Dearden, L.A.; Wilkinson, K.; Henley, J.M. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 2013, 32, 1514–1528. [Google Scholar] [CrossRef]
- Gu, J.; Fan, Y.; Liu, X.; Zhou, L.; Cheng, J.; Cai, R.; Xue, S. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1α-dependent pathway. Cardiovasc. Res. 2014, 104, 83–92. [Google Scholar] [CrossRef]
- Wagner, K.; Kunz, K.; Piller, T.; Tascher, G.; Hölper, S.; Stehmeier, P.; Keiten-Schmitz, J.; Schick, M.; Keller, U.; Muller, S.C. The SUMO Isopeptidase SENP6 Functions as a Rheostat of Chromatin Residency in Genome Maintenance and Chromosome Dynamics. Cell Rep. 2019, 29, 480–494. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M.J. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and Go Extraction Tips for Matrix-Assisted Laser Desorption/Ionization, Nanoelectrospray, and LC/MS Sample Pretreatment in Proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Michalski, A.; Iepsen, E.W. Software Lock Mass by Two-Dimensional Minimization of Peptide Mass Errors. J. Am. Soc. Mass Spectrom. 2011, 22, 1373–1380. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]


Sample Availability: The sample of compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotz, P.W.; Wiesnet, M.; Tascher, G.; Braun, T.; Müller, S.; Mendler, L. Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury. Molecules 2020, 25, 5571. https://doi.org/10.3390/molecules25235571
Hotz PW, Wiesnet M, Tascher G, Braun T, Müller S, Mendler L. Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury. Molecules. 2020; 25(23):5571. https://doi.org/10.3390/molecules25235571
Chicago/Turabian StyleHotz, Paul W., Marion Wiesnet, Georg Tascher, Thomas Braun, Stefan Müller, and Luca Mendler. 2020. "Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury" Molecules 25, no. 23: 5571. https://doi.org/10.3390/molecules25235571
APA StyleHotz, P. W., Wiesnet, M., Tascher, G., Braun, T., Müller, S., & Mendler, L. (2020). Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury. Molecules, 25(23), 5571. https://doi.org/10.3390/molecules25235571






