A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions
Abstract
1. Introduction
2. Theoretical Methods of Estimating the Energy of Intramolecular Interactions
2.1. Conformational Methods
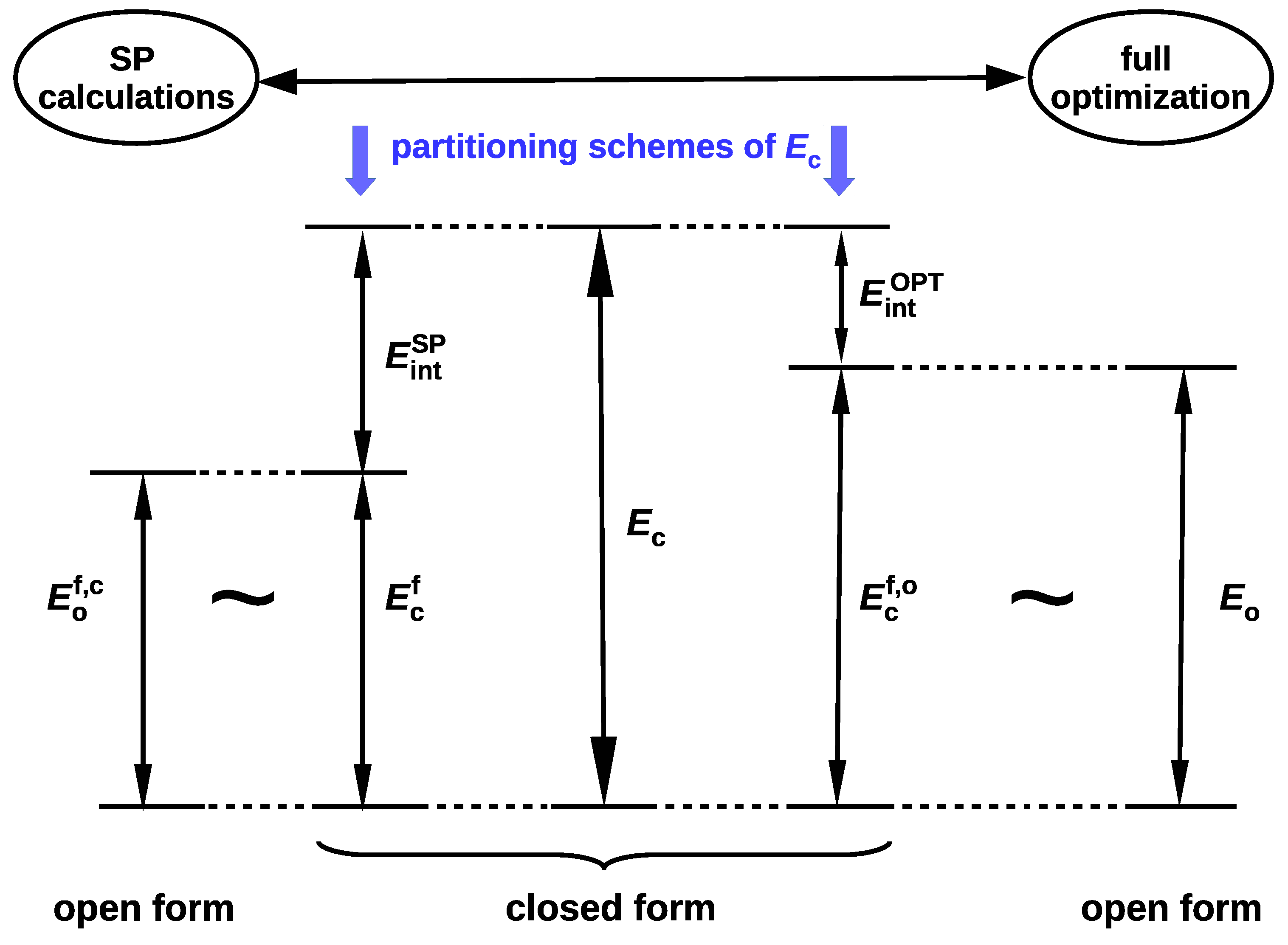
2.1.1. The Open-Closed Method (OCM)
2.1.2. Ortho-Para Method (opM)
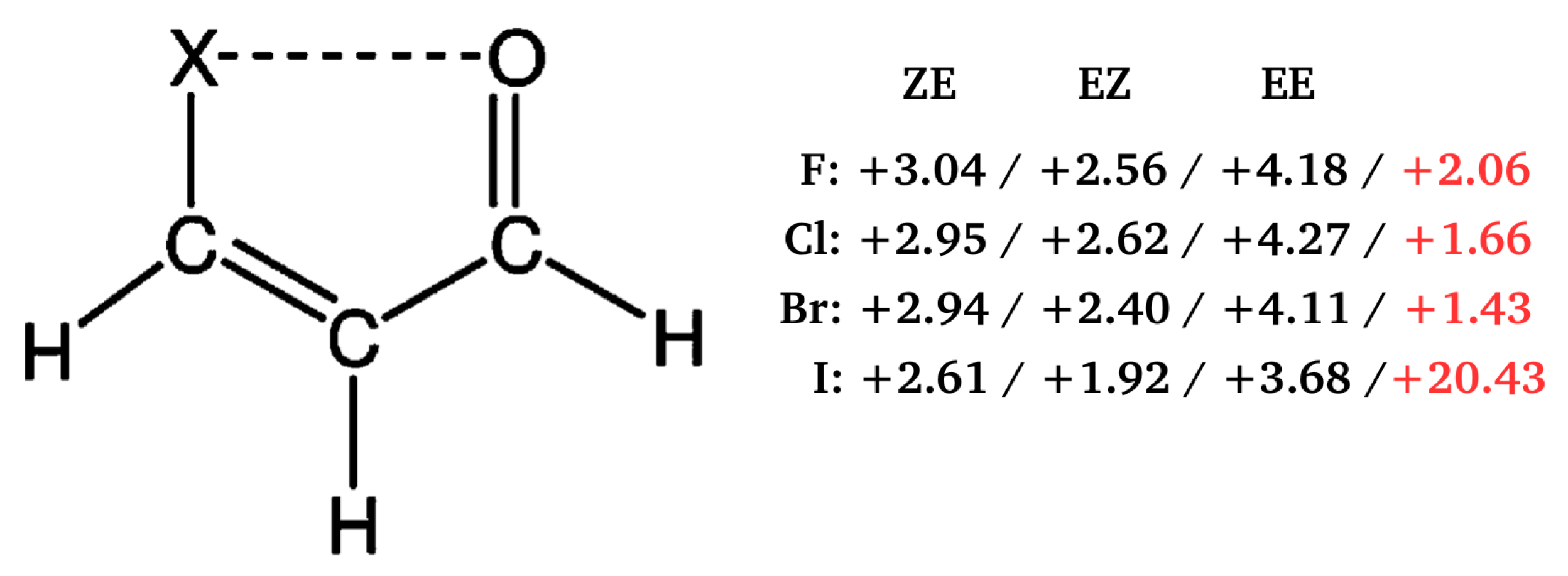
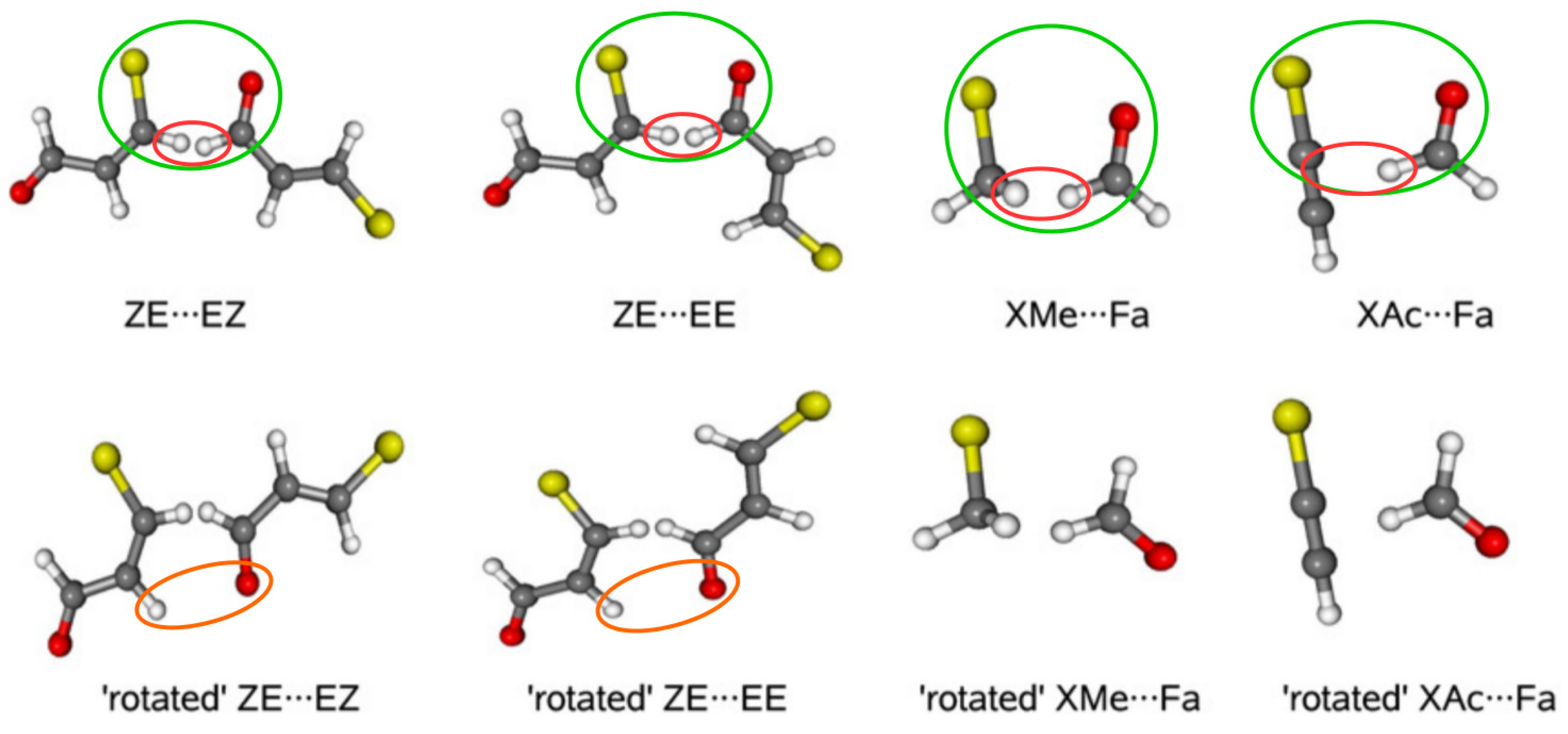
2.1.3. Related Rotamers Method (RRM)
2.1.4. Geometry-Corrected Method (GCM)
2.1.5. Geometry-Corrected Related Rotamer Method (GCRRM)
2.2. Rotation Barriers Method (RBM)
2.3. Dimer Model (DM)
2.4. Isodesmic Reactions Method (IRM)
2.5. QTAIM-Based Methods
2.5.1. Espinosa’s Method (EM)
2.5.2. Interacting Quantum Atoms (IQA)
2.6. Empirically-Based Methods
2.6.1. Iogansen’s Relationship
2.6.2. Chemical Shift—Based Method
3. Summary
Funding
Conflicts of Interest
Abbreviations
| OCM | Open-Closed Method |
| opM | Ortho-Para Method |
| RRM | Related Rotamers Method |
| GCM | Geometry-Corrected Method |
| GCRRM | Geometry-Corrected Related Rotamers Method |
| RBM | Rotation Barriers Method |
| DM | Dimer Model |
| IRM | Isodesmic Reactions Method |
| EM | Espinosa’s Method |
| QTAIM | Quantum Theory of Atoms in Molecules |
| IQA | Interacting Quantum Atoms |
| BP | bond path |
| BCP | bond critical point |
References
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: New York, NY, USA, 1960. [Google Scholar]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman & Co.: San Francisco, CA, USA, 1960. [Google Scholar]
- Hamilton, W.C.; Ibers, J.A. Hydrogen Bonding in Solids; W. A. Benjamin: New York, NY, USA, 1968. [Google Scholar]
- Vinogradov, S.N.; Linnell, R.H. Hydrogen Bonding; Van Nostrand-Reinhold: Princeton, NJ, USA, 1971. [Google Scholar]
- Schuster, P.; Zundel, G.; Sandorfy, C. (Eds.) The Hydrogen Bond. Recent Developments in Theory and Experiments; North Holland: Amsterdam, The Netherlands, 1976; Volumes I–III. [Google Scholar]
- Schuster, P. Intermolecular Interactions: From Diatomics to Biopolymers; Pullman, B., Ed.; Wiley-Interscience: New York, NY, USA, 1978. [Google Scholar]
- Hobza, P.; Zahradník, R. Weak Intermolecular Interactions in Chemistry and Biology; Academia: Prague, Czech Republic, 1980. [Google Scholar]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Hadži, D. (Ed.) Theoretical Treatments of Hydrogen Bonding; Wiley: Chichester, UK, 1997. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Scheiner, S. (Ed.) Molecular Interactions. From van der Waals to Strongly Bound Complexes; Wiley: Chichester, UK, 1997. [Google Scholar]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Grabowski, S.J. Hydrogen Bonding—New Insights. Challenges and Advances in Computational Chemistry and Physics; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Maréchal, Y. The Hydrogen Bond and the Water Molecule; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond. Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Frenking, G.; Shaik, S. (Eds.) The Chemical Bond; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014. [Google Scholar]
- Piela, L. Ideas of Quantum Chemistry; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Schuster, P. LCAO-MO-Beschreibung intramolekularer Wasserstoffbrücken. Monatshefte Chem. 1969, 100, 2084–2095. [Google Scholar] [CrossRef]
- Dietrich, S.W.; Jorgensen, E.C.; Kollman, P.A.; Rothenberg, S. A Theoretical Study of Intramolecular Hydrogen Bonding in Ortho-Substituted Phenols and Thiophenols. J. Am. Chem. Soc. 1976, 98, 8310–8324. [Google Scholar] [CrossRef]
- Emsley, J. The Composition, Structure and Hydrogen Bonding of the β-Diketones. Struct. Bond. 1984, 57, 147–191. [Google Scholar]
- Buemi, G.; Gandolfo, C. Malondialdehyde and Acetylacetone: An AM1 Study of their Molecular Structures and Keto-Enol Tautomerism. J. Chem. Soc. Faraday Trans. 1989, 85, 215–227. [Google Scholar] [CrossRef]
- Millefiori, S.; Di Bella, S. Hydrogen Bonding and Tautomerism in 3-Substituted β-Thioxoketones: An Ab Initio Molecular Orbital Study. J. Chem. Soc. Faraday Trans. 1991, 87, 1297–1302. [Google Scholar] [CrossRef]
- Craw, J.S.; Bacskay, G.B. Quantum-chemical Studies of Hydrogen Bonding involving Thioxoketones, Thienols, Thioformaldehyde and Hydrogen Sulfide with Specific Reference to the Strength of Intramolecular Hydrogen Bonds. J. Chem. Soc. Faraday Trans. 1992, 88, 2315–2321. [Google Scholar] [CrossRef]
- Luth, K.; Scheiner, S. Excited-State Energetics and Proton-Transfer Barriers in Malonaldehyde. J. Phys. Chem. 1994, 98, 3582–3587. [Google Scholar] [CrossRef]
- Lampert, H.; Mikenda, W.; Karpfen, A. Intramolecular Hydrogen Bonding in 2-Hydroxybenzoyl Compounds: Infrared Spectra and Quantum Chemical Calculations. J. Phys. Chem. 1996, 100, 7418–7425. [Google Scholar] [CrossRef]
- Scheiner, S.; Kar, T.; Čuma, M. Excited State Intramolecular Proton Transfer in Anionic Analogues of Malonaldehyde. J. Phys. Chem. A 1997, 101, 5901–5909. [Google Scholar] [CrossRef]
- Chung, G.; Kwon, O.; Kwon, Y. Theoretical Study on 1,2-Dihydroxybenzene and 2-Hydroxythiophenol: Intramolecular Hydrogen Bonding. J. Phys. Chem. A 1997, 101, 9415–9420. [Google Scholar] [CrossRef]
- Cuma, M.; Scheiner, S.; Kar, T. Effect of adjoining aromatic ring upon excited state proton transfer, o-hydroxybenzaldehyde. J. Mol. Struct. 1999, 467, 37–49. [Google Scholar] [CrossRef]
- Forés, M.; Scheiner, S. Effects of chemical substitution upon excited state proton transfer. Fluoroderivatives of salicylaldimine. Chem. Phys. 1999, 246, 65–74. [Google Scholar] [CrossRef]
- Koll, A.; Rospenk, M.; Jagodzińska, E.; Dziembowska, T. Dipole moments and conformation of Schiff bases with intramolecular hydrogen bonds. J. Mol. Struct. 2000, 552, 193–204. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Intramolecular Hydrogen Bonds in ortho-Substituted Hydroxybenzenes and in 8-Susbtituted 1-Hydroxynaphthalenes: Can a Methyl Group Be an Acceptor of Hydrogen Bonds? J. Phys. Chem. A 2001, 105, 10462–10467. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F. Is the intramolecular hydrogen bond energy valuable from internal rotation barriers? J. Mol. Struct. 2002, 581, 71–85. [Google Scholar] [CrossRef]
- Kovács, A.; Szabó, A.; Hargittai, I. Structural Characteristics of Intramolecular Hydrogen Bonding in Benzene Derivatives. Acc. Chem. Res. 2002, 35, 887–894. [Google Scholar] [CrossRef]
- Korth, H.-G.; de Heer, M.I.; Mulder, P. A DFT Study on Intramolecular Hydrogen Bonding in 2-Substituted Phenols: Conformations, Enthalpies, and Correlation with Solute Parameters. J. Phys. Chem. A 2002, 106, 8779–8789. [Google Scholar] [CrossRef]
- Grabowski, S.J. π-Electron delocalisation for intramolecular resonance assisted hydrogen bonds. J. Phys. Org. Chem. 2003, 16, 797–802. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonding strength–measures based on geometric and topological parameters. J. Phys. Org. Chem. 2004, 17, 18–31. [Google Scholar] [CrossRef]
- Estácio, S.G.; do Couto, P.C.; Costa Cabral, B.J.; Minas da Piedade, M.E.; Martinho Simões, J.A. Energetics of Intramolecular Hydrogen Bonding in Di-substituted Benzenes by the ortho–para Method. J. Phys. Chem. A 2004, 108, 10834–10843. [Google Scholar] [CrossRef]
- Jabłoński, M.; Kaczmarek, A.; Sadlej, A.J. Estimates of the Energy of Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M.; Sadlej, A.J. Blue-Shifting Intramolecular C–H⋯O Interactions. J. Phys. Chem. A 2007, 111, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. Blue-shifting intramolecular C–H⋯O(S) contacts in sterically strained systems. J. Mol. Struct. 2007, 820, 118–127. [Google Scholar]
- Jabłoński, M. Full vs. constrain geometry optimization in the open–closed method in estimating the energy of intramolecular charge-inverted hydrogen bonds. Chem. Phys. 2010, 376, 76–83. [Google Scholar] [CrossRef]
- Jabłoński, M. Energetic and Geometrical Evidence of Nonbonding Character of Some Intramolecular Halogen⋯Oxygen and Other Y⋯Y Interactions. J. Phys. Chem. A 2012, 116, 3753–3764. [Google Scholar] [CrossRef]
- Jabłoński, M.; Monaco, G. Different Zeroes of Interaction Energies As the Cause of Opposite Results on the Stabilizing Nature of C–H⋯O Intramolecular Interactions. J. Chem. Inf. Model. 2013, 53, 1661–1675. [Google Scholar] [CrossRef]
- Jabłoński, M. Strength of Si–H⋯B charge-inverted hydrogen bonds in 1-silacyclopent-2-enes and 1-silacyclohex-2-enes. Struct. Chem. 2017, 28, 1697–1706. [Google Scholar] [CrossRef]
- Honacker, C.; Kappelt, B.; Jabłoński, M.; Hepp, A.; Layh, M.; Rogel, F.; Uhl, W. Aluminium Functionalized Germanes: Intramolecular Activation of Ge-H Bonds, Formation of a Dihydrogen Bond and Facile Hydrogermylation of Unsaturated Substrates. Eur. J. Inorg. Chem. 2019, 3287–3300. [Google Scholar] [CrossRef]
- Jabłoński, M. Enaminoketony: Teoretyczne Badania Dynamiki Wewnątrzmolekularnej w Układzie Modelowym 3-Aminoakroleiny. Master’s Thesis, Nicolaus Copernicus University, Toruń, Poland, 2000. [Google Scholar]
- Buemi, G. AM1 Study of Tautomerism and Intramolecular Hydrogen Bonding in Thiomalondialdehyde and Thioacetylacetone. J. Chem. Soc. Faraday Trans. 1990, 86, 2813–2818. [Google Scholar] [CrossRef]
- Buemi, G. Intramolecular Hydrogen Bonding of Malondialdehyde and its Monothio and Dithio Analogues Studied by the PM3 Method. J. Mol. Struct. 1990, 208, 253–260. [Google Scholar] [CrossRef]
- Gonzales, L.; Mó, O.; Yáñez, M. High-Level ab Initio Calculations on the Intramolecular Hydrogen Bond in Thiomalonaldehyde. J. Phys. Chem. A 1997, 101, 9710–9719. [Google Scholar] [CrossRef]
- Jabłoński, M. Binding of X-H to the lone-pair vacancy: Charge-inverted hydrogen bond. Chem. Phys. Lett. 2009, 477, 374–376. [Google Scholar] [CrossRef]
- Jabłoński, M. Ten years of charge-inverted hydrogen bonds. Struct. Chem. 2020, 31, 61–80. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Tok, O.L.; Milius, W.; Khan, A.; Badshah, A. 1-Silacyclopent-2-enes and 1-silacyclohex-2-enes bearing functionally substituted silyl groups in 2-positions. Novel electron-deficient Si–H–B bridges. Appl. Organomet. Chem. 2006, 20, 99–105. [Google Scholar] [CrossRef]
- Pinto, S.S.; Diogo, H.P.; Guedes, R.C.; Costa Cabral, B.J.; Minas da Piedade, M.E.; Martinho Simões, J.A. Energetics of Hydroxybenzoic Acids and of the Corresponding Carboxyphenoxyl Radicals. Intramolecular Hydrogen Bonding in 2-Hydroxybenzoic Acid. J. Phys. Chem. A 2005, 109, 9700–9708. [Google Scholar] [CrossRef]
- Roy, D.; Sunoj, R.B. Quantification of Intramolecular Nonbonding Interactions in Organochalcogens. J. Phys. Chem. A 2006, 110, 5942–5947. [Google Scholar] [CrossRef]
- Hammett, L.P. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Hammett, L.P. Physical Organic Chemistry; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Stępień, B.T. Sigma- and Pi-Electron Delocalization: Focus on Substituent Effects. Chem. Rev. 2005, 105, 3482–3512. [Google Scholar] [CrossRef]
- Lipkowski, P.; Koll, A.; Karpfen, A.; Wolschann, P. An approach to estimate the energy of the intramolecular hydrogen bond. Chem. Phys. Lett. 2002, 360, 256–263. [Google Scholar] [CrossRef]
- Nowroozi, A.; Raissi, H.; Farzad, F. The presentation of an approach for estimating the intramolecular hydrogen bond strength in conformational study of β-Aminoacrolein. J. Mol. Struct. 2005, 730, 161–169. [Google Scholar] [CrossRef]
- Nowroozi, A.; Raissi, H.; Hajiabadi, H.; Mohammadzadeh Jahani, P. Reinvestigation of Intramolecular Hydrogen Bond in Malonaldehyde Derivatives: An Ab initio, AIM and NBO Study. Int. J. Quant. Chem. 2011, 111, 3040–3047. [Google Scholar] [CrossRef]
- Nowroozi, A.; Masumian, E. Comparative study of NH⋯O and NH⋯S intramolecular hydrogen bonds in β-aminoacrolein, β-thioaminoacrolein and their halogenated derivatives by some usual methods. Struct. Chem. 2017, 28, 587–596. [Google Scholar] [CrossRef]
- Tayyari, S.F.; Fazli, M.; Milani-nejad, F. Molecular conformation and intramolecular hydrogen bonding in 4-amino-3-penten-2-one. J. Mol. Struct. 2001, 541, 11–15. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F.; Venuvanalingam, P.; Ramalingam, M. Ab initio study of tautomerism and hydrogen bonding of β-carbonylamine in the gas phase and in water solution. Theor. Chem. Acc. 2000, 104, 226–234. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F. Molecular conformations and intramolecular hydrogen bonding of 3-formylmalondialdehyde and 3-formylacetylacetone. An ab initio study. Electr. J. Theor. Chem. 1997, 2, 118–129. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F.; Venuvanalingam, P.; Ramalingam, M.; Ammal, S.S.C. Ab initio study of formazan and 3-nitroformazan. J. Chem. Soc. Faraday Trans. 1998, 94, 3313–3319. [Google Scholar] [CrossRef][Green Version]
- Buemi, G. Ab initio DFT study of the hydrogen bridges in hexafluoro-acetylacetone, trifluoro-acetylacetone and some 3-substituted derivatives. J. Mol. Struct. 2000, 499, 21–34. [Google Scholar] [CrossRef]
- Buemi, G. Ab initio study of 2,4-dihalosubstituted malonaldehyde and 2-halo-phenols in gas phase and solution. Chem. Phys. 2002, 277, 241–256. [Google Scholar] [CrossRef]
- Buemi, G. Ab initio DFT study of the rotation barriers and competitive hydrogen bond energies (in gas phase and water solution) of 2-nitroresorcinol, 4,6-dinitroresorcinol and 2-nitrophenol in their neutral and deprotonated conformations. Chem. Phys. 2002, 282, 181–195. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F. DFT study of the intramolecular hydrogen bonds in the amino and nitro-derivatives of malonaldehyde. Chem. Phys. 2004, 306, 115–129. [Google Scholar] [CrossRef]
- Ramalingam, M.; Venuvanalingam, P.; Swaminathan, J.; Buemi, G. Ab initio and DFT studies on conformations, hydrogen bonding and electronic structures of glyoxalmonoxime and its methyl derivatives. J. Mol. Struct. 2004, 712, 175–185. [Google Scholar] [CrossRef]
- Palusiak, M.; Krygowski, T.M. Unusual electron density topology and intramolecular steric π–π interaction in 1,3,5,7-cyclooctatetraene. Chem. Phys. Lett. 2009, 481, 34–38. [Google Scholar] [CrossRef]
- Jabłoński, M.; Palusiak, M. The halogen⋯oxygen interaction in 3-halogenopropenal revisited—The dimer model vs. QTAIM indications. Chem. Phys. 2013, 415, 207–213. [Google Scholar] [CrossRef]
- Palusiak, M.; Grabowski, S.J. Do intramolecular halogen bonds exist? Ab initio calculations and crystal structures’ evidences. Struct. Chem. 2007, 18, 859–865. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Radom, L.; Pople, J.A. Molecular Orbital Theory of the Electronic Structure of Organic Compounds. V. Molecular Theory of Bond Separation. J. Am. Chem. Soc. 1970, 92, 4796–4801. [Google Scholar] [CrossRef]
- Radom, L.; Hehre, W.J.; Pople, J.A. Molecular Orbital Theory of the Electronic Structure of Organic Compounds. VII. A Systematic Study of Energies, Conformations, and Bond Interactions. J. Am. Chem. Soc. 1971, 93, 289–300. [Google Scholar] [CrossRef]
- Radom, L. Ab Initio Molecular Orbital Calculations on Anions. Determination of Gas Phase Acidities. J. Chem. Soc. Chem. Commun. 1974, 403–404. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. An Alternative Approach to the Problem of Assessing Stabilization Energies in Cyclic Conjugated Hydrocarbons. Theor. Chim. Acta 1975, 38, 121–129. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. Homodesmotic Reactions for the Assessment of Stabilization Energies in Benzenoid and Other Conjugated Cyclic Hydrocarbons. J. Chem. Soc. Perkin Trans. 1976, 2, 1222–1227. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. An Alternative Approach to the Problem of Assessing Destabilization Energies (Strain Energies) in Cyclic Hydrocarbons. Tetrahedron 1976, 32, 317–323. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Brett, A.M.; Bock, C.W. Comparison of Various lsodesmic and Homodesmotic Reaction Heats with Values derived from Published Ab initio Molecular Orbital Calculations. J. Chem. Soc. Perkin Trans. 1977, 2, 1036–1047. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; von Ragué Schleyer, P.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Varnali, T.; Hargittai, I. Geometrical consequences of resonance-assisted hydrogen bonding in 2-nitrovinyl alcohol and indication of a slight attractive O⋯H interaction in 2-nitroethanol. An ab initio molecular orbital investigation. J. Mol. Struct. 1996, 388, 315–319. [Google Scholar]
- Wiberg, K.B.; Ochterski, J.W. Comparison of Different Ab Initio Theoretical Models for Calculating Isodesmic Reaction Energies for Small Ring and Related Compounds. J. Comput. Chem. 1997, 18, 108–114. [Google Scholar] [CrossRef]
- Howard, S.T. Relationship between Basicity, Strain, and Intramolecular Hydrogen-Bond Energy in Proton Sponges. J. Am. Chem. Soc. 2000, 122, 8238–8244. [Google Scholar] [CrossRef]
- Sanz, P.; Mó, O.; Yáñez, M. Characterization of intramolecular hydrogen bonds and competitive chalcogen–chalcogen interactions on the basis of the topology of the charge density. Phys. Chem. Chem. Phys. 2003, 5, 2942–2947. [Google Scholar] [CrossRef]
- Cyrański, M.K. Energetic Aspects of Cyclic Pi-Electron Delocalization: Evaluation of the Methods of Estimating Aromatic Stabilization Energies. Chem. Rev. 2005, 105, 3773–3811. [Google Scholar] [CrossRef]
- Raab, V.; Gauchenova, E.; Merkoulov, A.; Harms, K.; Sundermeyer, J.; Kovačević, B.; Maksić, Z.B. 1,8-Bis(hexamethyltriaminophosphazenyl)naphthalene, HMPN: A Superbasic Bisphosphazene “Proton Sponge”. J. Am. Chem. Soc. 2005, 127, 15738–15743. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Strutynska, A.; Verevkin, S.P. Enthalpies of Formation and Strain of Chlorobenzoic Acids from Thermochemical Measurements and from ab Initio Calculations. J. Phys. Chem. A 2005, 109, 4375–4380. [Google Scholar] [CrossRef]
- Deshmukh, M.M.; Suresh, C.H.; Gadre, S.R. Intramolecular Hydrogen Bond Energy in Polyhydroxy Systems: A Critical Comparison of Molecular Tailoring and Isodesmic Approaches. J. Phys. Chem. A 2007, 111, 6472–6480. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N.; Schleyer, P.; Allen, W.D. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- ukomska, M.; Rybarczyk-Pirek, A.J.; Jabłoński, M.; Palusiak, M. The nature of NO-bonding in N-oxide group. Phys. Chem. Chem. Phys. 2015, 17, 16375–16387. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules. An Introduction; Longman: Singapore, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Appllcatlons. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Cioslowski, J.; Mixon, S.T.; Edwards, W.D. Weak Bonds in the Topological Theory of Atoms in Molecules. J. Am. Chem. Soc. 1991, 113, 1083–1085. [Google Scholar] [CrossRef]
- Cioslowski, J.; Mixon, S.T. Topological Properties of Electron Density in Search of Steric Interactions in Molecules: Electronic Structure Calculations on Ortho-Substituted Biphenyls. J. Am. Chem. Soc. 1992, 114, 4382–4387. [Google Scholar] [CrossRef]
- Haaland, A.; Shorokhov, D.J.; Tverdova, N.V. Topological Analysis of Electron Densities: Is the Presence of an Atomic Interaction Line in an Equilibrium Geometry a Sufficient Condition for the Existence of a Chemical Bond? Chem. Eur. J. 2004, 10, 4416–4421. [Google Scholar] [CrossRef]
- Cerpa, E.; Krapp, A.; Vela, A.; Merino, G. The Implications of Symmetry of the External Potential on Bond Paths. Chem. Eur. J. 2008, 14, 10232–10234. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. Hydrogen–Hydrogen Bonding in Planar Biphenyl, Predicted by Atoms-in-Molecules Theory, Does Not Exist. Chem. Eur. J. 2006, 12, 2889–2895. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. A Model of the Chemical Bond Must Be Rooted in Quantum Mechanics, Provide Insight, and Possess Predictive Power. Chem. Eur. J. 2006, 12, 2902–2905. [Google Scholar] [CrossRef]
- Dem’yanov, P.; Polestshuk, P. A Bond Path and an Attractive Ehrenfest Force Do Not Necessarily Indicate Bonding Interactions: Case Study on M2X2 (M = Li, Na, K; X = H, OH, F, Cl). Chem. Eur. J. 2012, 18, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Demy’anov, P.I.; Polestshuk, P.M. Forced Bonding and QTAIM Deficiencies: A Case Study of the Nature of Interactions in He@Adamantane and the Origin of the High Metastability. Chem. Eur. J. 2013, 19, 10945–10957. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.; Joubert, L. On the physical role of exchange in the formation of an intramolecular bond path between two electronegative atoms. J. Chem. Phys. 2013, 138, 024102. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.; Joubert, L. On critical points and exchange-related properties of intramolecular bonds between two electronegative atoms. Chem. Phys. Lett. 2013, 579, 122–126. [Google Scholar] [CrossRef]
- Shahbazian, S. Why Bond Critical Points Are Not “Bond” Critical Points. Chem. Eur. J. 2018, 24, 5401–5405. [Google Scholar] [CrossRef]
- Wick, C.R.; Clark, T. On bond-critical points in QTAIM and weak interactions. J. Mol. Model. 2018, 24, 142. [Google Scholar] [CrossRef]
- Jabłoński, M. Hydride-Triel Bonds. J. Comput. Chem. 2018, 39, 1177–1191. [Google Scholar] [CrossRef]
- Jabłoński, M. Bond Paths Between Distant Atoms Do Not Necessarily Indicate Dominant Interactions. J. Comput. Chem. 2018, 39, 2183–2195. [Google Scholar] [CrossRef]
- Jabłoński, M. On the Uselessness of Bond Paths Linking Distant Atoms and on the Violation of the Concept of Privileged Exchange Channels. ChemistryOpen 2019, 8, 497–507. [Google Scholar] [CrossRef]
- Jabłoński, M. Counterintuitive bond paths: An intriguing case of the C(NO2)3- ion. Chem. Phys. Lett. 2020, 759, 137946. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strength revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Blanco, M.A.; Pendás, A.M.; Francisco, E. Interacting Quantum Atoms: A Correlated Energy Decomposition Scheme Based on the Quantum Theory of Atoms in Molecules. J. Chem. Theory Comput. 2005, 1, 1096–1109. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Francisco, F.; Rocha-Rinza, T.; Pendás, A.M. Interacting Quantum Atoms—Review. Molecules 2020, 25, 4028. [Google Scholar] [CrossRef] [PubMed]
- Gatti, C.; May, E.; Destro, R.; Cargnoni, F. Fundamental Properties and Nature of CH··O Interactions in Crystals on the Basis of Experimental and Theoretical Charge Densities. The Case of 3,4-Bis(dimethylamino)-3-cyclobutene-1,2-dione (DMACB) Crystal. J. Phys. Chem. A 2002, 106, 2707–2720. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. Bridging QTAIM with vibrational spectroscopy: The energy of intramolecular hydrogen bonds in DNA-related biomolecules. Phys. Chem. Chem. Phys. 2012, 14, 7441–7447. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef] [PubMed]
- Pendás, A.M.; Blanco, M.A.; Francisco, E. The nature of the hydrogen bond: A synthesis from the interacting quantum atoms picture. J. Chem. Phys. 2006, 125, 184112. [Google Scholar] [CrossRef]
- García-Revilla, M.; Francisco, E.; Popelier, P.L.A.; Pendás, A.M. Domain-Averaged Exchange-Correlation Energies as a Physical Underpinning for Chemical Graphs. ChemPhysChem 2013, 14, 1211–1218. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Chávez-Calvillo, R.; García-Revilla, M.; Hernández-Trujillo, J.; Christiansen, O.; Francisco, E.; Pendás, A.M.; Rocha-Rinza, T. Hydrogen-Bond Cooperative Effects in Small Cyclic Water Clusters as Revealed by the Interacting Quantum Atoms Approach. Chem. Eur. J. 2013, 19, 14304–14315. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Romero-Montalvo, E.; Gómez, V.A.M.; Chávez-Calvillo, R.; García-Revilla, M.; Francisco, E.; Pendás, A.M.; Rocha-Rinza, T. Hydrogen bond cooperativity and anticooperativity within the water hexamer. Phys. Chem. Chem. Phys. 2016, 18, 19557–19566. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Mata, I.; Molins, E.; Espinosa, E. Charged versus Neutral Hydrogen-Bonded Complexes: Is There a Difference in the Nature of the Hydrogen Bonds? Chem. Eur. J. 2016, 22, 9226–9234. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Vela, J.M.; Romero-Montalvo, E.; Costales, A.; Pendás, A.M.; Rocha-Rinza, T. The nature of resonance-assisted hydrogen bonds: A quantum chemical topology perspective. Phys. Chem. Chem. Phys. 2016, 18, 26383–26390. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Vela, J.M.; Romero-Montalvo, E.; del Río Lima, A.; Pendás, A.M.; Hernández-Rodríguez, M.; Rocha Rinza, T. Hydrogen-Bond Weakening through π Systems: Resonance-Impaired Hydrogen Bonds (RIHB). Chem. Eur. J. 2017, 23, 16605–16611. [Google Scholar] [CrossRef] [PubMed]
- Romero-Montalvo, E.; Guevara-Vela, J.M.; Costales, A.; Pendás, A.M.; Rocha-Rinza, T. Cooperative and anticooperative effects in resonance assisted hydrogen bonds in merged structures of malondialdehyde. Phys. Chem. Chem. Phys. 2017, 19, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Dabbagh, H.A.; Eskandari, K. Nature of intramolecular interactions of vitamin C in view of interacting quantum atoms: the role of hydrogen bond cooperativity on geometry. Phys. Chem. Chem. Phys. 2016, 18, 18278–18288. [Google Scholar] [CrossRef]
- Pendás, A.M.; Francisco, E.; Blanco, M.A.; Gatti, C. Bond Paths as Privileged Exchange Channels. Chem. Eur. J. 2007, 13, 9362–9371. [Google Scholar] [CrossRef]
- Badger, R.M.; Bauer, S.H. Spectroscopic Studies of the Hydrogen Bond. II. The Shift of the O–H Vibrational Frequency in the Formation of the Hydrogen Bond. J. Chem. Soc. 1937, 5, 839–851. [Google Scholar] [CrossRef]
- Joesten, M.D.; Drago, R.S. The Validity of Frequency Shift-Enthalpy Correlations. I. Adducts of Phenol with Nitrogen and Oxygen Donors. J. Am. Chem. Soc. 1962, 84, 3817–3821. [Google Scholar] [CrossRef]
- Epley, T.D.; Drago, R.S. Calorimetric Studies on Some Hydrogen-Bonded Adducts. J. Am. Chem. Soc. 1967, 89, 5770–5773. [Google Scholar] [CrossRef]
- Odinokov, S.E.; Glazunov, V.P.; Nabiullin, A.A. Infrared Spectroscopic Studies of Hydrogen Bonding in Triethylammonium Salts. Part 3.—Strong Hydrogen Bonding. J. Chem. Soc. Faraday Trans. 1984, 80, 899–908. [Google Scholar] [CrossRef]
- Ratajczak, H.; Orville-Thomas, W.J.; Rao, C.N.R. Charge transfer theory of hydrogen bonds: Relations between vibrational spectra and energy of hydrogen bonds. Chem. Phys. 1976, 17, 197–216. [Google Scholar] [CrossRef]
- Jabłoński, M.; Sadlej, A.J. Infrared and Raman Intensities in Proper and Improper Hydrogen-Bonded Systems. Pol. J. Chem. 2007, 81, 767–782. [Google Scholar]
- Pecul, M.; Leszczynski, J.; Sadlej, J. Comprehensive ab initio studies of nuclear magnetic resonance shielding and coupling constants in XH⋯O hydrogen-bonded complexes of simple organic molecules. J. Chem. Phys. 2000, 112, 7930–7938. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Buckingham, A.D. Shift of proton magnetic shielding in ClH⋯Y dimers (Y = N2, CO, BF). Mol. Phys. 2006, 104, 2527–2531. [Google Scholar] [CrossRef]
- Scheiner, S. Interpretation of Spectroscopic Markers of Hydrogen Bonds. ChemPhysChem 2016, 17, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assessment of the Presence and Strength of H-Bonds by Means of Corrected NMR. Molecules 2016, 21, 1426. [Google Scholar] [CrossRef]
- Fliegl, H.; Lehtonen, O.; Sundholm, D. Kailaz, V.R.I. Hydrogen-bond strengths by magnetically induced currents. Phys. Chem. Chem. Phys. 2011, 13, 434–437. [Google Scholar] [CrossRef]
- Monaco, G.; Della Porta, P.; Jabłoński, M.; Zanasi, R. Topology of the magnetically induced current density and proton magnetic shielding in hydrogen bonded systems. Phys. Chem. Chem. Phys. 2015, 17, 5966–5972. [Google Scholar] [CrossRef]
- Busfield, W.K.; Ennis, M.P.; McEwen, I.J. An infrared study of intramolecular hydrogen bonding in α, ω diols. Spectrochim. Acta A 1973, 29, 1259–1264. [Google Scholar] [CrossRef]
- Schaefer, T. A Relationship between Hydroxyl Proton Chemical Shifts and Torsional Frequencies in Some Ortho-Substituted Phenol Derivatives. J. Phys. Chem. 1975, 79, 1888–1890. [Google Scholar] [CrossRef]
- Takasuka, M.; Matsui, Y. Experimental Observations and CNDO/2 Calculations for Hydroxy Stretching Frequency Shifts, Intensities, and Hydrogen Bond Energies of Intramolecular Hydrogen Bonds in ortho-Substituted Phenols. J. Chem. Soc. Perkin Trans. 2 1979, 1743–1750. [Google Scholar] [CrossRef]
- Shagidullin, R.R.; Chernova, A.V.; Shagidullin, R.R. Intramolecular hydrogen bonds and conformations of the 1,4-butanediol molecule. Russ. Chem. Bull. 1993, 42, 1505–1510. [Google Scholar] [CrossRef]
- Dziembowska, T.; Szczodrowska, B.; Krygowski, T.M.; Grabowski, S.J. Estimation of the OH⋯O interaction energy in intramolecular hydrogen bonds: A comparative study. J. Phys. Org. Chem. 1994, 7, 142–146. [Google Scholar] [CrossRef]
- Vashchenko, A.V.; Afonin, A.V. Comparative estimation of the energies of intramolecular C-H⋯O, N-H⋯O, and O-H⋯O hydrogen bonds according to the QTAIM analysis and NMR spectroscopy data. J. Struct. Chem. 2014, 55, 636–643. [Google Scholar] [CrossRef]
- Iogansen, A.V.; Rassadin, B.V. Dependence of Intensity and Shift in the Infrared Bands of the Hydroxyl Group on Hydrogen Bonding Energy. J. Appl. Spectrosc. 1969, 11, 1318–1325. [Google Scholar] [CrossRef]
- Iogansen, A.V. Direct proportionality of the hydrogen bonding energy and the intensification of the stretching ν(XH) vibration in infrared spectra. Spectrochim. Acta Part A 1999, 55, 1585–1612. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Ghomi, M.; Samijlenko, S.P.; Hovorun, D.M. Comprehensive Conformational Analysis of the Nucleoside Analogue 2’-β-Deoxy-6-azacytidine by DFT and MP2 Calculations. J. Phys. Chem. B 2007, 111, 6263–6271. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Ghomi, M.; Samijlenko, S.P.; Hovorun, D.M. How Many Conformers Determine the Thymidine Low-Temperature Matrix Infrared Spectrum? DFT and MP2 Quantum Chemical Study. J. Phys. Chem. B 2007, 111, 9655–9663. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Ghomi, M.; Samijlenko, S.P.; Hovorun, D.M. Ab Initio Comprehensive Conformational Analysis of 2’-Deoxyuridine, the Biologically Significant DNA Minor Nucleoside, and Reconstruction of Its Low-Temperature Matrix Infrared Spectrum. J. Phys. Chem. B 2008, 112, 1240–1250. [Google Scholar] [CrossRef]
- Gränacher, I. Einfluss der Wasserstoffbrückenbindung auf das Kernresonanzspektrum von Phenolen. Helv. Phys. Acta 1961, 34, 272–304. [Google Scholar]
- Hobza, P.; Halvas, Z. Blue-Shifting Hydrogen Bonds. Chem. Rev. 2000, 100, 4253–4264. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Lippincott, E.R.; Schroeder, R. One-Dimensional Model of the Hydrogen Bond. J. Chem. Phys. 1955, 23, 1099–1106. [Google Scholar] [CrossRef]
- Schroeder, R.; Lippincott, E.R. Potential Function Model of Hydrogen Bonds. II. J. Phys. Chem. 1957, 61, 921–928. [Google Scholar] [CrossRef]
- Musin, R.N.; Mariam, Y.H. An integrated approach to the study of intramolecular hydrogen bonds in malonaldehyde enol derivatives and naphthazarin: Trend in energetic versus geometrical consequences. J. Phys. Org. Chem. 2006, 19, 425–444. [Google Scholar] [CrossRef]



















| System | Rotated Group | SP | P1 | P2 | P3 | P4 | P5 | OPT |
|---|---|---|---|---|---|---|---|---|
| 1 | −SiH2 | −5.31 | −4.88 | −4.41 | −3.36 | −1.50 | −0.99 | −1.08 |
| −AlH2 | −7.01 | −6.61 | −5.79 | −4.41 | −3.24 | −1.75 | ||
| 2 | −SiH2 | −5.23 | −4.75 | −3.93 | −2.46 | −2.42 | −2.42 | Al |
| −AlH2 | −8.09 | −7.37 | −6.27 | −4.85 | −4.73 | −4.72 | ||
| 3 | −SiH2 | −6.04 | −5.03 | −3.47 | −1.41 | −1.40 | −1.40 | −4.97/−0.75 |
| −AlH2 | −10.61 | −10.05 | −8.44 | −6.23 | −6.19 | −6.19 |
| Y | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | 4.77 | 3.77 | 6.50 | −6.50 | −5.28 | 1.840 | 2.141 | −0.301 | −1.87 |
| S | 6.07 | 4.09 | 6.02 | −6.02 | −6.96 | 1.968 | 2.127 | −0.159 | −1.86 |
| Molecule | ||||||
|---|---|---|---|---|---|---|
| malondialdehyde | −14.0 | −10.7 | −14.1 | −14.0 | −12.4 | −12.9 |
| acetylacetone | −16.2 | −13.3 | −15.1 | −16.9 | −12.3 | −14.5 |
| X | ||||
|---|---|---|---|---|
| F | 1.82 | 1.97 | 0.52 | 0.44 |
| Cl | 2.98 | 3.34 | 1.06 | 0.38 |
| Br | 3.49 | 3.95 | 1.08 | 0.19 |
| Type | A | B | R | |
|---|---|---|---|---|
| O-H⋯O | −239 ± 2.2 | 3.09 ± 0.07 | 0.93 | 0.86 |
| O-H⋯N | −142 ± 2.1 | −1.72 ± 0.08 | 0.97 | 0.94 |
| N-H⋯O | −225 ± 12 | 2.03 ± 0.25 | 0.85 | 0.72 |
| O-H⋯C | −288 ± 19 | 0.29 ± 0.22 | 0.86 | 0.74 |
| together | −200 ± 2.2 | 1.70 ± 0.07 | 0.88 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. https://doi.org/10.3390/molecules25235512
Jabłoński M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules. 2020; 25(23):5512. https://doi.org/10.3390/molecules25235512
Chicago/Turabian StyleJabłoński, Mirosław. 2020. "A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions" Molecules 25, no. 23: 5512. https://doi.org/10.3390/molecules25235512
APA StyleJabłoński, M. (2020). A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules, 25(23), 5512. https://doi.org/10.3390/molecules25235512






